Treatment for breast cancer, including chemotherapy and radiation can have cardiovascular side effects. This study's objectives were to characterize changes in, and the association of exercise training with, clinical indices of cardiovascular autonomic function across the trajectory of breast cancer therapy.
Keywords: Exercise, Heart rate, Blood pressure, Breast neoplasms
Abstract
Background.
Cardiovascular autonomic dysfunction is an early marker for cardiovascular disease. Anthracycline chemotherapy and left‐sided radiation for breast cancer are associated with negative autonomic function changes. This study's objectives were to characterize changes in, and the association of exercise training with, clinical indices of cardiovascular autonomic function across the trajectory of breast cancer therapy.
Subjects, Materials, and Methods.
Seventy‐three patients receiving adjuvant chemotherapy participated to varying degrees in supervised aerobic and resistance exercise during chemotherapy ± radiation and for 20 weeks after. Resting heart rate (HRrest) and blood pressure were measured weekly during chemotherapy. HRrest, exercise heart rate recovery (HRrecovery), and aerobic fitness were measured at enrollment, end of chemotherapy ± radiation, and 10 and 20 weeks after treatment.
Results.
During chemotherapy, HRrest increased in a parabolic manner within a single treatment and with increasing treatment dose, whereas systolic and diastolic blood pressure decreased linearly across treatments. Tachycardia and hypotension were present in 32%–51% of participants. Factors associated with weekly changes during chemotherapy included receiving anthracyclines or trastuzumab, days since last treatment, hematocrit, and exercise attendance. Receipt of anthracyclines, trastuzumab, and left‐sided radiation individually predicted impairments of HRrest and HRrecovery during chemotherapy ± radiation; however, aerobic fitness change and at least twice‐weekly exercise attendance predicted improvement. By 10 weeks after treatment, HRrest and blood pressure were not different from prechemotherapy.
Conclusion.
In this study, chemotherapy resulted in increased HRrest and tachycardia, as well as decreased blood pressure and hypotension. Anthracyclines, trastuzumab, and left‐sided radiation were associated with HRrest elevations and impairments of HRrecovery, but exercise training at least twice a week appeared to mitigate these changes.
Implications for Practice.
This study characterized changes in clinically accessible measures with well‐established prognostic value for cardiovascular disease, and investigated associations with cardiotoxic treatments and the positive influence of exercise. The chemotherapy‐related incremental increase in resting heart rate, with tachycardia occurring in one third of patients, and decrease in blood pressure, with hypotension occurring in one half of the patients, is relevant to oncology practitioners for clinical examination or patient report of related symptoms (i.e., dizziness). The weekly dose of two 60‐minute sessions of moderate‐intensity aerobic and resistance exercise that was identified as protective of cardiovascular autonomic impairments can easily be prescribed to patients by oncologists.
摘要
背景。心血管自主神经功能障碍是心血管疾病的早期指标。针对乳腺癌的蒽环类药物化疗和左侧放疗与自主神经功能的负面变化有关。本研究的目的是描述在乳腺癌治疗过程中心血管自主神经功能临床指标的变化,及这些指标与运动训练之间的关联。
受试者、材料和方法。接受辅助化疗的 73 名患者在化疗 ± 放疗期间及 20 周之后参加了不同程度的指导性有氧及抗阻运动。化疗期间每周测量静息心率 (HRrest) 和血压。我们会在报名时、化疗 ± 放疗结束时,以及治疗后 10 周和 20 周测量 HRrest、运动心率恢复 (HRrecovery) 和有氧适能。
结果。在化疗期间,HRrest 在单次治疗中以抛物线形式随着治疗剂量的增加而增加,而收缩压和舒张压会在治疗过程中呈线性下降。有 32%‐51% 的参与者出现心动过速和低血压。与化疗期间每周变化相关的因素包括接受蒽环类药物或曲妥单抗、上次治疗后的天数、血细胞比容和参加运动的人数。接受蒽环类药物、曲妥单抗和左侧放疗分别预示出化疗 ± 放疗期间 HRrest 和 HRrecovery的障碍;但是,有氧健身的变化和每周至少两次的运动预计会有所改善。到治疗后 10 周,HRrest 和血压与化疗前相比没有分别。
结论。在本研究中,化疗导致 HRrest 增加、心动过速、血压下降和低血压。蒽环类药物、曲妥单抗和左侧放疗与 HRrest 升高和 HRrecovery 障碍有关,但每周进行两次运动似乎可以减缓这些变化。
对实践的启示:本研究根据心血管疾病的确定预后值来表现临床上可利用措施的变化,同时研究了与心脏毒性治疗和运动的积极影响之间的关联。与化疗相关的静息心率逐步增加,导致 1/3 的患者出现心动过速,血压降低,导致 1/2 患者出现低血压,这些均与肿瘤医生进行临床检查或患者报告相关症状(即头晕)相关。肿瘤医生可轻易给患者开具处方,每周进行两次 60 分钟的中等强度有氧及抗阻运动,这些运动被认为可以避免心血管自主神经损伤。
Introduction
Breast cancer survivors are at an elevated risk of cardiovascular disease [1] and are more likely to die of cardiovascular disease than women who have not had breast cancer [2]. Proposed contributing factors to this risk include presence of comorbid conditions at diagnosis, reduced physical activity and aerobic fitness during and after treatment, early menopause induced by chemotherapy, and direct cardiovascular effects from chemotherapy, mediastinal radiotherapy, and targeted therapy [3], [4]. Cardiovascular autonomic dysfunction, impairments in the normal regulation of sympathetic and parasympathetic nervous influences on the heart and vasculature, is an early marker for cardiovascular disease‐related morbidity and mortality [5]. Anthracycline chemotherapeutic agents and radiotherapy have been linked to negative autonomic changes [6]. The weight gain and reduced fitness common with breast cancer have also been associated with autonomic dysfunction in noncancer populations [6]. Aerobic and resistance exercise training have been suggested as potential therapies for prevention and treatment of autonomic impairment in breast cancer survivors [6].
Several simple, clinically accessible measures reflect cardiovascular autonomic function and have well‐established prognostic value for cardiovascular disease, including resting heart rate (HRrest), resting blood pressure, and heart rate recovery following exercise (HRrecovery) [7], [8]. The latter is a simple measure that could be acquired from a clinical stress test performed in conjunction with a cardio‐oncology clinic or by patient self‐monitoring using a physical activity device with telemetry (e.g., Fitbit). The study team has commonly observed large fluctuations in HRrest and blood pressure during chemotherapy among women with breast cancer in both clinical care and research settings. To our knowledge, these fluctuations have not previously been reported in the literature. These chemotherapy‐related changes in vital signs could have a number of implications for the clinical care of individuals receiving chemotherapy, including for clinical monitoring of patient response to treatment, interpreting patient‐reported symptoms (e.g., high heart rate or dizziness), and requirement for dose adjustments of prescribed exercise or medication (e.g., for hypertension). This study is an ancillary study of these clinically accessible measures reflecting cardiovascular autonomic function measured throughout the Nutrition and Exercise during adjuvant Treatment (NExT) trial [9]. NExT exercise programming was delivered in a real‐world setting, resulting in a wide range of adherence, and therefore a range of exercise volume received among participants, which enables dose‐response analysis. The primary objective of this ancillary study was to characterize and explain the changes in HRrest and resting blood pressure across the trajectory of adjuvant therapy in women with breast cancer participating in an exercise program. The secondary purpose was to assess whether receipt of cardiotoxic treatments and the extent of exercise performed are associated with changes in HRrest and HRrecovery during or after treatment.
Patients, Materials, and Methods
Study Design and Patients
Patients included women with stage I–IIIA breast cancer who were scheduled to receive adjuvant chemotherapy (NCT01806181). Patients were eligible to enroll up to 2 weeks before chemotherapy ("prechemotherapy") to within completion of half of planned chemotherapy treatments. Exclusion criteria included uncontrolled or unstable cardiovascular disease or diabetes, body mass index (BMI) >40 kg/m2, use of mobility aids, and stage IV/metastatic disease. Women with stage IV disease were excluded from the main trial because of the potential need for a different format of exercise programming (e.g., one‐on‐one training for increased supervision and more exercise prescription modification). The British Columbia Cancer Agency Research Ethics Board approved this study (#H12‐02504). Participants provided written informed consent.
All participants were invited to attend supervised sessions consisting of 20–30 minutes each of moderate‐to‐vigorous‐intensity (50%–75% of heart rate [HR] reserve/one‐repetition maximum) aerobic and whole‐body resistance exercise up to three times per week and were encouraged to perform 1–2 home‐based aerobic sessions throughout chemotherapy, and radiation if received (CT ± RT). Following CT ± RT completion, two supervised and three home‐based sessions per week were encouraged for 10 weeks, and then one supervised and four home‐based sessions per week for another 10 weeks (collectively referred to as "post‐CT ± RT"). During "post‐CT ± RT," otherwise healthy participants performed a combination of aerobic intervals (4×[4 minutes at 75%–85% + 4 minutes at 40%–65% VO2/HR reserve]) and continuous‐intensity exercise. Participants were encouraged to attend supervised sessions as often as possible, but no strict expectations were established around adherence.
Outcome Measures
Serial HRrest and Resting Blood Pressure Measurements During CT ± RT.
The timing of outcome measures relative to enrollment and the intervention is shown in Figure 1. During CT ± RT, HRrest and blood pressure were measured approximately once per week before supervised sessions at approximately the same time of day. HRrest was measured on an FT1 HR monitor (Polar, Lachine, Quebec) as the lowest HR (excluding ectopic beats) during the last 30 seconds of a 5‐minute period of quiet, seated rest, with back against the chair and feet flat on the floor, arms and legs uncrossed [10]. Test‐retest reliability (1–5 days apart) for this assessment is an error of 1.6 ± 0.6 (mean ± standard deviation) beats per minute (bpm), and a coefficient of variation of 1.7 ± 0.6%. Blood pressure was measured at the end of the 5 minutes of rest as the average of two measurements 60 seconds apart with a validated automatic monitor (Omron HEM‐907; Omron, Scarborough, Ontario) [11].
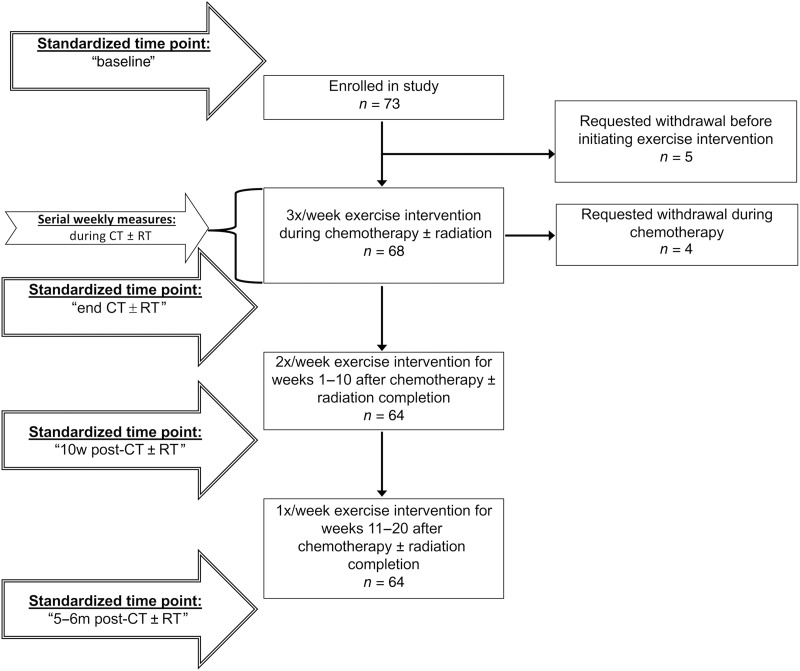
Figure 1.
Timing of standardized time points for assessment of resting heart rate, blood pressure, heart rate recovery after exercise, and weekly serial assessments of resting heart rate and blood pressure relative to study enrollment and the exercise intervention. Abbreviations: CT ± RT, chemotherapy with or without radiation; m, months; w, weeks.
Standardized Time Points of Physical Assessments.
The timing of four physical assessments was standardized across participants to assess changes across the treatment trajectory. Timing of the "baseline" study assessment varied from prechemotherapy to within the first half of chemotherapy treatments depending on patient recruitment and enrollment. The "end CT ± RT" assessment was within 1 week after completion of radiation, or for those not receiving radiation, was one chemotherapy cycle length (1–3 weeks) after the last treatment. The "10w post‐CT ± RT" assessment took place approximately 10 weeks after CT ± RT completion. The final, or "5–6m post‐CT ± RT," assessment corresponded with completion of the intervention, 5–6 months after completion of CT ± RT. Assessments were typically performed at the same time of day for each participant. Prior to each assessment, participants were asked to not eat, drink caffeine or alcohol, smoke cigarettes, or consume nonvital drugs for at least 3 hours, and in the previous 24 hours, to not exercise strenuously, but to aim to hydrate and sleep well.
During the physical assessment, HRrest and resting blood pressure were measured similar to methods described above. Aerobic fitness was assessed as estimated peak volume of oxygen consumption (VO2) by a modified Balke protocol [12] treadmill test with 3‐minute stages, that was terminated at 70% of age‐predicted [13] HR reserve (using current HRrest), followed by a cooldown at 2.0 mph and 0%. Participants were asked to abstain from talking or holding handrails during the test. HRs at the end of each stage, and at 2 minutes into the cooldown, were recorded. The VO2 corresponding to the treadmill speed and grade for each stage of the test was estimated using a metabolic equation for treadmill walking [14]. We have previously demonstrated accuracy of this equation for exercise prescription during and after chemotherapy for breast cancer [15]. The peak VO2 was estimated by extrapolating the linear relationship between the HR at the end of each stage and the corresponding estimated VO2 to age‐predicted peak HR. The error introduced by using age‐predicted HR is minimized by the use of change scores for these data. The HRrecovery was calculated as the difference between the peak HR during the test and the HR at 2 minutes into the cooldown. The HR response to the onset of exercise (HRonset) was calculated as the difference in HR between the end of the first stage and HRrest.
Prevalence of Abnormal HRrest and Resting Blood Pressures.
The prevalence of tachycardia (HRrest >100 bpm), bradycardia (HRrest <50 bpm), and systolic or diastolic hypertension (blood pressure >140 or >90 mmHg) or hypotension (blood pressure <100 or <60 mmHg) [16] was assessed for each physical assessment, and using all weekly measures during CT ± RT.
Physical Activity Performed.
Supervised exercise attendance was calculated as sessions attended divided by total possible sessions. A modified version of the Minnesota Leisure Time Physical Activity Questionnaire [17] was administered at the "baseline" and "5–6m post‐CT ± RT" assessments in reference to the previous 6 months. The “5–6m post‐CT+RT” questionnaire included both supervised and home‐based exercise. Moderate‐to‐vigorous physical activity (MVPA; all aerobic activity with metabolic equivalent ≥3.0) and metabolic equivalent (MET)‐hours per week for all reported activities were calculated.
Descriptive and Confounding Variables.
Demographics and menopausal status were collected at baseline by questionnaire. Treatment characteristics, complete blood counts, medical history, and medications were extracted from medical records. The length of time enrolled in the study (and exercising) concurrent to CT ± RT ("Study + CT ± RT length") was calculated as weeks between the "baseline" and "end CT ± RT" assessments, whereas the total length of chemotherapy treatment was time between the first and last treatment.
Statistical Analysis
The time course of HRrest and resting systolic (SBPrest) and diastolic blood pressure (DBPrest) was assessed independent of other factors first using mixed linear models with participant as a random effect. The number of days since the last chemotherapy treatment was used to characterize the trajectory within the first 14 days of all chemotherapy cycles. The number of days since the first chemotherapy treatment was used to characterize the trajectory across the course of chemotherapy. Parabolic relationships were investigated when the residuals distribution appeared non‐normal.
Second, the association of exercise, treatment, and other potential explanatory factors with the variation in change in HRrest, SBPrest, and DBPrest throughout chemotherapy treatment was assessed via computation of all possible combinations of mixed linear models and assignment of an Akaike information criterion score and weight. Independent variable importance was calculated by summing the weights of all models where the independent variable was included that summed to 95% of total weight.
Next, HRrest and blood pressure were compared across the treatment trajectory using the average of all available measures that occurred prior to chemotherapy ("prechemotherapy"), during chemotherapy, and during radiation, as well as the values measured at the "end CT ± RT," "10w post‐CT ± RT," and "5–6m post‐CT ± RT" assessments using linear mixed models and Tukey post hoc tests. A Cochran's Q test with McNemar's test for post hoc comparisons were used to compare prevalence of tachycardia, hypertension, and hypotension across these time points.
Lastly, treatment and exercise‐related predictors of ΔHRrest and ΔHRrecovery during CT ± RT ("end CT ± RT" minus "baseline" assessments) and post‐CT ± RT ("5–6m post‐CT ± RT" minus "end CT ± RT" assessments) were identified using univariate general linear models. The treatment‐related categorical independent variables tested included receiving anthracyclines, trastuzumab, radiation (either side), left‐sided radiation, radiation to either internal mammary chain, hormonal therapy, and hormonal therapy type (none/tamoxifen/aromatase inhibitor). Independent physical activity/fitness‐related continuous variables tested included supervised exercise attendance during CT ± RT and during chemotherapy alone, Δaerobic fitness, and MVPA and MET‐hours. Exercise attendance during CT ± RT was also categorized into tertiles, but then dichotomized to 0%–66%, and ≥67% due to low cell size for <33%. Other potentially explanatory independent variables tested included "baseline" and change values of body weight, BMI, waist circumference, blood pressures (systolic, diastolic, mean), aerobic fitness, HRrecovery, HRonset, and HRrest, as well as age, self‐reported baseline menopausal status, history of heart disease (of any type) or hypertension, number of comorbid conditions, currently receiving hypertensive medications, "study + CT ± RT length," and total length of chemotherapy treatment. Hormonal therapy and "5–6m post‐CT ± RT" MVPA and MET‐hours were used only for post‐CT ± RT. For post‐CT ± RT, the "end CT ± RT" test value was used as the baseline value, and change in variables during CT ± RT as well as concurrent changes during the post‐CT ± RT were tested as independent variables.
Significant or borderline significant (p ≤ .100) variables were then entered into a backward multiple regression with a p value for entry of .050 and p value for removal of .100. The Levene's test was used to ensure equality of error variances for the model. A collinearity tolerance statistic >0.2 was ensured for all variables in the final model. SPSS version 24.0 (IBM Corporation, Armonk, NY) and R version 3.3.1 were used for all analyses and figures.
Results.
Participants
One hundred nine patients were referred during study recruitment (August 2013 to October 2014); 16 were ineligible and 20 declined participation. Seventy‐three patients (78% of those eligible) enrolled. Nine participants (12%) withdrew; all available data were included. The primary results of the trial, including significant improvements in weekly minutes of MVPA and resistance training, are reported elsewhere [9]. As the "baseline" assessment was completed prior to starting chemotherapy ("prechemotherapy") in only 51% (n = 37) of participants as per eligibility for the primary trial, analyses were also completed including only these participants, but did not generally change overall trends; any discrepancies from results including all participants are noted. A post hoc power analysis was conducted conservatively using the n = 37 with "prechemotherapy" measures. Using R version 3.3.1, a p value of .05, and an F‐test with repeated measures design provided >80% power to detect the effect sizes in HRrest (power = 0.99), SBPrest (power = 0.8), and DBPrest (power = 0.84) that were observed in this study between "prechemotherapy" and chemotherapy.
Table 1 describes participant characteristics, whereas Figure 2A and 2B depict the changes in hemoglobin and hematocrit across chemotherapy. Ninety‐five percent of participants were anemic (<120 g/L hemoglobin) for at least one treatment cycle.
Table 1. Participant characteristics.
Missing n = 4 responses.
Abbreviations: CT ± RT, chemotherapy with or without radiation treatment; MI, myocardial infarction; MVPA, moderate‐to‐vigorous physical activity.
Figure 2.
Changes in hemoglobin and hematocrit across chemotherapy cycles and after completion. Hemoglobin (A), hematocrit (B). Bars denote mean and 95% confidence intervals. aSignificantly different from prechemotherapy. bSignificantly different from cycle 1. cSignificantly different from cycle 2. dSignificantly different from >1 month after chemotherapy.
HRrest Changes During Chemotherapy and Across the Adjuvant Treatment Trajectory
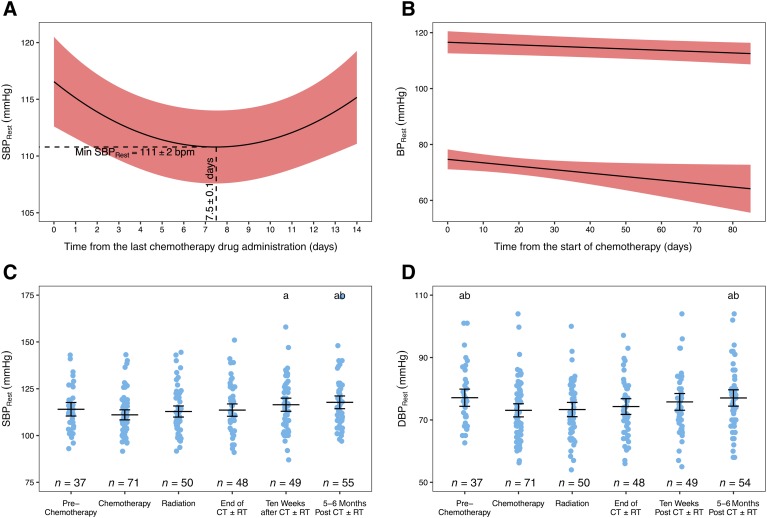
In terms of the time course of changes in HRrest during chemotherapy, there was a significant parabolic relationship with number of days since the last treatment (p < .001). The peak HRrest occurred at 7.6 days after receipt of a treatment (Fig. 3A). Across the first four cycles (minimum number received), there appeared to be sequentially higher HRrest peaks (Fig. 3B). Across the course of chemotherapy, a significant parabolic relationship was also demonstrated (p < .001), with the peak occurring at 76 days after the start (Fig. 3C).
Figure 3.
Changes in resting heart rate. HRrest during a chemotherapy cycle (A) and across the first four cycles (B), the course of chemotherapy (C), and the treatment trajectory (D). Solid line and shaded area denotes mean and 95% confidence intervals. aSignificantly different from chemotherapy. bSignificantly different from radiation. cSignificantly different from prechemotherapy. Abbreviations: CT ± RT, chemotherapy with or without radiation; HRrest, resting heart rate.
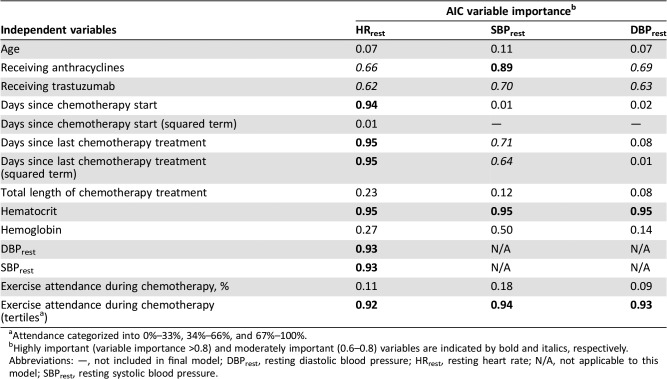
Days since last chemotherapy and since chemotherapy start were highly important variables when including other factors in the model explaining ΔHRrest during chemotherapy. Other highly important variables associated with ΔHRrest during chemotherapy included hematocrit, SBPrest, and DBPrest, as well as tertiles of exercise attendance during chemotherapy. Receiving anthracyclines and trastuzumab were moderately important variables (Table 2). When those without a "prechemotherapy" baseline were excluded, days since last chemotherapy, SBPrest, and DBPrest were no longer important variables.
Table 2. Independent variable importance for HRrest, SBPrest, and DBPrest during chemotherapy.
Attendance categorized into 0%–33%, 34%–66%, and 67%–100%.
Highly important (variable importance >0.8) and moderately important (0.6–0.8) variables are indicated by bold and italics, respectively.
Abbreviations: —, not included in final model; DBPrest, resting diastolic blood pressure; HRrest, resting heart rate; N/A, not applicable to this model; SBPrest, resting systolic blood pressure.
Across the adjuvant treatment trajectory, average HRrest was highest during chemotherapy compared with all other time points (all p < .001), and remained elevated above prechemotherapy during radiation (p < .001) and at "end CT ± RT" (p = .004; Fig. 3D). By "10w post‐CT ± RT," HRrest was not different than prechemotherapy (p = .941). Resting tachycardia was present at least once in 32% of participants during chemotherapy, which was significantly higher than all other time points (all 0%–1%, p < .001). Resting bradycardia did not occur.
SBPrest and DBPrest Changes During Chemotherapy and Across the Adjuvant Treatment Trajectory
In terms of the time course of changes in blood pressure during chemotherapy, there was a significant parabolic relationship (p < .001) between SBPrest and number of days since the last treatment, with the nadir occurring at day 7.5 (Fig. 4A). There was no significant within‐cycle effect on DBPrest. SBPrest and DBPrest exhibited significant negative linear relationships with the number of days since chemotherapy start (p = .018, p = .039, respectively; Fig. 4B).
Figure 4.
Changes in resting blood pressure. SBPrest during a chemotherapy cycle (A) and across the course of chemotherapy (B) and the treatment trajectory (C), with changes in DBPrest across the course of chemotherapy (B) and across the treatment the treatment trajectory (D). Solid line and shaded area denotes mean and 95% confidence intervals. aSignificantly different from chemotherapy. bSignificantly different from radiation.Abbreviations: BPrest, resting blood pressure; CT ± RT, chemotherapy with or without radiation; DBPrest, resting diastolic blood pressure; SBPrest, resting systolic blood pressure.
When other factors were included in the model explaining ΔSBPrest during chemotherapy, days since last chemotherapy treatment was moderately important and days since chemotherapy start was no longer an important variable. Highly important variables associated with ΔSBPrest during chemotherapy included receiving anthracyclines, the most recently measured hematocrit, and tertiles of exercise attendance during chemotherapy, whereas receiving trastuzumab was moderately important (Table 2). For DBPrest, time was not an important variable when other factors were included. Instead, hematocrit and tertiles of exercise attendance during chemotherapy were highly important, and treatment with anthracyclines and trastuzumab were moderately important variables explaining ΔDBPrest during chemotherapy (Table 2).
Across the adjuvant treatment trajectory, SBPrest did not significantly change from "prechemotherapy" to during chemotherapy (p = .307), but was significantly elevated at "10w post‐CT ± RT" and "5–6m post‐CT ± RT" relative to chemotherapy (both p < .001), and relative to radiation at "5–6m post‐CT ± RT" (p = .008; Fig. 4C). DBPrest was significantly reduced during chemotherapy relative to "prechemotherapy" (p = .002) and remained reduced during radiation (p = .015). By "10w post‐CT ± RT," DBPrest was not different than prechemotherapy (p = .922; Fig. 4D). The prevalence of systolic (3%–14%, p = .130) or diastolic (0%–10%, p = .183) hypertension was not different between time points. The prevalence of systolic hypotension was significantly higher during chemotherapy (51%) than radiation (19%, p = .003) and all other time points (3%–10%, all p < .001); prevalence during radiation was higher than the remaining time points (all p < .040). The prevalence of at least one instance of diastolic hypotension was also significantly higher during chemotherapy (29%) than radiation (12%, p = .008) and all other time points (3%–4%, all p < .001); prevalence during radiation was also higher than the remaining time points (all p < .040).
Factors Associated with Changes in HRrest and HRrecovery During CT ± RT and Post‐CT ± RT
The independent predictors and multiple regression models of ΔHRrest and ΔHRrecovery during CT ± RT and post‐CT ± RT are listed in Table 3. The treatment‐related factors that were predictive of impaired HRrecovery and elevated HRrest were receiving anthracyclines, trastuzumab, and left‐sided radiation. The physical activity/fitness‐related factors that were predictive of increased HRrecovery and less HRrest elevation were baseline and Δaerobic fitness, as well as supervised exercise attendance.
Table 3. Predictors of the ΔHRrest and ΔHRrecovery during and after treatment.
Effect size reported as partial eta squared. Interpretation: small = 0.01–0.08; medium = 0.09–0.024; large = ≥0.25.
90% CI reported for these borderline significant variables (0.10 ≤ p > .05).
Abbreviations: —, not significant for this model; bpm, beats per minute; CI, confidence interval; CT ± RT, chemotherapy with or without radiation; DBPrest, resting diastolic blood pressure; HRonset, cardiac response to the onset of exercise; HRrecovery, heart rate recovery after exercise; HRrest, resting heart rate; MAPrest, resting mean arterial pressure; mL/kg/minute, millilitres of oxygen per kilogram of body weight per minute; SBPrest, resting systolic blood pressure.
Discussion
The main findings in this study of early‐stage breast cancer patients receiving adjuvant chemotherapy and participating in an exercise program are the following: (a) HRrest increased within and successively across chemotherapy treatments; (b) within a chemotherapy cycle, the peak HRrest and the SBPrest nadir occurred at the 8th day after receipt of treatment; (c) SBPrest and DBPrest decreased linearly across the course of chemotherapy; (d) receiving cardiotoxic systemic therapies, hematocrit and hemoglobin levels, and supervised exercise attendance were associated with the changes in HRrest and blood pressure that occurred during chemotherapy; (e) during chemotherapy, tachycardia and hypotension occurred in one third and half of patients, respectively; and (f) receipt of cardiotoxic treatments was associated with elevated HRrest and impaired HRrecovery during CT ± RT, although objective indicators of exercise training during CT ± RT predicted improvements in these measures.
Tachycardia and diastolic hypotension occurred in almost one third and systolic hypotension in half of participants during chemotherapy treatment but were resolved by "end CT ± RT." Therefore, the primary implications of these conditions would be for practitioners providing oncology care or exercise guidance during chemotherapy. Oncologists and nurses performing physical examinations or subjective assessments of patient well‐being should be aware of the pattern of incremental increases in HRrest and decreases in blood pressure that result in high prevalence of tachycardia and hypotension. Both conditions could result in patient symptoms (e.g., dizziness or lightheadedness, difficulty in changing body position, feelings of high heart rate) and a potential need for hypertension medication dose adjustments. Exercise professionals working with patients receiving chemotherapy should be aware of the prevalence of tachycardia and hypotension, regularly monitor for them, and adjust exercise plans as necessary. For exercise prescription and monitoring in the current study, we followed the 2010 American College of Sports Medicine exercise guidelines for cancer patients [14] of using HRrest >100 bpm, SBPrest >145 mmHg, and DBPrest >95 mmHg as contraindications to exercise training or testing. SBPrest <85 mmHg is also recommended as a contraindication, and there is no minimum for DBPrest. We did observe (but did not quantify) instances of dizziness and lightheadedness throughout chemotherapy, but often participants felt quite well in the face of hypotension and were able to exercise safely when counseled to change body positions slowly (e.g., sit to stand, or bending over to pick up weights) and ensure performance of ≥5‐minute gradual warm‐up and cool‐down. Given the relatively common occurrence of hypotension, additional exercise guidelines may be required. We recommend monitoring HRrest and blood pressure after completion of an exercise session to ensure a normal return to pre‐exercise measures, especially in the case of hypotension. Practitioners could also provide general recommendations for management of autonomic or orthostatic intolerance disorders to this patient population. In addition to exercise, these include ensuring adequate hydration, increasing salt intake, and avoiding bed rest [18]. Further, if resting is required during days of ill health, seated in bed would be the preferred position over lying supine to prevent severe deconditioning [18].
The autonomic nervous system regulates the regional and systemic circulation via changes in HR, arterial blood pressure, and peripheral vascular tone [5]. HRrest is influenced by numerous factors, but the major determining factor of HRrest is a combination of sympathetic stimulation and parasympathetic withdrawal [7]. Consistent with our findings, cross‐sectional studies have reported elevated HRrest (+15–17 bpm) in breast cancer patients during and years after treatment relative to untreated breast cancer patients or healthy controls [3], [19], [20]. Elevated HRrest is of clinical interest as it is independently associated with increased risk of cardiovascular disease and mortality [21], [22]. In the current study, we demonstrated that HRrest increased with time since the last chemotherapy treatment, with some recovery prior to receiving the subsequent treatment, but that each subsequent treatment has a cumulative effect, such that there is a significant sustained average increase (+11 ± 1 bpm) over the course of chemotherapy. There is evidence that other chemotherapy treatment side effects including fatigue may follow a similar cyclical pattern in which the peak occurs several days after each treatment, followed by partial recovery, such that there is a cumulative increase over consecutive treatment cycles [23]. This pattern of treatment symptoms matches our clinical research observations. On this basis, we hypothesize that a relationship exists between the patterns in physiological variables and patient‐reported (i.e., subjective) treatment symptoms. In terms of implications for the exercise professionals working with cancer patients during chemotherapy, we suggest that exercise volume and target HRs for intensity prescription should be prescribed to accommodate for the cyclical fluctuations in chemotherapy symptoms and HRrest, respectively. If possible, we suggest that HRrest be measured prior to every exercise session or at least once per week.
Little is known regarding the mechanisms for elevated HRrest with chemotherapy treatment. Reduced hemoglobin is a logical mechanism mediating the increase in HRrest, given its role in oxygen delivery. However, hematocrit, but not hemoglobin, was an important explanatory variable for ΔHRrest during chemotherapy, suggesting a role for plasma volume. SBPrest and DBPrest were also highly important explanatory variables, and changes during chemotherapy were in opposite directions for HRrest and blood pressure, which may suggest a role for baroreflex mediation. Additionally, a worsening in HRrecovery and HRonset were predictive of >40% of the variance in ΔHRrest. Parasympathetic reactivation may be the predominant mechanism with 2+ minute HRrecovery measures [7], as within the current study. HRonset likely predominantly represents a reduction in vagal discharge [24]. Therefore, this finding indicates that changes in the regulation of vagal tone may also be an important mechanism for ΔHRrest.
Known cardiotoxic treatments were also predictors of ΔHRrest as well as ΔHRrecovery. Receiving radiation to the left breast was predictive of a 7‐beat increase in HRrest during CT ± RT, and a 4‐beat reduction in HRrecovery post‐CT ± RT. A similar effect was previously reported with mediastinal radiation treatment, in which high‐frequency HR variability (parasympathetic activity marker) was reduced, whereas the ratio of low‐to‐high‐frequency (sympatho‐vagal balance) was elevated [25].
Receiving anthracyclines and trastuzumab were moderately important explanatory variables for ΔHRrest and Δblood pressure during chemotherapy, and were significant predictors of a 5–8‐beat worsening of ΔHRrest and ΔHRrecovery during CT ± RT. Importantly, supervised exercise attendance ≥67% (i.e., at least twice per week) during CT ± RT was associated with a 6‐beat improvement in both variables, potentially counteracting the negative effect of these cardiotoxic therapies. Furthermore, supervised exercise attendance (as a continuous variable) was a predictor of improvement in HRrecovery, but not HRrest during chemotherapy alone or CT ± RT, suggesting a dose‐response relationship for ΔHRrecovery but a minimum threshold for effects on ΔHRrest.
Despite the strong established link between aerobic fitness and autonomic function in other populations, longitudinal studies assessing this relationship in cancer populations are lacking [6]. In the current study, a reduction in aerobic fitness during CT ± RT was the strongest independent predictor of an elevated HRrest and was also an independent predictor of impaired HRrecovery both during and post‐CT ± RT. However, given that our measure of aerobic fitness was estimated without gas analysis, these findings should be interpreted with caution and need to be confirmed using cardiopulmonary exercise testing.
A history of heart disease predicted a 12‐beat worsening of HRrecovery during CT ± RT, but neither a history of hypertension nor use of hypertension medications were predictive of any changes. Age and postmenopausal status are known to increase the risk of cardiovascular disease in women [26], and potentially in the breast cancer population [27], and were also not predictors. Lakoski et al. have hypothesized that the weight gain and visceral adiposity common in breast cancer patients could be a source of autonomic dysfunction [6]. We did not find relationships between baseline levels or changes in body weight, BMI, or waist circumference with HRrest or HRrecovery.
This study is the first to provide prospective data on changes in clinical indices that reflect cardiovascular autonomic function across and between adjuvant treatments for breast cancer. Our study sample is generalizable to the breast cancer population by including a wide range of ages, multiple ethnicities, comorbid conditions, and common treatments. However, this heterogeneity may also limit internal validity of our results. Although there is no nonexercise control group, the range of exercise attendance allowed for assessment of associations between exercise volume and our outcome measures. However, our data are likely biased by having fewer weekly measurements for those attending the exercise sessions less frequently. Our results regarding the influence of aerobic fitness are limited by our indirect measure. Lastly, our 5–6‐month follow‐up to completion of CT ± RT may not have been long enough to capture delayed cardiotoxic effects. Overall, these limitations should be considered in the interpretation of these results.
Conclusion
This was a longitudinal study of the association of cardiotoxic cancer therapies and exercise training with clinical indices of cardiovascular autonomic control. Among women with breast cancer enrolled in in an exercise program offered as supportive care, receiving anthracyclines, trastuzumab, and left‐sided radiation treatments were associated with elevations in HRrest, reductions in blood pressure, and impairments of HRrecovery. During chemotherapy, resting tachycardia and hypotension were common, occurring in one third and half of women. However, exercise training, particularly attendance of at least two out of three weekly, supervised sessions, and an improvement in aerobic fitness appear to mitigate the treatment‐related changes. These preliminary findings using clinical measures of cardiovascular autonomic function warrant future research into the role of exercise training during and after cardiotoxic cancer therapies using more rigorous assessment methods.
Acknowledgments
Kelcey Bland, Alis Bonsignore, Holly Wollmann, and the other exercise volunteers are acknowledged for their help in collecting these data.
Author Contributions
Conception/design: Amy A. Kirkham, Karen A. Gelmon, Donald C. McKenzie, Kristin L. Campbell
Provision of study material or patients: Karen A. Gelmon
Collection and/or assembly of data: Amy A. Kirkham
Data analysis and interpretation: Amy A. Kirkham, Matthew G. Lloyd, Victoria E. Claydon, Kristin L. Campbell
Manuscript writing: Amy A. Kirkham, Matthew G. Lloyd, Victoria E. Claydon, Kristin L. Campbell
Final approval of manuscript: Amy A. Kirkham, Matthew G. Lloyd, Victoria E. Claydon, Karen A. Gelmon, Donald C. McKenzie, Kristin L. Campbell
Disclosures
The authors indicated no financial relationships.
References
- 1.Hooning MJ, Botma A, Aleman BM et al. Long‐term risk of cardiovascular disease in 10‐year survivors of breast cancer. J Natl Cancer Inst 2007;99:365–375. [DOI] [PubMed] [Google Scholar]
- 2.Riihimaki M, Thomsen H, Brandt A et al. Death causes in breast cancer patients. Ann Oncol 2012;23:604–610. [DOI] [PubMed] [Google Scholar]
- 3.Jones LW, Courneya KS, Mackey JR et al. Cardiopulmonary function and age‐related decline across the breast cancer survivorship continuum. J Clin Oncol 2012;30:2530–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones LW, Haykowsky MJ, Swartz JJ et al. Early breast cancer therapy and cardiovascular injury. J Am Coll Cardiol 2007;50:1435–1441. [DOI] [PubMed] [Google Scholar]
- 5.Rosenwinkel ET, Bloomfield DM, Arwady MA et al. Exercise and autonomic function in health and cardiovascular disease. Cardiol Clin 2001;19:369–387. [DOI] [PubMed] [Google Scholar]
- 6.Lakoski SG, Jones LW, Krone RJ et al. Autonomic dysfunction in early breast cancer: Incidence, clinical importance, and underlying mechanisms. Am Heart J 2015;170:231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lahiri MK, Kannankeril PJ, Goldberger JJ. Assessment of autonomic function in cardiovascular disease. J Am Coll Cardiol 2008;51:1725–1733. [DOI] [PubMed] [Google Scholar]
- 8.Stamler J, Stamler R, Neaton JD. Blood pressure, systolic and diastolic, and cardiovascular risks. US population data. Arch Intern Med 1993;153:598–615. [DOI] [PubMed] [Google Scholar]
- 9.Kirkham AA, Van Patten CL, Gelmon KA et al. Effectiveness of oncologist‐referred exercise and healthy eating programming as a part of supportive adjuvant care for early breast cancer. The Oncologist 2018;23:105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palatini P, Benetos A, Grassi G et al. Identification and management of the hypertensive patient with elevated heart rate: Statement of a European Society of Hypertension consensus meeting. J Hypertens 2006;24:603–610. [DOI] [PubMed] [Google Scholar]
- 11.El Assaad MA, Topouchian JA, Darne BM et al. Validation of the Omron HEM‐907 device for blood pressure measurement. Blood Press Monit 2002;7:237–241. [DOI] [PubMed] [Google Scholar]
- 12.Pollock ML, Foster C, Schmidt D et al. Comparative analysis of physiologic responses to three different maximal graded exercise test protocols in healthy women. Am Heart J 1982;103:363–373. [DOI] [PubMed] [Google Scholar]
- 13.Gulati M, Shaw LJ, Thisted RA et al. Heart rate response to exercise stress testing in asymptomatic women: The St. James Women Take Heart Project. Circulation 2010;122:130–137. [DOI] [PubMed] [Google Scholar]
- 14.Thompson WR, Gordon NF, Pescatello LS, eds. ACSM's Guidelines for Exercise Testing and Prescription 8th ed Philadelphia: Wolters Kluwer Lippincott Williams & Wilkins, 2010. [Google Scholar]
- 15.Kirkham AA, Campbell KL, McKenzie DC. Comparison of aerobic exercise intensity prescription methods in breast cancer. Med Sci Sports Exerc 2013;45:1443–1450. [DOI] [PubMed] [Google Scholar]
- 16.Parati G, Di Rienzo M, Coruzzi P et al. Chronic hypotension and modulation of autonomic cardiovascular regulation. Hypertens Res 2009;32:931–933. [DOI] [PubMed] [Google Scholar]
- 17.Taylor HL, Jacobs DR, Schucker B et al. A questionnaire for the assessment of leisure time physical activities. J Chron Dis 1978;31:741–755. [DOI] [PubMed] [Google Scholar]
- 18.Arnold AC, Raj SR. Orthostatic hypotension: A practical approach to investigation and management. Can J Cardiol 2017;33:1725–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones LW, Haykowsky M, Pituskin EN et al. Cardiovascular reserve and risk profile of postmenopausal women after chemoendocrine therapy for hormone receptor–positive operable breast cancer. The Oncologist 2007;12:1156–1164. [DOI] [PubMed] [Google Scholar]
- 20.Jones LW, Haykowsky M, Peddle CJ et al. Cardiovascular risk profile of patients with HER2/neu‐positive breast cancer treated with anthracycline‐taxane‐containing adjuvant chemotherapy and/or trastuzumab. Cancer Epidemiol Biomarkers Prev 2007;16:1026–1031. [DOI] [PubMed] [Google Scholar]
- 21.Kannel WB, Kannel C, Paffenbarger RS Jr et al. Heart rate and cardiovascular mortality: The Framingham Study. Am Heart J 1987;113:1489–1494. [DOI] [PubMed] [Google Scholar]
- 22.Cooney MT, Vartiainen E, Laatikainen T et al. Elevated resting heart rate is an independent risk factor for cardiovascular disease in healthy men and women. Am Heart J 2010;159:612–619.e3. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz A. Daily fatigue patterns and effect of exercise in women with breast cancer. Cancer Pract 2000;8:16–24. [DOI] [PubMed] [Google Scholar]
- 24.Fagraeus L, Linnarsson D. Autonomic origin of heart rate fluctuations at the onset of muscular exercise. J Appl Physiol 1976;40:679–682. [DOI] [PubMed] [Google Scholar]
- 25.Hoca A, Yildiz M, Ozyigit G. Evaluation of the effects of mediastinal radiation therapy on autonomic nervous system. Med Oncol 2012;29:3581–3586. [DOI] [PubMed] [Google Scholar]
- 26.World Heart Federation. Cardiovascular Disease Risk Factors. Geneva: World Heart Federation, 2013. [Google Scholar]
- 27.Ewer MS, Glück S. A woman's heart: The impact of adjuvant endocrine therapy on cardiovascular health. Cancer 2009;115:1813–1826. [DOI] [PubMed] [Google Scholar]