Abstract
This commentary reviews the novel mechanism of action of TRC105 in the context of treatments currently available for metastatic renal cell carcinoma, highlighting the recent findings of Choueiri and colleagues.
Renal cell carcinoma (RCC) is one of the 10 most commonly diagnosed cancers in the U.S., and approximately 30% of patients with newly diagnosed RCC present with locally advanced or metastatic disease [1], [2]. Although metastatic RCC (mRCC) carries a poor prognosis, the treatment landscape for mRCC is evolving at a staggering pace due to improved understanding of the pathogenic mechanisms underlying mRCC [3]. Somatic mutations to VHL are seen in the majority of patients with clear cell mRCC. These mutations prevent ubiquitination of hypoxia‐inducible factor (HIF), which causes an accumulation of intracellular HIF and production of growth factors that facilitate angiogenesis, glycolysis, and tumorigenesis [4]. Subsequently, vascular endothelial growth factor (VEGF)‐targeted therapies were developed and improved survival for patients with mRCC. However, most patients treated with VEGF‐targeted therapy will eventually have disease progression. A number of other angiogenesis pathways have been identified as possible resistance mechanisms to VEGF targeted therapy, including interleukin‐6 (IL‐6), transforming growth factor‐β (TGF‐β), platelet‐derived growth factor (PDGF), basic fibroblast growth factor (bFGF), c‐MET, and angiopoietin. With this knowledge, a multitarget tyrosine kinase inhibitor (TKI), cabozantinib, was developed to target VEGF and c‐MET, and it improved survival compared to treatment with sunitinib, a VEGF only TKI [5].
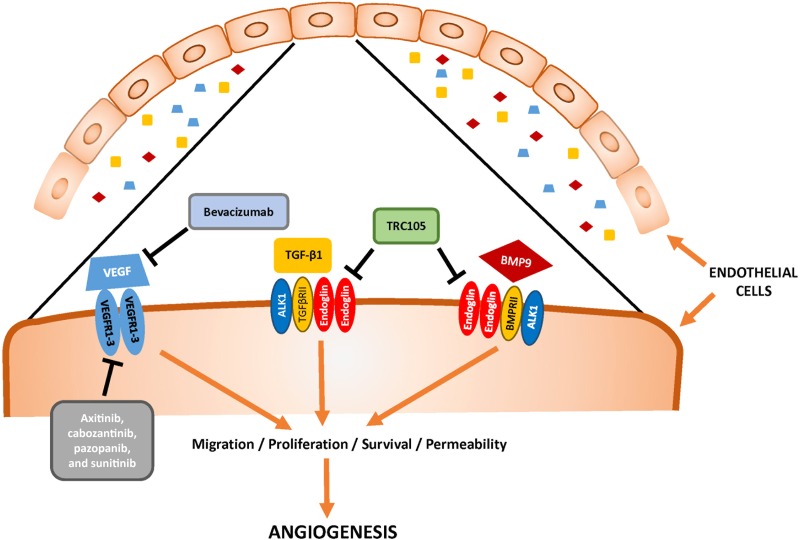
TRC105 (carotuximab) is a novel therapeutic that bears relationship to the underlying pathophysiology of Osler‐Weber‐Rendu syndrome, which is also referred to as hereditary hemorrhagic telangiectasia‐1 (HHT‐1). In HHT‐1, mutations to ENG result in defective endoglin. Endoglin (CD105) is a homodimeric TGF‐β coreceptor that is upregulated by HIF‐1‐α and is essential for normal vascular development [6], [7], [8]. Interestingly, patients with Osler‐Weber‐Rendu have improved survival across a number of cancers, likely due to defects in angiogenesis. TRC105 is a chimeric IgG1 monoclonal antibody that binds to endoglin, competitively inhibits bone morphogenic protein ligand binding required for endothelial signal transduction, induces antibody‐dependent cellular cytotoxicity of vascular endothelial cells and endoglin‐expressing tumor cells, and inhibits angiogenesis (Fig. 1) [9]. TRC105’s ability to target angiogenesis through the TGF‐β pathway suggests that it could cooperate with a VEGF‐targeted therapy to improve outcomes for patients with mRCC (Fig. 1).
Figure 1.
Mechanism of action for TRC105 plus axitinib and alternative pathways of angiogenesis in metastatic renal cell carcinoma.Abbreviations: ALK1, activin receptor‐like kinase 1; BMP, bone morphogenic protein; TGF‐β, transforming growth factor‐β; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
In the accompanying phase I clinical trial, Choueiri and colleagues clearly demonstrate that TRC105 can safely be combined with axitinib in patients with mRCC [10]. Nineteen patients with heavily pretreated mRCC who progressed following treatment with at least one VEGF‐targeted therapy were included. Patients received a median of three prior systemic therapies (range, one to six). In this cohort, TRC105 plus axitinib did not cause any dose‐limiting toxicities or increase the toxicity profiles of the individual drugs. Grade 3 toxicities attributed to TRC105 included anemia requiring blood transfusions and headache after the initial dose. Furthermore, TRC105 plus axitinib demonstrated promising efficacy in this small cohort of mRCC patients. In this cohort treated with TRC105 plus axitinib, median progression‐free survival (mPFS) was 11.3 months, objective response rate was 29.4% (5/17), and disease control rate was 88.2% (15/17). With the caveats of cross‐trial comparison, the mPFS of 11.3 months with TRC105 plus axitinib was an improvement compared with the original AXIS trial in which axitinib as monotherapy had a mPFS of 8.3 months [11]. Notably, in the AXIS trial, mRCC patients were allowed to receive only one prior systemic therapy, and the mPFS with axitinib in those with prior exposure to a VEGF TKI was 6.5 months.
With a good safety profile and encouraging efficacy data, TRC105 plus axitinib warrants further investigation as salvage therapy for patients with mRCC, and enrollment in a phase II registration trial is already complete (NCT01806064). However, these findings should be considered in the context of previous studies with TRC105 and another angiogenesis pathway inhibitor, dalantercept. TRC105 was initially combined with bevacizumab in a randomized phase II clinical trial of 59 patients with previously treated mRCC [12]. In that study, TRC105 plus bevacizumab did not improve mPFS compared to bevacizumab alone (2.8 vs. 4.6 months, p = .09). Dalantercept is an activin receptor‐like kinase 1 (ALK1) fusion protein that acts as a ligand trap for bone morphogenic proteins 9 and 10, which is similar to the pathophysiology behind hereditary hemorrhagic telangiectasia‐2. For dalantercept, the phase I clinical trial showed promising safety and efficacy data in previously treated mRCC; however, the phase II clinical trial revealed that dalantercept plus axitinib did not improve mPFS compared with axitinib plus placebo (6.8 months vs. 5.6 months; hazard ratio, 1.10; p = 0.67) [13]. With the benefit of hindsight, both studies have significant limitations that may have contributed to their negative results. In the TRC105 plus bevacizumab trial, bevacizumab is not a standard salvage‐line treatment for mRCC, and endoglin levels unexpectedly decreased after monotherapy with bevacizumab. For dalantercept, the physical manifestations of HHT were only seen in a minority of patients, suggesting an appropriate physiologic dose was not achieved.
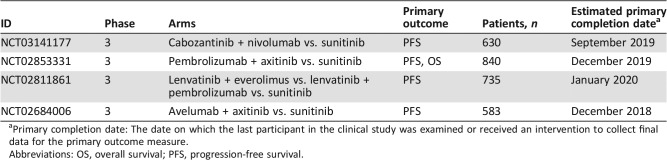
If the phase II registration trial of TRC105 plus axitinib is positive, oncologists will have a novel therapeutic class in their armamentarium for mRCC, yet it also raises the question of where TRC105 plus axitinib will fit in the increasingly crowded treatment landscape for mRCC. Currently, the options for salvage treatment of mRCC include cabozantinib, nivolumab, lenvatinib plus everolimus, and axitinib monotherapy. Without question, TRC105 plus axitinib could be utilized as an additional salvage treatment for mRCC. Currently, TRC105 plus axitinib is not being investigated as a first‐line treatment for mRCC. Newer regimens or agents, such as nivolumab plus ipilimumab and cabozantinib, have become standard of care first‐line treatment for most mRCC patients, and the combination of bevacizumab plus atezolizumab may be approved soon. Furthermore, novel combination regimens, such as pembrolizumab plus axitinib and avelumab plus axitinib, have shown promise for mRCC in early phase trials and are under investigation in phase III registration clinical trials (Table 1) [14], [15]. If the phase II registration trial of TRC105 plus axitinib leads to approval of this combination in the salvage therapy setting, could clinical trials evaluating first‐line triplet therapy with a checkpoint inhibitor plus axitinib plus TRC105 be initiated in the near future?
Table 1. Phase III clinical trials of novel combination regimens for first‐line therapy for metastatic renal cell carcinoma.
Primary completion date: The date on which the last participant in the clinical study was examined or received an intervention to collect final data for the primary outcome measure.
Abbreviations: OS, overall survival; PFS, progression‐free survival.
In conclusion, TRC105 targets endoglin, a non‐VEGF angiogenesis pathway that has the potential to complement VEGF targeted therapy. In the accompanying phase I trial, Choueiri and colleagues demonstrate that TRC105 plus axitinib is safe and has promising therapeutic activity in patients with mRCC. Due to the mixed history with targeting VEGF and alternative angiogenesis pathways, the jury will remain out on TRC105 plus axitinib until the phase II registration trial is reported.
Acknowledgments
The authors acknowledge Roberto H. Nussenzveig for help with figure preparation.
Footnotes
Editor's Note: See the related article, “An Open Label Phase Ib Dose Escalation Study of TRC105 (Anti‐Endoglin Antibody) with Axitinib in Patients with Metastatic Renal Cell Carcinoma,” by Toni K. Choueiri et al., on page 202 of this issue.
Disclosures
Sumanta K Pal: Genentech, Aveo, Eisai, Roche, Pfizer, Novartis, Ipsen, Bristol‐Myers Squib, Astellas Pharma (C/A); Neeraj Agarwal: Pfizer, Novartis, Merck, Genentech, Eisai, Exelixis, Clovis Oncology, EMD Serono, Inc., Bristol‐Myers Squibb, AstraZeneca, Astellas Pharma, Eli Lilly and Co. (C/A). The other author indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Stephenson AJ, Chetner MP, Rourke K et al. Guidelines for the surveillance of localized renal cell carcinoma based on the patterns of relapse after nephrectomy. J Urol 2004;172:58–62. [DOI] [PubMed] [Google Scholar]
- 3.Gill DM, Agarwal N, Vaishampayan U. Evolving treatment paradigm in metastatic renal cell carcinoma. Am Soc Clin Oncol Educ Book 2017;37:319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet 2009;373:1119–1132. [DOI] [PubMed] [Google Scholar]
- 5.Choueiri TK, Halabi S, Sanford BL et al. Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: The Alliance A031203 CABOSUN trial. J. Clin Oncol 2017;35:591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seon BK, Haba A, Matsuno F et al. Endoglin‐targeted cancer therapy. Curr Drug Deliv 2011;8:135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosen LS, Gordon MS, Robert F et al. Endoglin for targeted cancer treatment. Curr Oncol Rep 2014;16:365. [DOI] [PubMed] [Google Scholar]
- 8.Li DY, Sorensen LK, Brooke BS et al. Defective angiogenesis in mice lacking endoglin. Science 1999;284:1534–1537. [DOI] [PubMed] [Google Scholar]
- 9.Nolan‐Stevaux O, Zhong W, Culp S et al. Endoglin requirement for BMP9 signaling in endothelial cells reveals new mechanism of action for selective anti‐endoglin antibodies. PLoS One 2012;7:e50920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choueiri TK, Michaelson MD, Posadas EM et al. An open label phase 1b dose escalation study of TRC105 (anti‐endoglin antibody) with axitinib in patients with renal cell carcinoma. The Oncologist 2019;24:202–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Motzer RJ, Escudier B, Tomczak P et al. Axitinib versus sorafenib as second‐line treatment for advanced renal cell carcinoma: Overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol 2013;14:552–562. [DOI] [PubMed] [Google Scholar]
- 12.Dorff TB, Longmate JA, Pal SK et al. Bevacizumab alone or in combination with TRC105 for patients with refractory metastatic renal cell cancer. Cancer 2017;123:4566–4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voss MH, Bhatt RS, Plimack ER et al. The DART study: Results from the dose‐escalation and expansion cohorts evaluating the combination of dalantercept plus axitinib in advanced renal cell carcinoma. Clin Cancer Res 2017;23:3557–3565. [DOI] [PubMed] [Google Scholar]
- 14.Atkins MB, Plimack ER, Puzanov I et al. Axitinib in combination with pembrolizumab in patients with advanced renal cell cancer: A non‐randomised, open‐label, dose‐finding, and dose‐expansion phase 1b trial. Lancet Oncol 2018;19:405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choueiri TK, Larkin J, Oya M et al. Preliminary results for avelumab plus axitinib as first‐line therapy in patients with advanced clear‐cell renal‐cell carcinoma (JAVELIN Renal 100): An open‐label, dose‐finding and dose‐expansion, phase 1b trial. Lancet Oncol 2018;19:451–460. [DOI] [PubMed] [Google Scholar]