Abstract
Lessons Learned.
A shortened infusion of ramucirumab (from 60 to 20 minutes) was safe and feasible without infusion‐related reactions.
Twenty‐minute infusions of ramucirumab can be an option for patients with no infusion‐related reactions during the first 60‐minute treatment.
Background.
Ramucirumab is usually administered over 60 minutes, during which it is unlikely to cause infusion‐related reactions (IRRs). This prospective study evaluated the safety of a shortened infusion of ramucirumab.
Methods.
Patients who received their first dose of ramucirumab in a 60‐minute infusion without developing IRRs were eligible and received their second ramucirumab dose for 20 minutes. The primary study endpoint was incidence of IRR during the first short‐term infusion, and the secondary endpoints were incidence of IRR at any time and adverse events other than IRR.
Results.
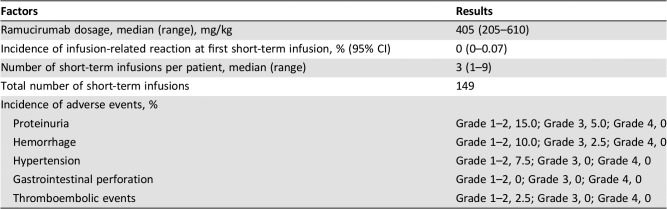
Of the 40 patients enrolled (median age, 68.5 years), 20 (55%) were male, 27 (67.5%) had stage IV gastric cancer, 25 (62.5%) received ramucirumab in combination with taxane‐based chemotherapy, and 24 (60%) received only a single administration of ramucirumab prior to their enrollment. Notably, no IRR was observed during the first short‐term infusion (IRR rate, 0%; 95% confidence interval [CI], 0%–0.72%). Among the 149 short‐term infusions performed, there were no instances of IRRs or unexpected adverse events related to the treatment (Table 1).
Conclusion.
For patients without development of IRRs upon the first ramucirumab administration, shortening infusion time (from 60 to 20 minutes) is safe and feasible.
Abstract
经验教训
• 雷莫芦单抗的短时(60 分钟到 20 分钟)注射是安全可行的,且没有注射相关反应。
• 对于前 60 分钟治疗期间未出现注射相关反应的患者,也可选择 20 分钟的雷莫芦单抗注射。
摘要
背景。通常会在 60 分钟内注射雷莫芦单抗,在此期间不太可能引起注射相关反应 (IRRs)。此项前瞻性研究评估了雷莫芦单抗短时注射的安全性。
方法。在 60 分钟注射过程中接受第一剂雷莫芦单抗注射而未出现 IRR 的患者可以接受第二剂 20 分钟雷莫芦单抗注射。主要研究终点是第一次短时注射期间 IRR 的发生率,次要终点是任何时间的 IRR 发生率和 IRR 以外的不良反应事件。
结果。在 40 位参与研究的患者中(中值年龄为 68.5 岁),有 20 人 (55%) 是男性,27 人 (67.5%) 患有 IV 期胃癌,25 人 (62.5%) 接受了雷莫芦单抗联合基于紫杉烷的化疗治疗,24 人 (60%) 在参与研究之前仅接受了雷莫芦单抗单次给药。值得注意的是,在第一次短时注射期间,未观察到 IRR(IRR 率,0%;95% 置信区间 [CI],0%‐0.72%)。在实施的 149 次短时注射中,未发现 IRR 实例或与治疗有关的意外不良反应事件(表 1)。
结论。对于在第一次雷莫芦单抗给药后未出现 IRR 的患者,缩短注射时间(60 到 20 分钟)是安全可行的。
Discussion
To the best of our knowledge, this is the first prospective study to demonstrate the safety of a shortened infusion of ramucirumab. We aimed to evaluate the safety of a short‐term infusion of ramucirumab. Our study met its primary endpoint, demonstrating that IRR rate during the first short‐term infusion was 0% (95% CI, 0%–0.72%). Additionally, among a total of 149 short‐term infusions, no IRR was observed. The frequency of ramucirumab‐related adverse events was consistent with that in previous reports, and no unexpected adverse events related to the study treatment were observed. These results demonstrated that a short‐term infusion of ramucirumab was safe. This can allow patients, particularly outpatients, to conveniently receive chemotherapy and reduce medical staff workload. We strongly believe our findings will be beneficial to both patients and medical staff.
Table 1. Results of a short‐term infusion of ramucirumab (n = 40).
Trial Information
- Disease
Advanced cancer/solid tumor only
- Stage of Disease/Treatment
Metastatic/advanced
- Prior Therapy
No designated number of regimens
- Type of Study ‐ 1
Phase II
- Type of Study ‐ 2
Single arm
- Primary Endpoint
Safety
- Secondary Endpoint
Safety
- Secondary Endpoint
Toxicity
- Additional Details of Endpoints or Study Design
Eligibility criteria included the following: (a) histologically proven gastrointestinal cancer; (b) first ramucirumab infusion administered over 60 minutes without development of an IRR; (c) no severe respiratory or cardiovascular comorbidities; and (d) no history of allergy or IRR to other chemotherapeutic agents. Our study was designed to have a maximum IRR rate of 15%, with α and β errors of .05 and .20, respectively, considering that the minimum sample size was 40 patients.
- Investigator's Analysis
Shortened infusion of ramucirumab is a safe and feasible method.
Drug Information
- Drug 1
- Generic/Working Name
Ramucirumab
- Trade Name
Cyramza
- Company Name
Eli Lilly
- Drug Type
Antibody
- Drug Class
Vascular endothelial glistItemPairth factor receptor (VEGFR)
- Dose
8 milligrams (mg) per kilogram (kg)
- Route
IV
- Schedule of Administration
Intravenous administration of ramucirumab over 20 minutes every 2 weeks in combination with paclitaxel, nanoparticle albumin‐bound paclitaxel, irinotecan with fluorouracil and leucovorin (FOLFIRI), or irinotecan.
Patient Characteristics
- Number of Patients, Male
22
- Number of Patients, Female
18
- Stage
Only metastatic or advanced; stage IV: 40 (100%)
- Age
Median (range): 68.5 (32–85)
- Number of Prior Systemic Therapies
Median (range): 1 (1–2)
- Performance Status: ECOG
-
0 — 16
1 — 23
2 — 1
3 — 0
- Cancer Types or Histologic Subtypes
Gastric cancer, 27; colorectal cancer, 13
Primary Assessment Method
- Number of Patients Screened
42
- Number of Patients Enrolled
40
- Number of Patients Evaluable for Toxicity
40
- Evaluation Method
Common Terminology Criteria for Adverse Events (CTCAE) version 4.0
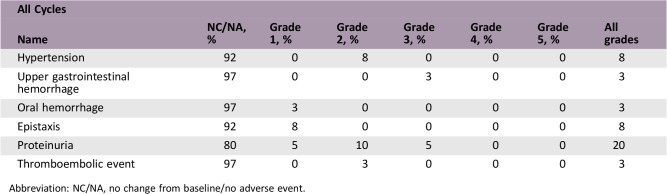
Adverse Events
Abbreviation: NC/NA, no change from baseline/no adverse event.
Assessment, Analysis, and Discussion
- Completion
Study completed
- Investigator's Assessment
Shortened infusion of ramucirumab is a safe and feasible method.
Ramucirumab is a fully human immunoglobulin G monoclonal antibody against vascular endothelial growth factor receptor‐2 (VEGFR‐2), a receptor for VEGF‐A, VEGF‐C, and VEGF‐D [1]. Ramucirumab has been shown to be effective in several cancer types, including gastric, colorectal, and non‐small cell lung cancer [2], [3], [4], [5]. In general, although antibody therapies are less toxic compared with cytotoxic agents, they have peculiar toxicity profiles. A typical adverse event is infusion‐related reaction (IRR), The symptoms of IRR include fever, chills, headache, pruritus, rash, cough, collapse, angioedema, and, in rare cases, life‐threating events such as respiratory disturbance or circulatory failure. Its mechanism is considered to be different from IgE‐mediated hypersensitivity due to type 1 allergic reaction [6]. Because infusion duration of antibody therapies may affect IRR occurrence, monoclonal antibodies are gradually administered. Ramucirumab has been administered for over 60 minutes, but no robust evidence supports this duration. Ramucirumab is a fully human protein, and IRR occurrence due to its use has been reported to be markedly low (0.4%–5.8%) [2], [3]. Several studies have shown that rapid infusion of other antibodies was safe. Salar et al. reported rapid administration of rituximab, which is more closely associated with IRR, and proposed that a 90‐minute infusion schedule was well tolerated and safe [7]. Sehn et al. also examined that a 90‐minute rituximab administration for more than 1,200 cases and reported that no grade 3 or 4 infusion reactions were observed [8]. As for bevacizumab, trastuzumab, and panitumumab, similar studies have been performed and indicated that it is possible to shorten infusion time [9], [10], [11], [12]. Therefore, we hypothesized that it is possible to shorten the infusion duration of ramucirumab for patients without IRR during the first administration.
The primary endpoint, IRR rate during the first short‐term infusion, was 0% (95% confidence interval, 0%–0.72%). Additionally, among the 149 short‐term infusions, no IRR was observed. In total, 13 patients (32.5%) developed adverse events related to ramucirumab. One (2.5%) and two (5.0%) patients developed grade 3 upper gastrointestinal hemorrhage and proteinuria, respectively. The incidence of these adverse events was consistent with that in previous reports. Therefore, our findings demonstrated that a short‐term infusion of ramucirumab was safe. In cases with no IRR upon the first administration of ramucirumab over 60 minutes, a shorter 20‐minute infusion can be used for the subsequent administrations. When ramucirumab is administered in combination with other cytotoxic drugs, it requires considerable time, increasing the burden on patients and medical staff. A shorter infusion can be beneficial to patients and medical staff.
There were a few limitations to the present study. First, data on pharmacokinetics of ramucirumab were not obtained. However, in a previous report, shortening the infusion duration did not affect the blood concentration of panitumumab [12], another fully human antibody. Second, this study included patients with differing treatment regimens, resulting in differences in terms of premedication. Our results should be validated in a larger sample sufficiently representing each regimen; however, this is the first study demonstrating that a 20‐minute ramucirumab infusion is safe and feasible for patients with gastrointestinal cancer despite these limitations. The shortened infusion time can reduce the burden of both patients and medical staff. Further studies are warranted to confirm the safety of short‐term ramucirumab infusion.
Contributed equally
Footnotes
UMIN Clinical Trial Registry: UMIN000029318
Sponsor(s): Aichi Cancer Research Foundation
Principal Investigators: Seiichiro Mitani, Naoya Hashimoto
IRB Approved: Yes
Disclosures
Seiichiro Mitani: Eli Lilly & Co. (H); Hiroya Taniguchi: Eli Lilly & Co. (H); Toshiki Masuishi: Eli Lilly & Co. (H); Shigenori Kadowaki: Eli Lilly Japan K.K. (RF, H); Kei Muro: Eli Lilly & Co., Chugai Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ono Pharmaceutical Co., Ltd., Taiho, Bayer (H), Gilead Sciences, Ono Pharmaceutical Co., Ltd., Merck Sharp & Dohme, Shionogi, Kyoma Hakko Kirin, Daiichi Sanyo (RF). The other authors reported no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Lu D, Jimenez X, Zhang H et al. Selection of high affinity human neutralizing antibodies to VEGFR2 from a large antibody phage display library for antiangiogenesis therapy. Int J Cancer 2002;20:393–399. [DOI] [PubMed] [Google Scholar]
- 2.Wilke H, Muro K, Van Cutsem E et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro‐oesophageal junction adenocarcinoma (RAINBOW): A double‐blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224–1235. [DOI] [PubMed] [Google Scholar]
- 3.Fuchs CS, Tomasek J, Yong CJ et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro‐oesophageal junction adenocarcinoma (REGARD): An international, randomized, multicenter, placebo‐controlled, phase 3 trial. Lancet 2014;383:31–39. [DOI] [PubMed] [Google Scholar]
- 4.Tabernero J, Takayuki Y, Cohn AL et al. Ramucirumab versus placebo in combination with second‐line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first‐line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): A randomized, double‐blind, multicenter, phase 3 study. Lancet Oncol 2015;16:499–508. [DOI] [PubMed] [Google Scholar]
- 5.Garon EB, Ciuleanu TE, Arrieta O et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second‐line treatment of stage IV non‐small‐cell lung cancer after disease progression on platinum‐based therapy (REVEL): A multicenter, double‐blind, randomised phase 3 trial. Lancet 2014;23:665–673. [DOI] [PubMed] [Google Scholar]
- 6.Cheifetz A, Smedley M, Martin S et al. The incidence and management of infusion reactions to infliximab: a large center experience. Am J Gastroenterol 2003;98:1315–1324. [DOI] [PubMed] [Google Scholar]
- 7.Salar A, Casao D, Cervera M et al. Rapid infusion of rituximab with or without steroid‐containing chemotherapy: 1‐yr experience in a single institution. Eur J Haematol 2006;77:338–340. [DOI] [PubMed] [Google Scholar]
- 8.Sehn LH, Donaldson J, Filewich A et al. Rapid infusion rituximab in combination with corticosteroid‐containing chemotherapy or as maintenance therapy is well tolerated and can safely be delivered in the community setting. Blood 2007;109:4171–4173. [DOI] [PubMed] [Google Scholar]
- 9.Reidy DL, Chung KY, Timoney JP et al. Bevacizumab 5 mg/kg can be infused safely over 10 minutes. J Clin Oncol 2007;25:2691–2695. [DOI] [PubMed] [Google Scholar]
- 10.Yanmaz MT, Guner SI, Satılmıs B et al. Thirty‐minutes infusion rate is safe enough for bevacizumab; no need for initial prolong infusion. Med Oncol 2014;31:276. [DOI] [PubMed] [Google Scholar]
- 11.Abe H, Umeda T, Kawai Y et al. Adjuvant trastuzumab can be infused safely over 30 minutes [in Japanese]. Gan To Kagaku Ryoho 2010;37:1887–1891. [PubMed] [Google Scholar]
- 12.Stephenson JJ, Gregory C, Burris H et al. An open‐label clinical trial evaluating safety and pharmacokinetics of two dosing schedules of panitumumab in patients with solid tumors. Clin Colorectal Cancer 2009;8:29–37. [DOI] [PubMed] [Google Scholar]