Abstract
Lessons Learned.
Patients with hepatocellular carcinoma (HCC) often have limited therapeutic responses to the vascular endothelial growth factor (VEGF) tyrosine kinase inhibitor sorafenib, which is standard of care in advanced HCC. Targeting the activin receptor‐like kinase 1 (ALK1) and VEGF pathways simultaneously by combining the ALK1 ligand trap dalantercept with sorafenib may result in more effective angiogenic blockade and delay tumor progression in patients with advanced HCC.
Although the combination was generally well tolerated, there was no additive antitumor activity with the combination of dalantercept plus sorafenib in patients with advanced HCC. No complete or partial responses were observed, and overall survival ranged from 1.9 to 23.3 months.
These results suggest that, in this patient population, further development of the possible limited benefits of combination therapy with dalantercept plus sorafenib is not warranted.
Background.
Targeting the activin receptor‐like kinase 1 (ALK1) and vascular endothelial growth factor (VEGF) pathways may result in more effective angiogenic blockade in patients with hepatocellular carcinoma (HCC).
Methods.
In this phase Ib study, patients with advanced HCC were enrolled to dose‐escalation cohorts, starting at 0.6 mg/kg dalantercept subcutaneously every 3 weeks plus 400 mg sorafenib orally once daily, or to a dose expansion cohort. The primary objective was to determine the safety and tolerability and the dalantercept maximum tolerated dose (MTD) level. Secondary objectives were to assess the preliminary activity and the association of pharmacodynamic biomarkers with tumor response.
Results.
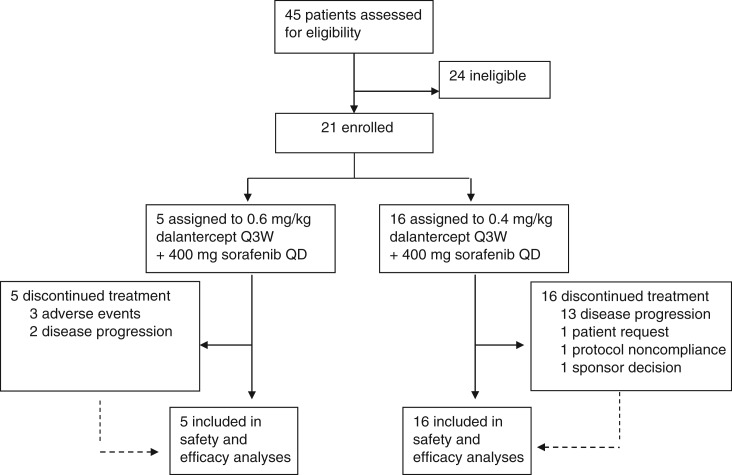
A total of 21 patients were enrolled in the study. Five patients received 0.6 mg/kg dalantercept in the first dose escalation cohort. Based on the initial safety results, the dose level was de‐escalated to 0.4 mg/kg in the second cohort (n = 6). The MTD was identified as 0.4 mg/kg and used for the dose expansion cohort (n = 10). At this dose level, the combination was generally well tolerated. Overall survival ranged from 1.9 to 23.3 months, and the best overall response was stable disease.
Conclusion.
The addition of dalantercept to sorafenib did not improve antitumor activity in patients with HCC. The dalantercept program in this population was discontinued.
Abstract
经验教训
• 肝癌 (HCC) 患者对血管内皮生长因子 (VEGF) 酪氨酸激酶抑制剂索拉非尼的治疗反应通常有限,这是晚期 HCC 的标准治疗方法。通过将 ALK1 配体陷阱 dalantercept 与索拉非尼组合,同时靶向激活素受体样激酶 1 (ALK1) 和 VEGF 通路可以导致更有效的血管生成阻滞,并延迟晚期 HCC 患者的肿瘤进展。
• 虽然该组合通常耐受良好,但对于晚期 HCC 患者,联合使用 dalantercept 与索拉非尼没有增加抗肿瘤活性。未观察到完全或部分反应,总生存期为 1.9 至 23.3 个月。
• 这些结果表明,对于这一患者群体,不建议进一步研究联合 dalantercept 和索拉非尼进行治疗带来的可能有限的疗效。
摘要
背景。靶向激活素受体样激酶 1 (ALK1) 和血管内皮生长因子 (VEGF) 通路可对肝癌 (HCC) 患者产生更有效的血管生成阻滞。
方法。在该 Ib 期研究中,患有晚期 HCC 的患者参加剂量递增队列,最初每 3 周皮下注射一次 0.6 mg/kg dalantercept,每日口服一次 400mg 索拉非尼,或者剂量扩增队列。主要目标是确定安全性和耐受性,以及 dalantercept 最大耐受剂量 (MTD) 水平。次要目标是评估初步活性和药效学生物标志物与肿瘤反应的关联。
结果。共有 21 名患者参加了该研究。5 名患者在第一次剂量递增队列中接受 0.6 mg/kg dalantercept。基于初始安全性结果,在第二队列中,剂量水平降低至 0.4 mg/kg (n = 6)。MTD 被确定为 0.4 mg/kg,并用于剂量扩增队列 (n = 10)。在该剂量水平下,该组合通常具有良好的耐受性。总生存期为 1.9 至 23.3 个月,最佳总体反应为病情稳定。
结论。在索拉非尼中加入 dalantercept 并未改善 HCC 患者的抗肿瘤活性。已经停止对该人群实施 dalantercept 计划。
Discussion
Dalantercept is a soluble ALK1 receptor fusion protein that acts as a ligand trap by binding bone morphogenetic protein 9 and 10, disrupting the formation of mature blood vessels through a mechanism distinct from the VEGF pathway [1], [2]. Targeting the ALK1 and VEGF pathways by combining dalantercept and the multikinase and VEGF receptor tyrosine kinase inhibitor (TKI), sorafenib, may result in more effective angiogenic blockade and delay tumor progression in patients with advanced HCC.
Figure 1.
Study design and patient disposition.Abbreviations: Q3W, every 3 weeks; QD, once daily.
Preclinical and early clinical studies suggest that dalantercept in combination with VEGF pathway inhibitors may maximize growth inhibition in tumors that are sensitive to antiangiogenic agents [3], [4]. This phase Ib study was designed to determine the maximum tolerated dose of dalantercept in combination with sorafenib for phase II studies. The starting dose level was 0.6 mg/kg dalantercept subcutaneously every 3 weeks (Q3W) plus 400 mg sorafenib orally once daily (QD).
Although dose levels of dalantercept ranging from 0.6 mg/kg to 1.6 mg/kg were generally well tolerated in other clinical studies [5], [6], [7], including 0.9 mg/kg in combination with the TKI axitinib [4], in this study the incidence and severity of volume‐related events at the 0.6 mg/kg dose level, including peripheral edema (40%), increased weight (60%), and one dose‐limiting toxicity, grade 4 hyponatremia, led to the de‐escalation of the dalantercept dose level to 0.4 mg/kg.
The combination of 0.4 mg/kg dalantercept Q3W plus 400 mg sorafenib QD was generally well tolerated in the 16 patients treated at this dose level. The safety profile was similar to that reported in other clinical studies [4], [5], [6], [7]. The most common treatment‐emergent adverse events were constipation, diarrhea, palmar‐plantar erythrodysesthesia syndrome, abdominal pain, fatigue, nausea, cough, peripheral edema, and increased lipase. There were no events higher than grade 3 and no study treatment discontinuation due to adverse events reported in this treatment group.
However, antitumor activity was minimal. Overall survival ranged from 1.9 to 23.3 months, and the best overall response was stable disease, reported in 53.3% of patients. In comparison, sorafenib alone in patients with advanced HCC has a median overall survival of 10.7 months (95% confidence interval [CI] 9.4–13.3) and time to progression of 5.5 months (95% CI 4.1–6.9) [8].
Although this combination was generally well tolerated, it did not improve upon the efficacy of sorafenib in patients with advanced HCC. Thus, there are no further clinical studies of this combination planned in patients with HCC.
Trial Information
- Disease
Hepatocellular carcinoma
- Stage of Disease/Treatment
Metastatic/advanced
- Prior Therapy
None
- Type of Study ‐ 1
Phase I
- Type of Study ‐ 2
3 + 3
- Primary Endpoint
Safety
- Primary Endpoint
Tolerability
- Secondary Endpoint
Efficacy
- Secondary Endpoint
Pharmacokinetics
- Secondary Endpoint
Pharmacodynamic
- Investigator's Analysis
Level of activity did not meet planned endpoint
Drug Information
- Drug 1
- Generic/Working Name
Dalantercept
- Company Name
Acceleron Pharma
- Drug Type
Antibody
- Drug Class
ALK
- Dose
0.6 mg/kg
- Route
Other
- Schedule of Administration
Subcutaneously every 3 weeks
- Drug 2
- Generic/Working Name
Sorafenib
- Trade Name
Nexavar
- Company Name
Bayer
- Drug Type
Biological
- Drug Class
Tyrosine kinase inhibitor
- Dose
400 mg per flat dose
- Route
p.o.
- Schedule of Administration
Daily
Dose‐Escalation Table
Patient Characteristics for Phase I Control
- Number of Patients, Male
14
- Number of Patients, Female
7
- Stage
IV
- Age
Median: 64
- Number of Prior Systemic Therapies
0
- Performance Status: ECOG
-
0 — 9
1 — 12
2 —
3 —
Unknown —
Primary Assessment Method
- Number of Patients Screened
45
- Number of Patients Enrolled
21
- Number of Patients Evaluable for Toxicity
21
- Number of Patients Evaluated for Efficacy
18
- Evaluation Method
RECIST 1.1
- Response Assessment CR
n = 0 (0%)
- Response Assessment PR
n = 0 (0%)
- Response Assessment SD
n = 10 (56%)
- Response Assessment PD
n = 8 (44%)4
- (Median) Duration Assessments OS
1.9–23.3 months
Serious Adverse Events
A serious adverse event was defined as an adverse event regardless of causality that resulted in death, was life threatening, required inpatient hospitalization or prolongation of hospitalization, resulted in persistent or significant disability or incapacity, was a congenital anomaly or birth defect, or was an important medical event that may jeopardize the patient and require medical or surgical intervention. Patients with multiple unique events were counted once per each unique event.
Dose‐Limiting Toxicities for Phase I Control
Assessment, Analysis, and Discussion
- Completion
Study completed
- Investigator's Assessment
Level of activity did not meet planned endpoint
Activin receptor‐like kinase 1 (ALK1) is a type I receptor of the transforming growth factor beta superfamily that is selectively expressed on the surface of activated endothelial cells [9], [10]. When activated by ligands bone morphogenetic protein (BMP) 9 and BMP10, ALK1 signals via phosphorylation [9], [11] of the Smad 1/5/8 to activate genes involved in vascular morphogenesis [10]. ALK1/BMP9 signaling promotes vascular stabilization and maturation, which are downstream from the proliferative stages of angiogenesis that are driven primarily by vascular endothelial growth factor (VEGF) [11].
Dalantercept is a soluble ALK1 receptor fusion protein that acts as a ligand trap by binding BMP9 and 10, inhibiting signaling through the ALK1 receptor. This disrupts the formation of mature blood vessels through a mechanism that is distinct from the VEGF pathway and impairs basic fibroblast growth factor and VEGF‐A‐stimulated angiogenesis both in vivo and in vitro [11], [12]. In preclinical models, dalantercept displayed potent antitumor activity accompanied by decreased tumor vascularity [12], [13], [14], [15]. In a phase I study of dalantercept in 37 patients with advanced solid tumors, dalantercept monotherapy demonstrated antitumor activity. One patient with squamous cell carcinoma of the head and neck had a partial response, and eight patients had prolonged stable disease [7]. Taken together, these results suggest that dalantercept may be effective in hepatocellular carcinoma (HCC).
ALK1 has been detected in the vasculature of many human tumor types, including HCC. BMP9 is overexpressed in HCC compared with normal hepatocytes and is a proliferative and survival factor in HepG2 HCC cells [16], [17]. A dalantercept analog (ALK1‐Fc) reduced proliferation rates in Huh7, Hep3B, and HepG2 cell lines [17]. In the BEL‐7402 preclinical model of HCC, a cell line derived from a primary human tumor from a patient with no prior chemotherapy, dalantercept monotherapy (15 mg/kg three times weekly) completely inhibited tumor growth compared with vehicle. Combination therapy with dalantercept (10 mg/kg twice weekly) plus sorafenib (5–15 mg/kg once daily [QD]) resulted in additive tumor growth inhibition [3]. The processes involved in vascular maturation include vessel stabilization via incorporation of pericytes and other stromal cells, which are commonly downstream of the proliferative stage processes driven by VEGF and other proangiogenic factors. Furthermore, ALK1 expression is elevated in neovascular endothelium during tumor growth, in contrast to the VEGF/VEGF receptor axis, which is constitutively expressed in new and established blood vessels and in other tissues [18]. In addition, the BMP9/BMP10/ALK1 pathway regulates development of lymphatic vessels [19], which has implications for metastatic spread of tumor cells through lymphatic vasculature [20]. Preclinical and early clinical studies suggest that dalantercept in combination with VEGF pathway inhibitors may maximize growth inhibition in tumors that are sensitive to antiangiogenic agents [3], [4]. Further, the safety profile of dalantercept is distinct from that of VEGF tyrosine kinase inhibitors (TKIs), which include fatigue, weight loss, rash/desquamation, hand‐foot skin reaction, alopecia, diarrhea, anorexia, nausea, and abdominal pain [8]. The most common toxicities with dalantercept include fatigue, peripheral edema, and anemia [7].
Thus, targeting the ALK1 and VEGF pathways simultaneously by combining dalantercept with sorafenib may result in more effective angiogenic blockade and delay tumor progression in patients with HCC.
This study aims to evaluate the safety and tolerability of dalantercept plus sorafenib and to determine the optimal dose of dalantercept in this combination to be studied in phase II trials.
Although dose levels of dalantercept ranging from 0.6 to 1.6 mg/kg were generally well tolerated in other clinical studies [5], [6], [7], including 0.9 mg/kg in combination with the TKI axitinib [2], in this study, a dose‐limiting toxicity (DLT), grade 4 hyponatremia, occurred at 0.6 mg/kg dose.
Although this DLT was not judged to be related to dalantercept, the Safety Review Team recommended de‐escalation of dalantercept from a dose level of 0.6 mg/kg to a dose level of 0.4 mg/kg because of the incidence and severity of volume‐related events, including peripheral edema (40%) and increased weight (60%), at the 0.6 mg/kg dose.
No DLTs or AEs higher than grade 3 occurred in the 0.4 mg/kg dose escalation cohort, leading to the determination of 0.4 mg/kg dose level as the maximum tolerated dose. Thus, the expansion cohort was enrolled at this dose level for a total of 16 patients at the 0.4 mg/kg dose level.
The combination of 0.4 mg/kg dalantercept every 3 weeks plus 400 mg sorafenib QD was generally well tolerated, with a safety profile similar to that reported in other clinical studies [4], [5], [6]; there were no events higher than grade 3 and no study treatment discontinuation due to adverse events.
However, tumor response at the 0.4 mg/kg dose level was poor; overall survival ranged from 1.9 to 23.3 months, and no patient achieved a complete or partial response.
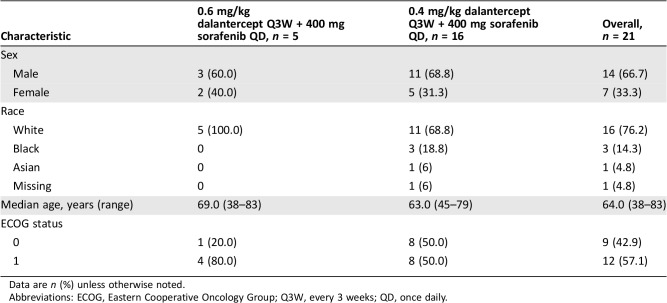
Table
Table 1. Demographic and baseline characteristics.
Data are n (%) unless otherwise noted.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; Q3W, every 3 weeks; QD, once daily.
Footnotes
ClinicalTrials.gov Identifier: NCT02024087
Sponsor(s): Acceleron Pharma
Principal Investigator: Ghassan K. Abou‐Alfa
IRB Approved: Yes
Disclosures
Ghassan K. Abou‐Alfa: Bayer (C/A), Acceleron (RF); Rebecca A. Miksad: Flatiron Health (E), Ipsen (C/A); Stephen Williamson: Acceleron (RF); Olugbenga O. Olowokure: Bayer, Celgene, Bristol‐Meyers Squibb (C/A, H); Matthew L. Sherman: Acceleron Pharma (E, IP, C/A, OI); Shuchi S. Pandya: Acceleron (OI). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Townson S, Martinez‐Hackert E, Greppi C et al. Specificity and structure of a high affinity activin receptor‐like kinase 1 (ALK1) signaling complex. J Biol Chem 2012;287:27313–27325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown MA, Zhao Q, Baker KA et al. Crystal structure of BMP‐9 and functional interactions with pro‐region and receptors. J Biol Chem 2005;280:25111–25118. [DOI] [PubMed] [Google Scholar]
- 3.Wang X, Solban N, Khanna P et al. Inhibition of ALK1 signaling with dalantercept combined with VEGFR TKI leads to tumor stasis in renal cell carcinoma. Oncotarget 2016;7:41857–41869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voss M, Bhatt R, Plimack E et al. The DART study: Results from the dose‐escalation and expansion cohorts evaluating the combination of dalantercept plus axitinib in advanced renal cell carcinoma. Clin Cancer Res 2017;23:3557–3565. [DOI] [PubMed] [Google Scholar]
- 5.Jimeno A, Posner MR, Wirth LJ et al. A phase 2 study of dalantercept, an activin receptor‐like kinase‐1 ligand trap, in patients with recurrent or metastatic squamous cell carcinoma of the head and neck. Cancer 2016;122:3641–3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makker V, Filiaci VL, Chem LM et al. Phase II evaluation of dalantercept, a soluble recombinant activin receptor‐like kinase 1 (ALK1) receptor fusion protein, for the treatment of recurrent or persistent endometrial cancer: An NRG oncology/gynecologic oncology group study 0229N. Gynecol Oncol 2015;138:24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bendell J, Gordon M, Hurwitz H et al. Safety, pharmacokinetics, pharmacodynamics, and antitumor activity of dalantercept, an activin receptor‐like kinase‐1 ligand trap, in patients with advanced cancer. Clin Cancer Res 2014;20:480–489. [DOI] [PubMed] [Google Scholar]
- 8.Llovet JM, Ricci S, Mazzaferro V et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378–390. [DOI] [PubMed] [Google Scholar]
- 9.Seki T, Yun J, Oh SP. Arterial endothelium‐specific activin receptor‐like kinase 1 expression suggests its role in arterialization and vascular remodeling. Circ Res 2003;93:682–689. [DOI] [PubMed] [Google Scholar]
- 10.Shi Y, Massague J. Mechanisms of TGF‐beta signaling from cell membrane to the nucleus. Cell 2003;113:685–700. [DOI] [PubMed] [Google Scholar]
- 11.Oh S, Seki T, Goss K et al. Activin receptor‐like kinase 1 modulates transforming growth factor‐beta 1 signaling in the regulation of angiogenesis. Proc Natl Acad Sci U S A 2000;97:2626–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell D, Pobre E, Mullvor A et al. ALK1‐Fc inhibits multiple mediators of angiogenesis and suppresses tumor growth. Mol Cancer Ther 2010;9:379–388. [DOI] [PubMed] [Google Scholar]
- 13.Hu‐Lowe D, Chen E, Zhang L et al. Targeting activin receptor‐like kinase 1 inhibits angiogenesis and tumorigenesis through a mechanism of action complementary to anti‐VEGF therapies. Cancer Res 2011;71:1362–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cunha SI, Pardali E, Thorikay M et al. Genetic and pharmacological targeting of activin receptor‐like kinase 1 impairs tumor growth and angiogenesis. J Exp Med 2010;207:85–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cunha S, Pietras K. ALK1 as an emerging target for antiangiogenic therapy of cancer. Blood 2011;117:6999–7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The Human Protein Atlas . ACVRL1. Available at http://www.proteinatlas.org/ENSG00000139567‐ACVRL1/cancer. Accessed January 25, 2018.
- 17.Li Q, Gu X, Weng H et al. Bone morphogenetic protein‐9 induces epithelial to mesenchymal transition in hepatocellular carcinoma cells. Cancer Sci 2013;104:398–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roodhart JM, Langenberg MH, Witteveen E et al. The molecular basis of class side effects due to treatment with inhibitors of the VEGF/VEGFR pathway. Curr Clin Pharmacol 2008;3:132–143. [DOI] [PubMed] [Google Scholar]
- 19.Niessen K, Zhang G, Ridgway JB et al. ALK1 signaling regulates early postnatal lymphatic vessel development. Blood 2010:115:1654–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duong T, Koopman P, Francois M. Tumor lymphangiogenesis as a potential therapeutic target. J Oncol 2012;2012:204946–204969. [DOI] [PMC free article] [PubMed] [Google Scholar]