Medication management and vaccinations are particularly challenging in an older cancer population, and incorporating clinical pharmacists into the cancer care of older patients represents a potential strategy to enhance clinical outcomes for the geriatric oncology population. This article reports on the feasibility and efficacy of integrating pharmacists into the care of older adults with cancer to improve medication management and vaccination administration.
Keywords: Geriatric oncology, Cancer, Pharmacy, Inappropriate prescribing, Vaccination
Abstract
Background.
Oncology clinicians often struggle with managing medications and vaccinations in older adults with cancer. We sought to demonstrate the feasibility and preliminary efficacy of integrating pharmacists into the care of older adults with cancer to enhance medication management and vaccination administration.
Methods.
We randomly assigned patients aged ≥65 years with breast, gastrointestinal, or lung cancer receiving first‐line chemotherapy to the pharmacy intervention or usual care. Patients assigned to the intervention met with a pharmacist once during their second or third chemotherapy infusion. We obtained information about patients' medications and vaccinations via patient report and from the electronic health record (EHR) at baseline and week 4. We determined the number of discrepant (difference between patient report and EHR) and potentially inappropriate (Beers Criteria assessed by nonintervention pharmacists blinded to group assignment) medications. We defined the intervention as feasible if >75% of patients enrolled in the study and received the pharmacist visit.
Results.
From January 17, 2017, to October 27, 2017, we enrolled and randomized 60 patients (80.1% of patients approached). Among those assigned to the intervention, 96.6% received the pharmacist visit. At week 4, intervention patients had higher rates of acquiring vaccinations for pneumonia (27.6% vs. 0.0%, p = .002) and influenza (27.6% vs. 0.0%, p = .002) compared with usual care. Intervention patients had fewer discrepant (5.82 vs. 8.07, p = .094) and potentially inappropriate (3.46 vs. 4.80, p = .069) medications at week 4, although differences were not significant.
Conclusion.
Integrating pharmacists into the care of older adults with cancer is feasible with encouraging preliminary efficacy for enhancing medication management and improving vaccination rates.
Implications for Practice.
Results of this study showed the feasibility, acceptability, and preliminary efficacy of an intervention integrating pharmacists into the care of older adults with cancer. Notably, patients assigned to the intervention had fewer discrepant medications and were more likely to acquire vaccinations for pneumonia and influenza. Importantly, this work represents the first randomized controlled trial involving the integration of pharmacists into the outpatient oncologic care of older adults with cancer. In the future, a larger randomized trial is needed to demonstrate the efficacy of this care model to enhance medication management and improve vaccination outcomes for older patients with cancer.
Introduction
Cancer disproportionately affects older adults, and older patients with cancer often present a distinct set of challenges for the clinicians caring for them [1]. A complex constellation of medical, physiologic, psychological, and social support needs can make caring for older patients with cancer challenging [2], [3], [4], [5], [6], [7]. Medication management and vaccinations are particularly challenging in an older population with cancer, often related to these patients' comorbid conditions, cognitive issues, and immunocompromised state [8], [9], [10], [11]. Chemotherapy and associated supportive care medications often add to the risk of adverse drug effects, drug–drug, and drug‐disease interactions, as well as the risk for impaired immunity and infections in these patients [12], [13], [14], [15], [16]. Although evidence suggests that older adults with cancer often have complicated medication regimens and vaccination schedules [9], [12], [13], [15], [16], [17], models of care focused on these needs are lacking.
Most efforts to date addressing medication management and vaccinations in older adults with cancer have utilized retrospective data or secondary data analysis [9], [18], [19], [20], [21]. Notably, these studies have demonstrated high rates (over one fifth in most studies) of polypharmacy and potentially inappropriate medication use and low rates (under half) of influenza and pneumonia vaccinations among older adults with cancer [8], [9], [18], [19], [20]. In addition, data suggest that poor medication management and lack of vaccinations are associated with adverse clinical outcomes, such as increased risk of falls, treatment toxicity, hospitalizations, and even death [9], [20], [22], [23]. Thus, interventions to address medication management and vaccinations among older adults with cancer are critically needed in order to enhance care delivery and outcomes for the rapidly growing geriatric oncology population.
Incorporating clinical pharmacists into the cancer care of older patients represents a potential option to improve medication management and vaccination administration for these individuals [18], [24], [25], [26], [27]. However, clinical pharmacists are rarely part of older patients' cancer care team, despite guidelines recommending their involvement given their expertise in medication management and appropriate vaccinations [28]. Additionally, prospective studies are lacking regarding the impact of clinical pharmacists on these patients' care. Therefore, further research is needed to develop and test care models that incorporate clinical pharmacists into the cancer care of older patients.
We conducted a pilot randomized controlled trial of an intervention integrating pharmacists into the care of older adults with cancer, which we called “Pharmacist Reconciliation to Improve Medication Management in the Elderly” (PRIME). Specifically, we sought to assess the feasibility, acceptability, and preliminary efficacy of PRIME for improving medication management and receipt of appropriate vaccinations in older adults with breast, gastrointestinal, and lung cancers. We focused on patients with these cancers, as these are highly prevalent in the geriatric oncology population [29], [30], [31]. We hypothesized that PRIME would be feasible to deliver and that patients would find the intervention acceptable. We also explored the preliminary efficacy for PRIME to improve the accuracy of medication documentation in the electronic health record (EHR), decrease use of potentially inappropriate medications, and increase administration of vaccinations for pneumonia and influenza.
Materials and Methods
Study Design and Procedures
From January 17, 2017 to October 17, 2017, we enrolled patients at Massachusetts General Hospital (MGH) in a nonblinded, randomized controlled trial of PRIME versus usual care (ClinicalTrials.gov identifier NCT02871115). Trained study staff identified and recruited consecutive patients during the study period by screening the oncology clinic and infusion schedules. Study staff sent opt‐out emails to the treating oncologist to confirm eligibility prior to approaching eligible patients. After patients provided written informed consent, study staff asked them to complete baseline study measures. Following completion of baseline study measures, the Office of Data Quality randomly assigned patients in a 1:1 fashion to receive the PRIME intervention or usual care, stratified by cancer type. The Dana‐Farber/Harvard Cancer Center Institutional Review Board approved the study protocol.
Participants
Patients eligible for study participation included those who were at least 65 years of age and receiving outpatient first‐line intravenous chemotherapy at MGH for any‐stage breast, gastrointestinal, or lung cancer. Study participants also had to be able to read and respond to study questionnaires in English or with minimal assistance from an interpreter. We excluded patients who were already receiving pharmacy services (e.g., had already met with a clinical pharmacist at the cancer center) or who had significant psychiatric or other comorbid disease (e.g., cognitive impairment) that their oncology clinician felt would prohibit participation.
PRIME Intervention
Patients assigned to PRIME participated in one in‐person visit with a clinical pharmacist during their second or third chemotherapy infusion, in which the pharmacist (a) performed a detailed medication and vaccination history (including contacting patients' primary care clinicians to inquire about vaccinations); (b) evaluated patients' medications, medication indications, potentially inappropriate medications or doses, medication duplications, potential prescribing omissions and/or lack of appropriate medications (e.g., bowel regimen if prescribed narcotics for pain), drug interactions (e.g., drug–drug, drug‐food, and drug‐disease interactions), alternative or herbal medications, medication adherence, and patient understanding of safe medication handling (e.g., oral chemotherapy); (c) documented their findings in the medical record (e.g., any medication updates, potential interactions, and recommendations for medication changes and/or vaccinations needed based on guidelines [32]); and (d) communicated their recommendations with the patients' oncology team either in person or via phone call and/or email.
Usual Care
Participants receiving usual care could meet with a pharmacy clinician and/or geriatrician upon request by the oncologist, patient, or family. Patients with breast, gastrointestinal, and lung cancers do not routinely receive pharmacist or geriatric consultation as part of standard care at MGH. All patients, regardless of group assignment, continued to receive routine oncology care throughout the study period, and no other initiatives to improve influenza and pneumonia vaccination rates were active during this time.
Study Measures
Sociodemographic and Clinical Characteristics.
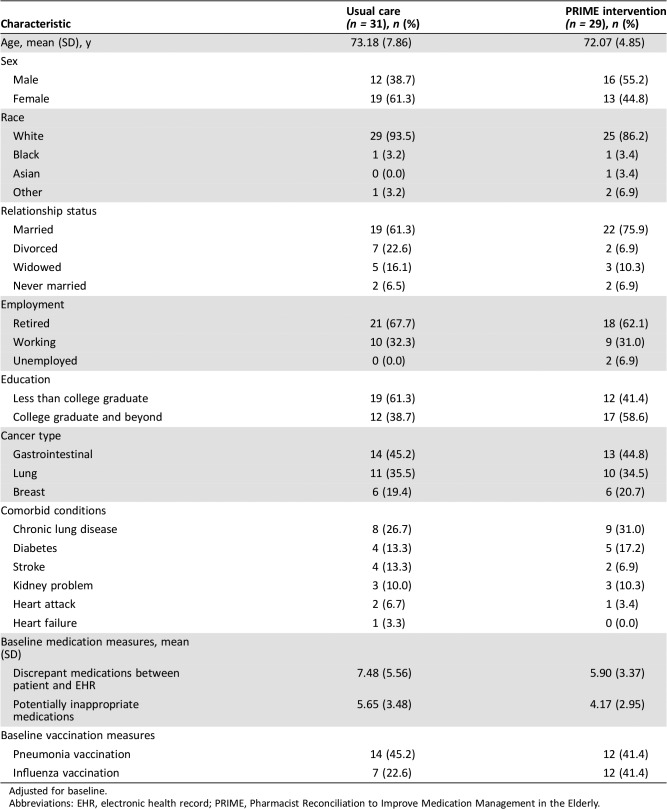
Participants completed baseline study measures prior to randomization. To describe participant characteristics, we asked patients to self‐report their sex, race, relationship status, employment, education, and comorbid conditions. We obtained information about participants' age and cancer from the EHR.
Medications.
We obtained information about patients' medications, including herbal/alternative medications and vitamins, via patient report and from the EHR at baseline and week 4. We determined the number of discrepant medications, defined as the difference between patient‐report and the EHR. Consistent with prior work, we categorized medications as potentially inappropriate, based on the 2015 Beers Criteria, as determined by nonintervention pharmacists blinded to group assignment [33], [34].
Vaccinations.
We obtained information about patients' vaccinations from the EHR at baseline, week 4, and week 8. Specifically, we investigated vaccination rates for pneumonia and influenza at baseline, and then assessed again at weeks 4 and 8 whether unvaccinated patients had acquired their pneumonia or influenza vaccinations. We focused on pneumonia and influenza vaccinations because these are both recommended for older adults with cancer [9], [16].
Acceptability of the Intervention.
As part of the week 4 assessment, we asked patients assigned to PRIME to complete a survey inquiring about the timing and utility of the intervention. Specifically, we asked patients about their perceptions of the visit frequency and length and whether they perceived the visit with the pharmacist as helpful.
Statistical Analysis
The primary endpoint of the study was feasibility. We defined the intervention as feasible if at least 75% of approached patients enrolled in the study (95% confidence interval of 65%–83%) and if at least 75% of those assigned to the intervention received the visit with the pharmacist (95% confidence interval of 63%–84%).
In addition, secondary endpoints included an evaluation of the number of discrepant medications between patient report and the EHR and the number of potentially inappropriate medications at week 4. We also investigated whether patients had acquired their pneumonia and influenza vaccinations by week 4 and week 8. To assess the effect of PRIME on the number of discrepant and potentially inappropriate medications at week 4, we used univariate t tests and multivariable linear regression, controlling for the respective baseline number of each outcome. To investigate intervention effects on rates of acquiring pneumonia and influenza vaccinations by week 4 and week 8, we used chi‐squared tests. We used conservative (α = 0.05) and liberal (α = 0.25) values to assess statistical significance given the pilot nature of this study [35], [36], [37]. Pilot preliminary efficacy studies are not formally powered to assess efficacy but rather focus on hypothesis generation and help to inform the design of larger confirmatory studies. We used SPSS for Windows version 20 (IBM, Armonk, NY) for statistical analyses.
Results
Participant Characteristics
Patients had a median age of 71.74 years (range, 65.07–91.49), and the majority were white (90.0%), female (59.3%), married (68.3%), and retired (65.0%; Table 1). The most common comorbid conditions were chronic lung disease (28.3%), diabetes (15.0%), stroke (10.0%), and kidney problems (10.0%).
Table 1. Participant characteristics.
Adjusted for baseline.
Abbreviations: EHR, electronic health record; PRIME, Pharmacist Reconciliation to Improve Medication Management in the Elderly.
Baseline Study Measures
Overall, patients had an average of 13.33 (SD = 5.87) medications on their medication list. Patients had an average of 6.72 (SD = 4.66) medications discrepant between patient report and the EHR and 4.93 (SD = 3.29) potentially inappropriate medications on their medication list in the EHR. At baseline, 43.3% and 31.7% of participants had received pneumonia and influenza vaccinations, respectively.
Feasibility and Acceptability of the Intervention
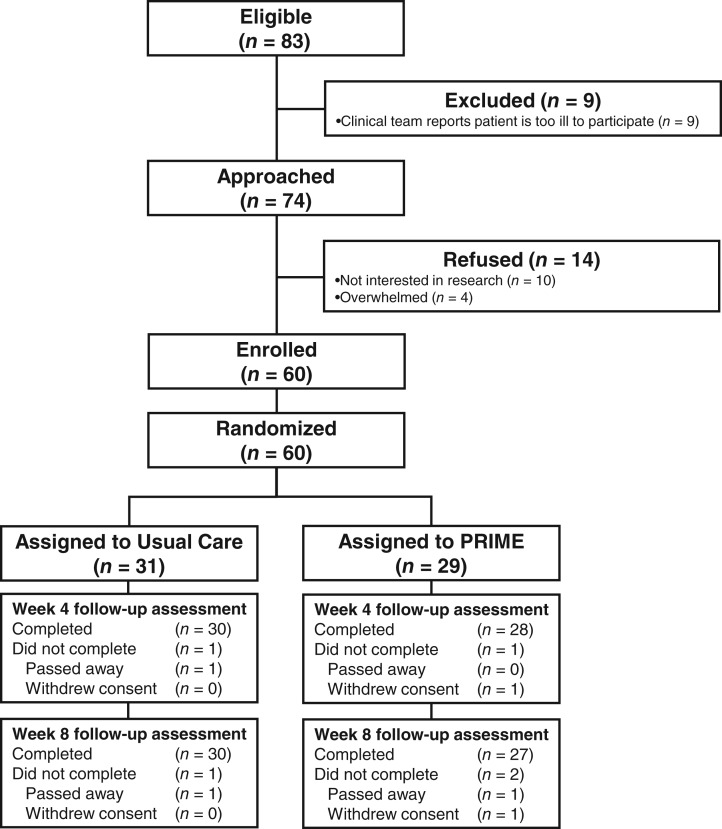
We enrolled 81.1% (60/74) of patients approached (Fig. 1). Among patients assigned to the intervention, 96.6% (28/29) received the pharmacist visit. We had five clinical pharmacists who saw patients for the study, and they reported spending approximately 30–45 minutes preparing for each study visit. The median length of the pharmacist visit was 55 minutes (range, 30–75) per patient.
Figure 1.
CONSORT diagram.
Abbreviation: PRIME, Pharmacist Reconciliation to Improve Medication Management in the Elderly.
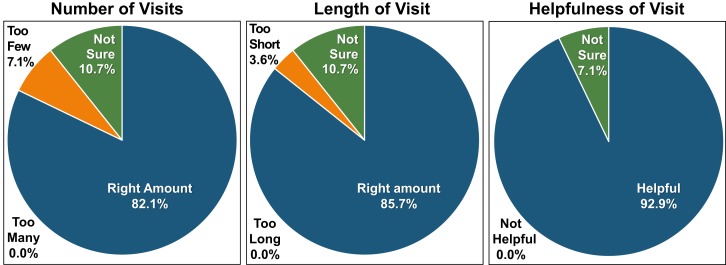
Most participants reported that the number of visits was the “right amount” (82.1% [23/28]), the length of the pharmacy visit was the “right amount” (85.7% [24/28]), and the pharmacy visit was “helpful” (92.9% [26/28]; Fig. 2). No patients reported that the number of visits was “too many,” the length of the visit was “too long,” or the visit was “not helpful.”
Figure 2.
Intervention acceptability ratings.
Intervention Effect on Patients' Medications
With a liberal α of 0.25, intervention patients had fewer discrepant (5.82 vs. 8.07, p = .094) and potentially inappropriate (3.46 vs. 4.80, p = .069) medications compared with usual care at week 4 in unadjusted analyses. In multivariable models controlling for baseline values, patients assigned to PRIME had fewer discrepant medications (B, −1.50; 95% confidence interval [95% CI], −4.04 to 1.04; p = .242), when using a liberal α of 0.25 (Table 2). However, multivariable models demonstrated no between‐group differences in the number of potentially inappropriate medications (B, −0.49; 95% CI, −1.65 to 0.68; p = .408).
Table 2. Intervention effects on medication outcomes.
Adjusted for baseline.
Abbreviations: CI, confidence interval; EHR, electronic health record.
Intervention Effect on Patients' Vaccinations
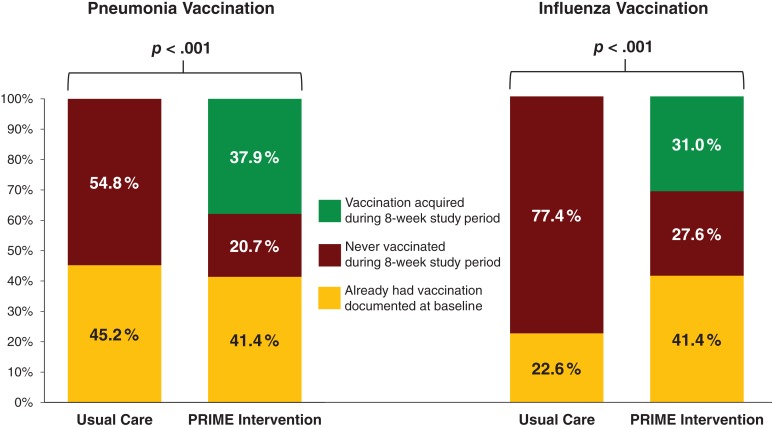
At week 4, patients assigned to PRIME had higher rates of obtaining vaccinations for pneumonia (27.6% vs. 0.0%, p = .005) and influenza (27.6% vs. 0.0%, p < .001) compared with patients assigned to usual care. Similarly, at week 8, intervention patients had higher rates of obtaining vaccinations for pneumonia (37.9% vs. 0.0%, p < .001) and influenza (31.0% vs. 0.0%, p < .001). Fig. 3 displays the proportion of patients with vaccinations for pneumonia and influenza acquired during the 8‐week study period, the proportion who already had vaccinations at baseline, and the proportion who were never vaccinated during the study period.
Figure 3.
Intervention effects on the rates of pneumonia and influenza vaccinations.
Abbreviation: PRIME, Pharmacist Reconciliation to Improve Medication Management in the Elderly.
Discussion
In this pilot randomized controlled trial, we investigated the feasibility, acceptability, and preliminary efficacy of PRIME for improving medication management and receipt of recommended vaccinations among older adults with cancer. We enrolled over 80% of patients approached, and nearly every patient assigned to the intervention received the pharmacist visit. In addition, patients found the intervention to be highly acceptable. Importantly, patients assigned to PRIME had fewer discrepant medications and were more likely to acquire vaccinations for pneumonia and influenza. Collectively, these data demonstrate that PRIME is feasible and acceptable and demonstrates encouraging preliminary efficacy for improving patients' medication management and rates of vaccinations.
To our knowledge, the current study is the first randomized controlled trial involving the integration of pharmacists into the outpatient oncologic care of older adults with cancer. Importantly, our results demonstrate the feasibility and acceptability of an intervention integrating pharmacists into the oncologic care of older adults with cancer. Although prior randomized trials have demonstrated the efficacy of integrating pharmacists into the care of patients within cardiovascular, pulmonology, and primary care settings [38], [39], [40], [41], [42], efforts to conduct randomized trials of pharmacist interventions among older adults with cancer are lacking. Furthermore, with the rapidly growing geriatric oncology population and the challenges of addressing these patients' distinct and complex needs, it is imperative that we develop and test interventions targeting issues unique to this population, such as medication management and vaccination administration [9], [18], [43]. The current intervention ensures that older adults with cancer receive focused attention to their complicated medication regimens and vaccination schedules by incorporating pharmacists into their outpatient oncologic care. Notably, PRIME yielded encouraging results using just a single visit with a pharmacist, thus reflecting an intervention that could be widely disseminated and readily incorporated into the clinical care of older adults with cancer.
Importantly, we found that patients assigned to PRIME experienced significant improvements in their rates of acquiring pneumonia and influenza vaccinations. Over one fourth of the intervention patients acquired pneumonia and influenza vaccinations by week 4, with further improvements by week 8. Notably, none of the patients assigned to usual care received vaccinations for pneumonia or influenza during the study period, which highlights the critical need for efforts to address vaccinations in this population. Prior research suggests that pneumonia and influenza vaccinations in older patients with cancer are associated with fewer infections, reduced chemotherapy interruptions, decreased hospitalizations, and potentially enhanced survival [9]. Thus, our findings that PRIME resulted in higher vaccination rates for pneumonia and influenza have important clinical implications and underscore the potential for this intervention to affect additional outcomes, such as infection risk and treatment duration, which merit further investigation in future studies.
We also investigated the preliminary efficacy of PRIME for enhancing medication management among older adults with cancer. Using a liberal p value cutoff as per guidelines for pilot studies [35], [36], [37], we found that patients assigned to the intervention had fewer discrepant and potentially inappropriate medications at week 4. The medications in our EHR reflect patients' medication lists for all hospitals affiliated with MGH but not those outside our health system. Thus, PRIME helped clarify discrepancies between patient‐reported medications and the list documented in the EHR, which is critically important, clinically, as this helps foster safe prescribing and drug interaction monitoring. Furthermore, prior work has demonstrated that inadequate medication management among older adults with cancer is associated with poor clinical outcomes, such as increased risk of hospitalizations and diminished survival [20], [21]. Notably, we enrolled patients early in their treatment course, which can have implications for enhancing downstream outcomes, such as treatment adherence and avoidance of drug interactions, which could potentially affect patients' cancer outcomes, including survival [9]. Therefore, additional research is needed to further test the efficacy of PRIME on medication management outcomes and also to investigate intervention effects on other outcomes, such as treatment adherence, hospitalization rates, and survival.
In addition, our work highlights the high number of discrepant and potentially inappropriate medications, as well as the considerably low rates of vaccinations for pneumonia and influenza, among older patients with cancer. Patients in our sample had an average of over 13 medications on their medication list, with nearly 7 discrepant and almost 5 potentially inappropriate medications. These high numbers of potentially inappropriate medications are consistent with prior work describing medication management in older adults with cancer [18], [20], [21], [34]. Moreover, we found that under half of patients had received pneumonia and influenza vaccinations at the time of study enrollment, which is also consistent with previous research [9], [44], [45], [46]. With such high baseline numbers of discrepant and potentially inappropriate medications and low baseline vaccination rates, our findings further underscore the importance of efforts to address medication management and vaccination administration for older patients with cancer. Importantly, these findings also highlight the potential for interventions such as PRIME to enhance care outcomes for the geriatric oncology population.
Our study has several limitations. First, we conducted this trial at a single institution with limited diversity, which may limit the generalizability of our results to other care settings and clinical populations. Additionally, we may lack information about vaccinations if patients received vaccinations outside of our institution and this was not documented in the EHR. Second, although we had a high enrollment rate, the pilot nature of this study limited our ability to demonstrate definitive intervention effects on patients' medication outcomes. Third, we lack information about some potential factors that may influence the impact of PRIME, such as patients' social supports, cognition, and ability to independently manage their own medications. Future work should investigate whether these and other important factors, such as comorbidity, cancer type, and concurrent geriatric consultation, may influence the effects of PRIME on patient outcomes. Furthermore, in the current study, we did not collect data on herpes zoster vaccination rates, accuracy of patients' allergy lists, or potential prescribing omissions, all of which will be important to track in future studies. We also lack information about oncology clinicians' perceptions regarding the utility of PRIME, and future efforts to fully integrate pharmacists into the cancer care team should consider the clinician perspective, as well as the funding and space required to support pharmacists. Moreover, future work should determine the efficacy of PRIME for enhancing vaccination rates during the height of influenza season and should include information about rates of influenza and pneumonia infections.
Conclusion
In this study, we demonstrated the feasibility and acceptability of an intervention that integrates pharmacists into the care of older adults with cancer. Among the patients we asked to participate in our study, over 80% enrolled, and all but one patient assigned to the intervention received the pharmacist visit. Importantly, we found promising preliminary efficacy for PRIME to improve medication management and vaccination acquisition in our sample. In addition, our data highlight the substantially high rates of discrepant and potentially inappropriate medication use, as well as low rates of obtaining recommended vaccinations among older adults with cancer, thus underscoring the critical importance of efforts to address medication management and vaccinations in the geriatric oncology population. A larger randomized controlled trial to demonstrate the efficacy of this care model to enhance medication management and improve vaccination outcomes for older patients with cancer is clearly warranted.
Acknowledgments
This work was supported by NCI K24 CA181253 (Jennifer S. Temel) and MGH Cancer Center Funds (Jennifer S. Temel). It was presented as an oral presentation at the 2018 ASCO Annual Meeting in Chicago, IL.
Author Contributions
Conception/design: Ryan D. Nipp, Margaret Ruddy, Charn‐Xin Fuh, Mark L. Zangardi, Christine Chio, E. Bridget Kim, Barbara Kong Mui Li, Ying Long, Gayle C. Blouin, Daniel Lage, David P. Ryan, Joseph A. Greer, Areej El‐Jawahri, Jennifer S. Temel
Collection and/or assembly of data: Ryan D. Nipp, Margaret Ruddy, Charn‐Xin Fuh, Mark L. Zangardi, Christine Chio, E. Bridget Kim, Barbara Kong Mui Li, Ying Long, Gayle C. Blouin, Daniel Lage, David P. Ryan, Joseph A. Greer, Areej El‐Jawahri, Jennifer S. Temel
Data analysis and interpretation: Ryan D. Nipp, Margaret Ruddy, Charn‐Xin Fuh, Mark L. Zangardi, Christine Chio, E. Bridget Kim, Barbara Kong Mui Li, Ying Long, Gayle C. Blouin, Daniel Lage, David P. Ryan, Joseph A. Greer, Areej El‐Jawahri, Jennifer S. Temel
Manuscript writing: Ryan D. Nipp, Margaret Ruddy, Charn‐Xin Fuh, Mark L. Zangardi, Christine Chio, E. Bridget Kim, Barbara Kong Mui Li, Ying Long, Gayle C. Blouin, Daniel Lage, David P. Ryan, Joseph A. Greer, Areej El‐Jawahri, Jennifer S. Temel
Final approval of manuscript: Ryan D. Nipp, Margaret Ruddy, Charn‐Xin Fuh, Mark L. Zangardi, Christine Chio, E. Bridget Kim, Barbara Kong Mui Li, Ying Long, Gayle C. Blouin, Daniel Lage, David P. Ryan, Joseph A. Greer, Areej El‐Jawahri, Jennifer S. Temel
Disclosures
The authors indicated no financial relationships.
References
- 1.Smith BD, Smith GL, Hurria A et al. Future of cancer incidence in the United States: Burdens upon an aging, changing nation. J Clin Oncol 2009;27:2758–2765. [DOI] [PubMed] [Google Scholar]
- 2.Ommundsen N, Wyller TB, Nesbakken A et al. Frailty is an independent predictor of survival in older patients with colorectal cancer. The Oncologist 2014;1268–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamaker ME, Vos AG, Smorenburg CH et al. The value of geriatric assessments in predicting treatment tolerance and all‐cause mortality in older patients with cancer. The Oncologist 2012;17:1439–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Puts MT, Santos B, Hardt J et al. An update on a systematic review of the use of geriatric assessment for older adults in oncology. Ann Oncol 2014;25:307–315. [DOI] [PubMed] [Google Scholar]
- 5.Cheung WY, Le LW, Gagliese L et al. Age and gender differences in symptom intensity and symptom clusters among patients with metastatic cancer. Support Care Cancer 2011;19:417–423. [DOI] [PubMed] [Google Scholar]
- 6.Linden W, Vodermaier A, Mackenzie R et al. Anxiety and depression after cancer diagnosis: Prevalence rates by cancer type, gender, and age. J Affect Disord 2012;141:343–351. [DOI] [PubMed] [Google Scholar]
- 7.Mor V, Allen S, Malin M. The psychosocial impact of cancer on older versus younger patients and their families. Cancer 1994;74(suppl 7):2118–2127. [DOI] [PubMed] [Google Scholar]
- 8.Prithviraj GK, Koroukian S, Margevicius S et al. Patient characteristics associated with polypharmacy and inappropriate prescribing of medications among older adults with cancer. J Geriatr Oncol 2012;3:228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Earle CC. Influenza vaccination in elderly patients with advanced colorectal cancer. J Clin Oncol 2003;21:1161–1166. [DOI] [PubMed] [Google Scholar]
- 10.Hurria A, Rosen C, Hudis C et al. Cognitive function of older patients receiving adjuvant chemotherapy for breast cancer: A pilot prospective longitudinal study. J Am Geriatr Soc 2006;54:925–931. [DOI] [PubMed] [Google Scholar]
- 11.Stilley CS, Bender CM, Dunbar‐Jacob J et al. The impact of cognitive function on medication management: Three studies. Health Psychol 2010;29:50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riechelmann RP, Tannock IF, Wang L et al. Potential drug interactions and duplicate prescriptions among cancer patients. J Natl Cancer Inst 2007;99:592–600. [DOI] [PubMed] [Google Scholar]
- 13.Puts MT, Monette J, Girre V et al. Potential medication problems in older newly diagnosed cancer patients in Canada during cancer treatment: A prospective pilot cohort study. Drugs Aging 2010;27:559–572. [DOI] [PubMed] [Google Scholar]
- 14.Scripture CD, Figg WD. Drug interactions in cancer therapy. Nat Rev Cancer 2006;6:546–558. [DOI] [PubMed] [Google Scholar]
- 15.Lees J, Chan A. Polypharmacy in elderly patients with cancer: Clinical implications and management. Lancet Oncol 2011;12:1249–1257. [DOI] [PubMed] [Google Scholar]
- 16.Rubin LG, Levin MJ, Ljungman P et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2014;58:309–318. [DOI] [PubMed] [Google Scholar]
- 17.Esposito S, Bonanni P, Maggi S et al. Recommended immunization schedules for adults: Clinical practice guidelines by the Escmid Vaccine Study Group (EVASG), European Geriatric Medicine Society (EUGMS) and the World Association for Infectious Diseases and Immunological Disorders (WAidid). Hum Vaccin Immunother 2016;12:1777–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nightingale G, Hajjar E, Swartz K et al. Evaluation of a pharmacist‐led medication assessment used to identify prevalence of and associations with polypharmacy and potentially inappropriate medication use among ambulatory senior adults with cancer. J Clin Oncol 2015;33:1453–1459. [DOI] [PubMed] [Google Scholar]
- 19.Maggiore RJ, Dale W, Gross CP et al. Polypharmacy and potentially inappropriate medication use in older adults with cancer undergoing chemotherapy: Effect on chemotherapy‐related toxicity and hospitalization during treatment. J Am Geriatr Soc 2014;62:1505–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karuturi MS, Holmes HM, Lei X et al. Potentially inappropriate medication use in older patients with breast and colorectal cancer. Cancer 2018;124:3000–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park JW, Roh JL, Lee SW et al. Effect of polypharmacy and potentially inappropriate medications on treatment and posttreatment courses in elderly patients with head and neck cancer. J Cancer Res Clin Oncol 2016;142:1031–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turner JP, Jamsen KM, Shakib S et al. Polypharmacy cut‐points in older people with cancer: How many medications are too many? Support Care Cancer 2016;24:1831–1840. [DOI] [PubMed] [Google Scholar]
- 23.Woopen H, Richter R, Ismaeel F et al. The influence of polypharmacy on grade III/IV toxicity, prior discontinuation of chemotherapy and overall survival in ovarian cancer. Gynecol Oncol 2016;140:554–558. [DOI] [PubMed] [Google Scholar]
- 24.Kaur S, Mitchell G, Vitetta L et al. Interventions that can reduce inappropriate prescribing in the elderly: A systematic review. Drugs Aging 2009;26:1013–1028. [DOI] [PubMed] [Google Scholar]
- 25.Sessions JK, Valgus J, Barbour SY et al. Role of oncology clinical pharmacists in light of the oncology workforce study. J Oncol Pract 2010;6:270–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah S, Dowell J, Greene S. Evaluation of clinical pharmacy services in a hematology/oncology outpatient setting. Ann Pharmacother 2006;40:1527–1533. [DOI] [PubMed] [Google Scholar]
- 27.Biganzoli L, Wildiers H, Oakman C et al. Management of elderly patients with breast cancer: Updated recommendations of the International Society of Geriatric Oncology (SIOG) and European Society of Breast Cancer Specialists (EUSOMA). Lancet Oncol 2012;13:e148–160. [DOI] [PubMed] [Google Scholar]
- 28.Mohile SG, Dale W, Somerfield MR et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO Guideline for Geriatric Oncology summary. J Oncol Pract 2018;14:442–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel SS, Nelson R, Sanchez J et al. Elderly patients with colon cancer have unique tumor characteristics and poor survival. Cancer 2013;119:739–747. [DOI] [PubMed] [Google Scholar]
- 30.Yancik R. Population aging and cancer: A cross‐national concern. Cancer J 2005;11:437–441. [DOI] [PubMed] [Google Scholar]
- 31.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- 32.Baden LR, Swaminathan S, Angarone M et al. Prevention and treatment of cancer‐related infections, version 2.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2016;14:882–913. [DOI] [PubMed] [Google Scholar]
- 33.American Geriatrics Society Beers Criteria Update Expert Panel . American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015;63:2227–2246. [DOI] [PubMed] [Google Scholar]
- 34.Reis CM, Dos Santos AG, de Jesus Souza P et al. Factors associated with the use of potentially inappropriate medications by older adults with cancer. J Geriatr Oncol 2017;8:303–307. [DOI] [PubMed] [Google Scholar]
- 35.Lee EC, Whitehead AL, Jacques RM et al. The statistical interpretation of pilot trials: Should significance thresholds be reconsidered? BMC Med Res Methodol 2014;14:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schoenfeld D. Statistical considerations for pilot studies. Int J Radiat Oncol Biol Phys 1980;6:371–374. [DOI] [PubMed] [Google Scholar]
- 37.Moore CG, Carter RE, Nietert PJ et al. Recommendations for planning pilot studies in clinical and translational research. Clin Transl Sci 2011;4:332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Green BB, Cook AJ, Ralston JD et al. Effectiveness of home blood pressure monitoring, Web communication, and pharmacist care on hypertension control: A randomized controlled trial. JAMA 2008;299:2857–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koshman SL, Charrois TL et al. Pharmacist care of patients with heart failure: A systematic review of randomized trials. Arch Intern Med 2008;168:687–694. [DOI] [PubMed] [Google Scholar]
- 40.Weinberger M, Murray MD, Marrero DG et al. Effectiveness of pharmacist care for patients with reactive airways disease: A randomized controlled trial. JAMA 2002;288:1594–1602. [DOI] [PubMed] [Google Scholar]
- 41.Krska J, Cromarty JA, Arris F et al. Pharmacist‐led medication review in patients over 65: A randomized, controlled trial in primary care. Age Ageing 2001;30:205–211. [DOI] [PubMed] [Google Scholar]
- 42.Margolis KL, Asche SE, Bergdall AR et al. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: A cluster randomized clinical trial. JAMA 2013;310:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol 2007;25:1824–1831. [DOI] [PubMed] [Google Scholar]
- 44.Klabunde CN, Meissner HI, Wooten KG et al. Comparing colorectal cancer screening and immunization status in older Americans. Am J Prev Med 2007;33:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vinograd I, Eliakim‐Raz N, Farbman L et al. Clinical effectiveness of seasonal influenza vaccine among adult cancer patients. Cancer 2013;119:4028–4035. [DOI] [PubMed] [Google Scholar]
- 46.Shenson D, Bolen J, Adams M et al. Are older adults up‐to‐date with cancer screening and vaccinations? Prev Chronic Dis 2005;2:04A. [PMC free article] [PubMed] [Google Scholar]