Abstract
Objective:
The aim of this study was to perform a systematic review and meta-analysis to assess the accuracy of different surgical axillary staging procedures compared with ALND.
Summary of Background Data:
Optimal axillary staging after neoadjuvant systemic therapy (NST) in node-positive breast cancer is an area of controversy. Several less invasive procedures, such as sentinel lymph node biopsy (SLNB), marking axillary lymph node with radioactive iodine seed (MARI), and targeted axillary dissection (a combination of SLNB and a MARI-like procedure), have been proposed to replace the conventional axillary lymph node dissection (ALND) with its concomitant morbidity.
Methods:
PubMed and Embase were searched for studies comparing less invasive surgical axillary staging procedures to ALND to identify axillary burden after NST in patients with pathologically confirmed node-positive breast cancer (cN+). A meta-analysis was performed to compare identification rate (IFR), false-negative rate (FNR), and negative predictive value (NPV).
Results:
Of 1132 records, 20 unique studies with 2217 patients were included in quantitative analysis: 17 studies on SLNB, 1 study on MARI, and 2 studies on a combination procedure. Overall axillary pathologic complete response rate was 37%. For SLNB, pooled rates of IFR and FNR were 89% and 17%. NPV ranged from 57% to 86%. For MARI, IFR was 97%, FNR 7%, and NPV 83%. For the combination procedure, IFR was 100%, FNR ranged from 2% to 4%, and NPV from 92% to 97%.
Conclusion:
Axillary staging by a combination procedure consisting of SLNB with excision of a pre-NST marked positive lymph node appears to be most accurate for axillary staging after NST. More evidence from prospective multicenter trials is needed to confirm this.
Keywords: axillary staging, breast cancer, iodine seed, neoadjuvant systemic therapy, node-positive, sentinel lymph node biopsy
De-escalation of axillary surgery in clinically node positive (cN+) breast cancer patients is a topic of debate. The significant number of patients with axillary pathologic complete response (ax-pCR) resulting from increased use of neoadjuvant systemic therapy (NST) urges the need for a less invasive procedure to replace the conventional axillary lymph node dissection (ALND) in order to diminish unnecessary morbidity in patients with ax-pCR.1–3 Seeking for a less invasive procedure, both prevention of unnecessary morbidity and preservation of oncologic safety are of utmost importance.
Until recently, ALND was routinely performed after NST in cN+ patients, irrespective of axillary response to NST. Recent surveys among members of the American Society of Breast Surgeons as well as Dutch surgeons reported changes in axillary surgery in cN+ patients treated with NST.4,5 The majority of specialists were willing to replace ALND by a less invasive staging procedure in patients with a favorable treatment response. A wide variety of less invasive staging procedures such as sentinel lymph node biopsy (SLNB), removal of a marked pathologically-proven positive lymph node (MARI and MARI-like procedures), or a combination of these two procedures (eg, targeted axillary dissection) are incorporated in clinical practice according to local preferences.6–9 These results denote the lack of consensus on the preferred procedure for axillary staging after NST in pretreatment cN+ patients. Up to now, it is not clear which procedure is optimal.
The aim of this review is to provide an overview of different less invasive procedures for axillary staging after NST in pretreatment cN+ patients, which are currently in use. By evaluating the accuracy of different less invasive axillary staging procedures, we aim to determine the optimal procedure for axillary staging after NST in cN+ breast cancer to safely replace ALND.
METHODS
Criteria for Considering Studies for This Review
This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for diagnostic test accuracy.10 A systematic literature search was performed by the first author (JM) for randomized controlled trials, cohort studies, and case-control studies testing less invasive axillary staging procedures after NST in cN+ breast cancer patients treated with NST. Studies were only included if nodal positivity was pathologically confirmed before starting with NST. Any study in which a less invasive axillary staging procedure was compared with the gold standard, that is, ALND, was included. In case completion ALND (cALND) was not performed routinely, studies were not considered for quantitative analysis. If relevant studies included both cN0 and cN+ patients, only the cN+ patients were considered for analysis. In case only part of the study population consisted of patients with pathologic confirmed nodal positivity, only the pathologically confirmed cN+ patients were considered for analysis. When it was not possible to discriminate between cN0 and cN+ patients or between pathologically confirmed and non-pathologically confirmed cN+ patients, studies were excluded. Reviews, case reports, conference abstracts, and editorials were excluded. In case of inclusion of the same study population in 2 or more papers, the most extensive paper was included. Studies reporting small study populations (10 patients or less) and studies in which nodal positivity was confirmed by SLNB before NST were excluded. The primary outcome was the overall ax-pCR rate and the accuracy of the studied less invasive axillary staging procedure. Studies were therefore excluded if reported data did not allow construction of a 2 x 2 contingency table with absolute numbers of true positive (TP), true negative (TN), false-positive (FP), and false-negative (FN) test results. FP is always 0, as the index test and reference test are considered the same in case of a positive index test result (ie, presence of residual axillary disease). The secondary outcome was the identification rate (IFR) of the studied less invasive axillary staging procedure.
Search Methods for Identification of Studies
The following electronic databases were searched until April 20, 2018, with no restriction on language or date of publication: Medline (via PubMed) and EMBASE (via EMBASE.com).
A health sciences librarian was consulted to help develop a detailed search strategy. Details of the full search strategies in both databases are provided in Appendix 1. The reference lists of included studies and existing reviews were manually checked for additional relevant studies.
Selection of Studies and Quality Assessment
Duplicate references were identified and removed with Endnote. Titles and abstracts of all remaining references were scanned independently by 2 authors (JS and TvN). Subsequently, these 2 authors independently assessed the full text papers of all potentially eligible studies. Disagreement was resolved by mutual consensus.
Eligible studies were assessed for quality using the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS- 2) questionnaire.11 The QUADAS-2 was tailored to our analysis, as described in the guideline. Finally, all included studies were evaluated for quality by the 2 independent authors.
Data Extraction and Analysis
For each included study, the following parameters were extracted: first author, year of publication, type of hospital, study design, sample size, characteristics of trial participants (including primary tumor type, TNM-stage, type of evaluation of axillary involvement, and NST regimens), type of less invasive axillary staging procedure after NST and characteristics of the procedure, type of pathological assessment of lymph nodes (including use of immunohistochemistry (IHC), definition of ax-pCR, ax-pCR rate, accuracy, and IFR of the less invasive axillary staging procedure.
For each less invasive axillary staging procedure, the ax-pCR rate, IFR, false-negative rate (FNR), and negative predictive value (NPV) were calculated. Rate of ax-pCR was based on data of the contingency tables. The IFR was defined as the number of successful procedures divided by the total number of patients in whom the procedure was attempted. The FNR was defined as the number of FN divided by the total number of patients with presence of residual axillary disease [FN / (FN + TP)]. The NPV was defined as the number of TN divided by the total number of patients with a negative test result [TN / (TN + FN)]. As FP cannot occur, numbers of TP, TN, and FN were documented for each procedure and FP was always documented to be 0 (in case FP was reported to be > 0 in the record, this number was added up to the TP number). Statistical analysis was performed with Stata/SE Statistical Software for Windows, version 14.2 (StataCorp LP, College Station, TX). To calculate pooled proportions for ax-pCR rate, IFR and FNR random-effects models for meta-analysis were used with 95% exact confidence intervals (CIs) with help of the metaprop command.12 All considered outcomes are presented in forest plots including pooled estimates. Chi-squared test was performed to test for statistical heterogeneity and was quantified by I2-index.13 As recommended in the Cochrane Handbook for Diagnostic Test Accuracy Reviews,14 reporting bias (eg, publication bias) was not assessed.
Subgroup analyses were performed to evaluate the impact of several factors on FNR. Factors that were considered relevant were number of examined lymph nodes, sampling method for SLNB, ycN status, definition of ax-pCR, and use of IHC in addition to standard H&E evaluation. Statistical significance was considered as P values (2-sided) ≤ 0.05.
RESULTS
Study Selection
In total, 1920 records were identified through database searching and reference checking. After deduplication, 1132 records were screened, which resulted in the selection of 116 records for retrieval of full texts. Assessment of full text for eligibility yielded 27 records for qualitative synthesis; a total of 20 records were included in quantitative synthesis. See Fig. 1 for a flow chart depicting the study selection process.
FIGURE 1.

Flow diagram depicting the study selection process.
Study Characteristics
Index Tests
Three different axillary staging procedures were identified: SLNB, excision of a pretreatment marked biopsy-proven positive lymph node (hereinafter all such procedures are referred to as ML), and a combination procedure involving both SLNB and ML. A total of 2217 patients were included (2002 for SLNB, 95 for ML, 120 for the combination procedure) in whom the axillary staging procedure was successful and followed by cALND. See Table 1 for general characteristics of all studies included for qualitative analysis.
TABLE 1.
General Characteristics of All Studies Included in Qualitative Analysis Sorted by Type of Procedure
| First Author | Year of Publication | Study Type | Index Test | Reference Test | Sample Size* | cN-Stage | ycN-Stage | Definition ax-pCR | IHC |
| Alvarado | 2012 | R, S | SLNB | ALND | 121 | N1-N3 | Any | NR | NR |
| Boileau | 2015 | P, M | SLNB | ALND | 127 | N1-2 | Any | ypN0/itc+ | Yes, if H&E negative |
| Boughey | 2013 | P, M | SLNB | ALND | 637 | N1-2 | Any | ypN0/itc+ | No |
| Brown | 2010 | R, S | SLNB | ALND | 86 | N1-3 | Any | ypN0/itc+ | No |
| Carrera | 2016 | P, M | SLNB | ALND | 48 | N1-2 | ycN0 (MRI +/- US) | NR | Yes, always |
| Enokido | 2016 | P, M | SLNB | ALND | 130 | N1 | ycN0 (imaging) | ypN0 | NR |
| Ge | 2014 | P, S | SLNB | ALND | 43 | N1-3 | Any | NR | Yes, but not routinely |
| Kang | 2011 | R, S | SLNB | ALND | 58 | N1-3 | Any | NR | Yes, always |
| Kuehn | 2013 | P, M | SLNB | ALND | 123 | N1-2 | ycN0 (PE +/-US) | ypN0/itc+ | No |
| Ozmen | 2010 | R, S | SLNB | ALND | 71 | N1-2 | ycN0 (PE and imaging) | ypN0 | Yes, if H&E negative |
| Park | 2013 | R, S | SLNB | ALND | 169 | N1-3 | Any | ypN0/itc+ | Yes, but not routinely |
| Pinero-Madrona | 2015 | P, M | SLNB | ALND | 38 | N1-3 | Any | NR | NR |
| Shen | 2007 | P, S | SLNB | ALND | 56 | N1-3 | Any | NR | No |
| Thomas | 2011 | P, S | SLNB | ALND | 26 | N+ | ycN0 (PE) | NR | Yes, always |
| Yagata | 2013 | P, S | SLNB | ALND | 81 | N1-3 | ycN0 (MRI; including rPR) | ypN0 | Yes, if H&E negative |
| Yu | 2016 | R, S | SLNB | ALND | 46 | N+ | ycN0 (PE) | ypN0/itc+/mi+ | Yes, always |
| Zetterlund | 2017 | P, M | SLNB | ALND | 152 | N1 | Any | ypN0 | Yes, but not routinely |
| Donker | 2015 | P, S | ML | ALND | 95 | N1-3 | Any | ypN0 | Yes, but not routinely |
| Caudle | 2016 | R, S | Combi | ALND | 85 | N1-3 | Any | ypN0 | Yes, but not routinely |
| Dashevsky | 2017 | R, S | Combi | NA | 21 | N1-2 | NR | ypN0/itc+ | No |
| Diego | 2016 | R, S | Combi | NA | 29 | N1 | ycN0 (PE) | ypN0 | Yes, but not routinely |
| Kim | 2017 | P, S | Combi | NA | 11 | N1-2 | Any | ypN0/itc+ | No |
| Nguyen | 2017 | R, S | Combi | NA | 20 | N1-3 | NR | NR | NR |
| Park | 2017 | P, S | Combi | NA | 20 | N1-3 | NR | ypN0/itc+ | Yes, but not routinely |
| Plecha | 2015 | R, S | Combi | NA | 19 | N1-3 | NR | NR | NR |
| Siso | 2017 | P, S | Combi | ALND | 35 | N1-3 | Any | ypN0 | Yes, always |
| Taback | 2018 | P, S | Combi | NA | 19 | N1-2 | NR | ypN0 | NR |
Combi indicates combination procedure; H&E, hematoxylin and eosin stain; IHC, immunohistochemistry; M, multicenter; NA, not applicable; NR, not reported; P, prospective; PE, psychical examination; R, retrospective; rPR, radiologic partial response; S, single-center.
*Number of patients in whom the less invasive axillary staging procedure was successful and in whom this procedure was followed by cALND (if applicable).
Reference Tests
In 20 studies, the axillary staging procedure was always followed by ALND as part of trial protocol. A total of 17 trials investigated accuracy of SLNB,15–31 1 trial investigated ML,8 and 2 trials investigated a combination procedure.6,32
Studies validating the combination procedure were scarce, yet several studies did report on cohorts of patients in whom a combination procedure was performed without routine cALND. Therefore, we decided to include these studies, 7 in total, in the qualitative analysis.7,33–38 See Table 2 for detailed characteristics of these studies. In this table, we have included results of a study by our own research group (manuscript submitted). As cALND was not routinely performed in these studies, they were excluded from quantitative analysis.
TABLE 2.
Characteristics of Studies Involving the Combination Procedure Without Routine ALND
| Author | Sample Size* | Pre-NST Marking at Time of FNA/CNB | Pre-NST Marking After FNA/CNB | Post-NST Marking | Sampling SLNB | IFR†, % | ML is SLN, % | Confirmation Removal ML | Lymph Nodes‡, Median (Range) | ALND, % | Ax-pCR, % |
| Dashevsky | 21 | Clip | NA | Wire | Tc + blue | 100.0 | NR | XR | NR | 0.0 | 33.3 |
| Diego | 29 | Clip | NA | Iodine seed | Tc + blue | 100.0 | 91.0 | XR | 4 (1–11) | 23.3 | 63.0 |
| Kim | 11 | Clip | NA | Wire | Tc + blue | 100.0 | NR | XR/palpation | NR | 45.5 | 36.4 |
| Nguyen | 20 | NA | Clip | Iodine seed | Tc and/or blue | 100.0 | NR | XR | NR | NR | NR |
| Park | 20 | NA | Charcoal | NA | Tc and/or blue | 100.0 | 75.0 | NA | 3 (1–12) | 60.0 | 50.0 |
| Plecha | 19 | Clip | NA | Wire | Tc ± blue | 100.0 | 100.0 | XR/PA | 5.7 (mean) | NR | NR |
| Simons (data submitted) | 139 | NA | Clip/Iodine Seed | Iodine seed/wire | Tc and/or blue | 99.3 | 64.6 | XR/PA | 2 (1–9) | 22.3 | 36.0 |
| Taback | 19 | Clip (78.9%) | Electromagnetic Reflector | NA | Tc + blue | 100.0 | 63.2 | XR | 4 (2–10) | 31.6 | 31.6 |
NA indicates not applicable; NR, not reported (a pathologic assessment); Tc, technetium; XR, specimen radiography.
*Patients who underwent successful ML in combination with SLNB.
†IFR refers to proportion of patients in whom at least 1 lymph node could be identified with the combination procedure.
‡Number of lymph nodes of the combination procedure and not the number of lymph nodes of either ML or either SLNB.
Risk of Bias and Applicability
Figure 2 shows the methodological quality of all included studies. In general, studies included in the quantitative analysis showed a lower risk of bias than studies included only in the qualitative analysis.
FIGURE 2.
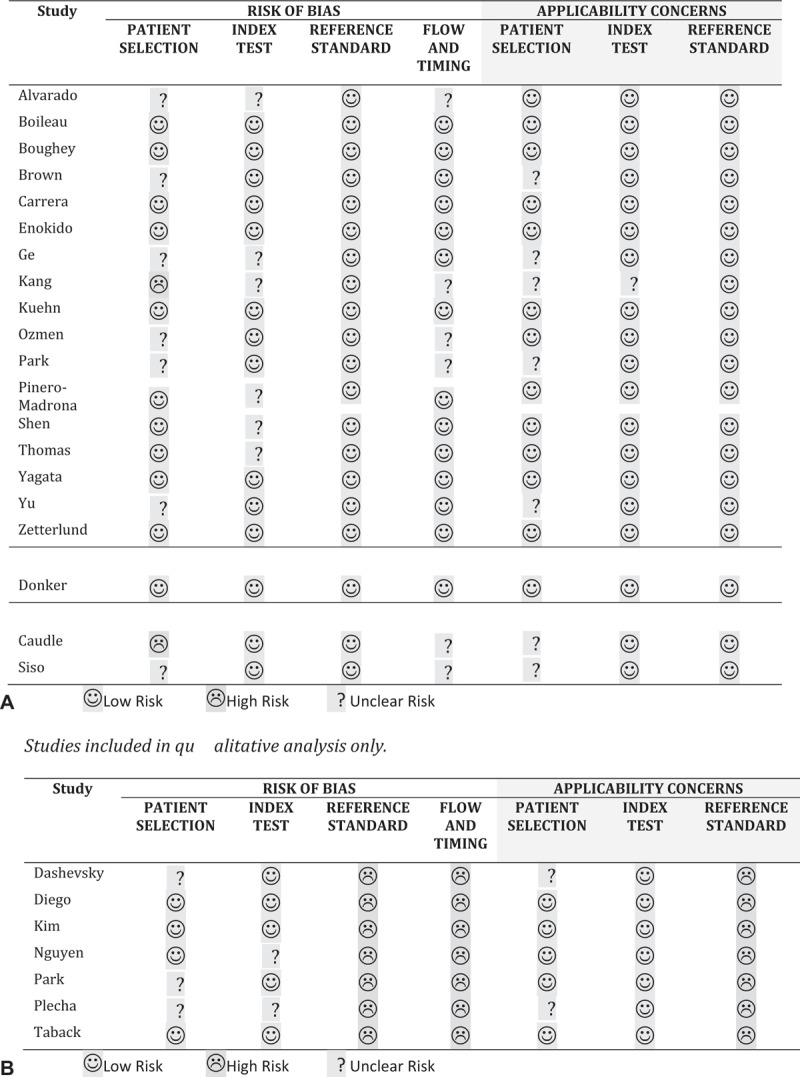
Assessment of risk of bias.
Results of Individual Studies Included in Quantitative Analysis
Pooled Prevalence of ax-pCR
The overall prevalence of ax-pCR in all 20 included studies was 37% (see Appendix 2). The I2-statistic was 57.08% (P < 0.01). Test for heterogeneity between subgroups based on staging procedure was not significant, supporting the pooling of all studies in 1 overall rate.
SLNB
The IFR of SLNB was available for 16 of 17 studies. The overall IFR was 89% in a pooled sample of 2154 patients (see Appendix 3). For all studies, data to calculate FNR and NPV were available for a total of 2002 patients. An overall FNR of 17% was found (see Fig. 3) and NPV ranged from 57% to 86% (see Table 3 SLNB). The I2 statistic revealed values of variation due to heterogeneity of 68.3% for IFR and 38.7% for FNR (P < 0.01 and P = 0.05, respectively).
FIGURE 3.
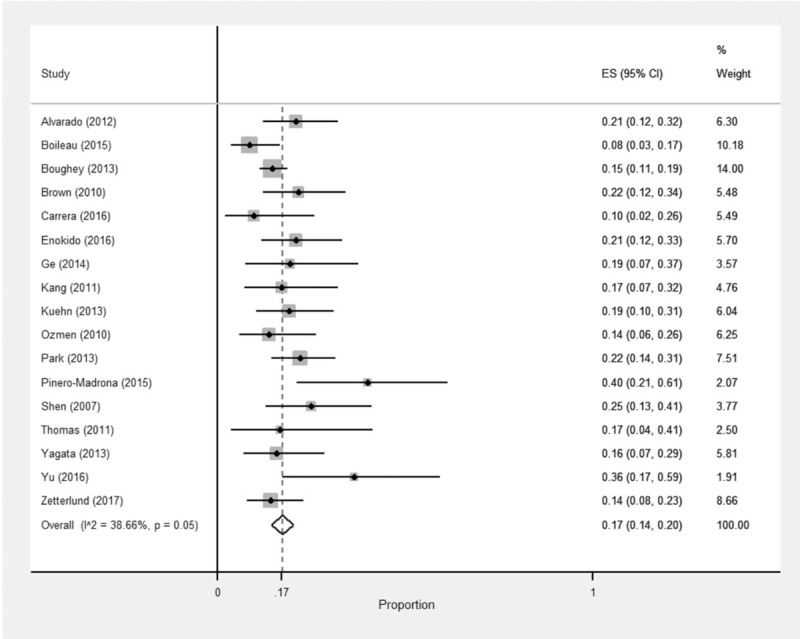
Forest plot for the FNR of SLNB.
TABLE 3.
Overview and Diagnostic Accuracy Sorted by Type of Procedure
| SLNB | ||||||||||
| Author | Identification Rate | Sampling | SLNs, Median (range) | Ax-pCR %* | TP | FP | FN | TN | FNR % (CI) | NPV % (CI) |
| Alvarado | 92.7 | Tc and/or blue | 2 (1–7) | 35 | 57 | 0 | 15 | 39 | 21 (12–32) | 72 (58–84) |
| Boileau | 87.6 | Tc and/or blue | 2.7 (mean) | 35 | 76 | 0 | 7 | 44 | 8 (3–17) | 86 (74–94) |
| Boughey | 92.7 | Tc and/or blue | NR | 40.0 | 326 | 0 | 56 | 255 | 15 (11–19) | 82 (77–86) |
| Brown | NR | Tc and/or blue | 2 (1–10) | 30.2 | 47 | 0 | 13 | 26 | 22 (12–34) | 67 (50–81) |
| Carrera | 90.5 | Single radioactive | 2.2 (mean) (1–6) | 35.4 | 28 | 0 | 3 | 17 | 10 (2–26) | 85 (62–97) |
| Enokido | 90.9 | Tc and/or blue | 1.6 (mean) | 52 | 49 | 0 | 13 | 68 | 21 (12–33) | 84 (74–91) |
| Ge | 84.3 | Tc and/or blue | 2.4 (1–7) | 27.9 | 25 | 0 | 6 | 12 | 19 (7–37) | 67 (41–87) |
| Kang | 87.9 | Tc and/or blue | 2.8 (mean) (1–8) | 28.8 | 34 | 0 | 7 | 17 | 17 (7–32) | 71 (49–87) |
| Kuehn | 82.6 | Tc ± blue | NR | 48.8 | 51 | 0 | 12 | 60 | 19 (10–31) | 83 (73–91) |
| Ozmen | 92.2 | Tc + blue | 2.1 (1–5) | 28 | 44 | 0 | 7 | 20 | 14 (6–26) | 74 (54–89) |
| Park | 94.9 | Single, radioactive | 2,1 (mean) (1–12) | 40.8 | 78 | 0 | 22 | 69 | 22 (14–31) | 76 (66–84) |
| Pinero-Madrona | 84.0 | Tc ± blue | NR | 34.2 | 15 | 0 | 10 | 13 | 40 (21–61) | 57 (34–77) |
| Shen | 92.8 | Tc and/or blue | 2 (1–10) | 28.6 | 30 | 0 | 10 | 16 | 25 (13–41) | 62 (41–80) |
| Thomas | 86.7 | Single blue | 1,57 (mean) (1–4) | 31 | 15 | 0 | 3 | 8 | 17 (4–41) | 73 (39–94) |
| Yagata | 85.3 | Tc + blue | 2 (1–7) | 37 | 43 | 0 | 8 | 30 | 16 (7–29) | 79 (63–90) |
| Yu | 95.8 | Single: Blue | 1,48 (mean) (1–4) | 52.2 | 14 | 0 | 8 | 24 | 36 (17–59) | 75 (57–89) |
| Zetterlund | 77.9 | Tc and/or Blue | 2 (1–5) | 39.5 | 79 | 0 | 13 | 60 | 14 (8–23) | 82 (71–90) |
| ML | |||||||||||
| Author | IFR % | Pre-NST Marker | Post-NST Marker | N Lymph Nodes | Ax-pCR %* | TP | FP | FN | TN | FNR % (CI) | NPV % (CI) |
| Donker | 97 | Iodine seed | NA | 1 | 26 | 65 | 0 | 5 | 25 | 7 (2 – 16) | 83 (65–94) |
| Combination | |||||||||||||
| Author | IFR % | Sampling SLNB | Pre-NST Marker | Post-NST Marker | N Lymph Nodes | Ax-pCR %* | ML is SLN % | TP | FP | FN | TN | FNR% (CI) | NPV% (CI) |
| Caudle | 100% | Tc and/or blue | Clip | Iodine seed | NR | 41 | 77† | 49 | 0 | 1 | 35 | 2 (0–11) | 97 (85–1) |
| Siso | NR | Tc and/or blue | Clip | NA* | 3 median | 31.7 | 77 | 23 | 0 | 1 | 11 | 4 (0–21) | 92 (62–1) |
NA indicates not applicable; NR, not reported; Tc, technetium.
*Ax-pCR rate based on data of 2 × 2 contingency tables.
†Rate is based on 134 patients with a clipped node that underwent SLNB (it was documented if the clipped node was identified as an SLN). Eighty-five patients actually underwent TAD followed by cALND.
Ten studies documented the definition of ax-pCR: overall FNR was 16% when ax-pCR was defined as ypN0 and 17% when ax-PCR was defined as ypN0/itc+ (P = 0.61, 1 study defined ax-pCR as ypN0/itc+/mi+). FNR was reported for single versus dual-tracer sampling separately in 5 studies and another 6 studies used either single-tracer or dual-tracer sampling in 100% of patients. Pooled FNR was 13% for dual-tracer sampling and 16% for single-tracer sampling (P = 0.53). A total of 14 studies reported on the use of IHC: overall FNR was 15% when IHC was used (either always or in selected patients) versus 17% when IHC was not used (P = 0.47). In 6 studies, FNR was reported separately in relation to the number of SLN(s): all 6 studies reported FNR for excision of 3 or more SLNs (NB: in 1 study, this was 2 or more SLNs) and 5 of 6 studies also reported FNR for excision of <3 SLNs. Overall FNR was 8% with removal of at least 3 SLNs and 22% with removal of < 3 SLN(s) (P < 0.0001). In 7 studies, only cN+ patients with ycN0 status were included. In addition to these studies, another 4 studies reported on FNR separately for patients with any ycN status versus ycN0 status. Overall FNR was 14% when only patients with ycN0 status were taken into account versus 18% when patients irrespective of ycN status were taken into account (P = 0.14).
ML
One study reported on a ML procedure: this study involved the validation of the MARI procedure (marking axillary lymph nodes with radioactive iodine seeds) in 95 patients. In this study, the pathologically proven positive lymph node was marked with an I-125 seed pre-NST. After completion of NST, at the time of surgery, the lymph node with the Iodine seed was removed. The IFR was 97%, the FNR 7%, and the NPV 83.3%. See Table 3 (ML) for characteristics of this procedure.
Combination Procedure
Two studies investigated a combination procedure: one involved clipping of the positive lymph node pre-NST later followed by I-125 seed localization of the clipped node post-NST in combination with SLNB and one involved clipping of the positive lymph node pre-NST followed by US-guided excision of the clipped-node in combination with SLNB. Table 3 (Combination) shows values for FNR and NPV for the 2 combination procedures. As only 2 studies were available for analysis, pooling of proportions was not performed. The studies appeared clinically similar, as they targeted the same population in terms of inclusion criteria, definition of ax-pCR, and use of IHC.
DISCUSSION
In this systematic review, the accuracy of 3 different procedures for axillary staging after NST in cN+ patients was evaluated. This is the first review up to now that compared all these different less invasive staging procedures with the gold standard ALND. The goal was to provide an overview of currently available procedures in order to guide decision making regarding replacing ALND in selected cN+ patients.
The SLNB for axillary staging after NST in cN+ patients has been extensively studied over the past years. The SLNB procedure is widely accepted as axillary staging procedure in cN0 patients. Even when performed after NST, the accuracy of SLNB for cN0 patients is accepted.39 SLNB for axillary staging after NST in pre-treatment cN+ patients, however, is associated with unacceptably high rates of FNR. In 2015, the accuracy of SLNB in cN+ patients after NST was evaluated in a systematic review that included 8 studies with pathologically proven cN+ patients.40 That review reported an overall FNR of 15% and the NPV of SLNB did not exceed 86%.40 In the current meta-analysis, a total of 17 studies with 2002 patients (the 8 studies of the previous review were also included) were analyzed. The overall FNR is 17% and the NPV still does not exceed 86%: in case SLNB predicts ax-PCR, residual axillary disease is actually missed in at least 1 in 6 patients. The overall IFR is 89%. Previous studies reported multiple factors that may improve IFR and accuracy of SLNB, for example, using dual-tracer sampling technique, evaluating the SLNs with IHC in addition to standard H&E evaluation and removing 3 or more SLNs. As IHC and single- versus dual-tracer sampling was not used consistently within and between studies, it is not possible to draw definite conclusions from this review on whether/or not specific sampling and pathologic evaluation methods should be promoted. Our results did show that FNR was favorable (yet not statistically significant) for both dual-tracer sampling and pathologic evaluation with IHC. FNR was also favorable for patients with ycN0 status based on physical examination and/or imaging compared with any ycN status (FNR of 14% vs 18%, P = 0.14). Regarding the number of SLNs, removing ≥ 3 lymph nodes was associated with a significantly better FNR in our meta-analysis (8% vs 22%, P < 0.0001). However, removing ≥ 3 SLNs is not achievable in a significant number of patients17 and whether this will be achieved is unpredictable preoperatively. This renders SLNB impractical, as random node-picking should be discouraged. Currently recruiting studies as Alliance 11202 and NSABP-51/RTOG 1304 will determine whether the SLNB, despite its rather poor overall accuracy and shortcomings, can have a place in axillary staging after NST in cN+ patients.41,42
The MARI procedure was the first ML procedure to be proposed as an alternative to SLNB for axillary staging after NST in cN+ patients.8 By marking the pathologically proven positive lymph node before start of NST, it was expected to enable accurate assessment of treatment response after completion of NST. The MARI procedure was validated in 1 single-center trial with 95 patients. This study reported an improved FNR (7%), but the NPV of 83.3% was less favorable, that is, in 1 of 6 patients with a negative MARI, axillary residual disease is left behind. Therefore, equally to SLNB, MARI as a stand-alone procedure is insufficiently accurate to safely replace ALND. Despite the shortcomings of MARI (potential of missing residual axillary disease and limited evidence by 1 single—dedicated breast cancer—center trial to support this procedure), it is already implemented in clinical practice. A recent publication suggests combining information on axillary burden on pre-NST PET-CT (ie, number of suspicious lymph nodes: <4 vs ≥ 4) with MARI outcome9 to determine adjuvant axillary treatment: no further axillary treatment, axillary radiotherapy, or cALND with axillary radiotherapy. Results of 1 prospective implementation study showed that this treatment strategy indeed results in a major reduction of ALND.43 Data of longer follow-up have to determine whether implementation of this protocol is untimely and whether it does not only reduce morbidity but also preserves oncologic safety in terms of disease-free and overall survival. Prospective trials with sufficient follow-up are therefore urgently needed.
In the Z1071 trial, a clip was placed in the positive lymph node before NST in a subset of patients.44 The trial protocol did not require surgeons to selectively target and remove the clipped node at time of surgery, but did encourage surgeons and pathologists to document whether the clipped node was located in the SLNB or ALND specimen. In 141 of 170 patients with a clipped node, the location of the clipped node was documented: 75.9% in the SLNB specimen and 24.1% in the ALND specimen. This suggested that removing the clipped node together with SLN(s) at time of surgery may improve accuracy of SLNB and may possibly overcome shortcomings associated with SLNB or MARI if used as stand-alone procedures. Up to now, only 2 trials evaluated accuracy of such a combination procedure and were included in our meta-analysis.6,32 This procedure is associated with excellent IFRs. Caudle et al6 confirmed that the clipped node does not necessarily have to be a SLN, as this was the case in only 77%. Furthermore, FNR is low (2% to 4%) and NPV is high (92% to 97%). These results are promising: when ax-pCR is predicted, residual axillary disease is missed in 1 in 12 to 33 patients. The evidence for this procedure is yet limited with only 2 trials available (1 retrospective and 1 prospective study), involving small sample sizes and single-center study designs. The ongoing Dutch RISAS trial (NCT02800317 at https://clinicaltrials.gov) will prove whether the promising results of a combination procedure can be confirmed in a large, prospective, multicenter trial.45
Although evidence to support replacing ALND by less invasive procedures is limited, several reports have been recently published on implementation of such procedures, especially procedures involving excision of the ML and SLNs. A variety of methods are used to target the pathologically proven positive lymph node: marking with a clip pre-NST followed by placing an iodine seed or wire in the clipped node post-NST7,33–35,37 and primary marking with an iodine seed, clip, charcoal, or electromagnetic reflector.36,38 Also, the time of marking the lymph node pre-NST differs: either immediately at time of FNAC/CNB,7,33,34,37 at a second appointment once metastatic burden of the punctured lymph node is confirmed by the pathologist35,36 or even at both occasions.38 Currently, further research has to define which combination procedure is most accurate, patient-friendly, and cost-effective. Identification of the ML at time of surgery is highly feasible, provided that clipping (with/without secondary localization of the clip) of the node was successful. Success rates of this part of the procedure are often not sufficiently reported and may be improved to further optimize combination procedures.
The abovementioned 3 different staging procedures intend to offer a less invasive strategy compared with the conventional ALND, yet ≥ 10 lymph nodes are removed in some patients with SLNB and combination procedures. It is important to realize that these procedures serve as a staging procedure to identify ax-pCR and not as a managing procedure to remove all residual diseases. Hence, it should be the primary goal to remove as few lymph nodes as possible. In this way, patients with ax-pCR can truly benefit from less invasive staging procedures. At the same time, when these procedures identify axillary residual disease, adjuvant axillary treatment plans should consist of cALND. Results of the Alliance 11202 and NSABP-51/RTOG 1304 trials have to be awaited to determine whether cALND may be replaced by axillary radiation therapy.41,42
As this review is limited by the heterogeneity of included studies, results of the review should be interpreted with caution. The random effects model that was used for statistical analysis takes in account that, although similar interventions were studied, different populations were included. Factors such as definition of ax-PCR, sampling method for SLNB, and use of IHC for pathologic assessment of lymph nodes may all impact accuracy of the studied intervention. These factors differed widely among included studies and further research is necessary to determine, among others, what should be the preferred definition of ax-pCR. The prognostic impact of residual ITCs and micrometastasis may be different for patients treated in the neoadjuvant compared with adjuvant setting, as they might be therapy-resistant. A retrospective study of cN+ patients treated with NAC and always followed by ALND suggested that patients with residual ITCs and micrometastases carry a similar prognosis as patients with ypN0.46 These results have yet to be confirmed in trials where patients with ypN0 and ITCs or micrometastasis did not undergo ALND. In addition, the value of IHC has not yet been thoroughly studied, as most studies that used IHC in addition to standard H&E evaluation, did so randomly, and not in a routine matter. Contrary to improving accuracy of detecting residual axillary disease, a potential undesired result of IHC may be detection of residual disease that would have otherwise been left undetected (of which implications on prognosis and need for adjuvant treatment are unknown). The question whether IHC may not only result in improved accuracy but may also result in overtreatment is yet left unanswered.
In this review, we only included patients in whom cN+ status was pathologically proven before NST. This is particularly important to determine true accuracy of the different staging procedures. When patients who are expected to be cN+ based on physical examination or imaging only, the number of true negatives rises and the chance to have false negatives decreases. This may result in a false impression of improved rates of FNR and NPV.
In conclusion, the SLNB as well as ML procedures seem insufficiently accurate as stand-alone procedures for axillary staging after NST in cN+ patients. Accuracy of these procedures may improve by taken in account axillary burden on pre-NST and/or post-NST imaging. A combination procedure involving excision of the ML and SLNs appears most accurate for axillary staging and has the lowest risk of missing axillary residual disease when ax-pCR is predicted. More evidence from prospective multicenter trials is needed to confirm this.
Acknowledgments
We thank the following individuals for assistance with search strategies and statistical analysis: P.H. Wiersma and F.P. Weijdema, health science librarians, Utrecht University Library.
J.B. Reitsma, clinical epidemiologist, Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht.
R.J.P.M. Scholten, professor of clinical epidemiology, Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht.
Appendix 1
PubMed search strategy
(“Breast Neoplasms”[Mesh] OR ((carcinoma OR carcinomas OR tumor OR tumours OR tumour OR tumours OR neoplasm OR neoplasms OR malignancy OR adenocarcinoma OR cancer) AND (breast OR mamma))) AND (“Sentinel Lymph Node Biopsy”[Mesh] OR sentinel lymph node biopsy OR slnb OR (MARI[tiab]) OR (Axilla∗ AND staging[tiab]) OR targeted axillary dissection) AND (sensitivity OR specificity OR “Sensitivity and Specificity”[Mesh] OR “Predictive Value of Tests”[Mesh] OR negative predictive value OR positive predictive value OR likelihood ratio OR diagnosis OR “false-negative” OR “false-positive”) AND (“Lymph Nodes”[Mesh] OR “Lymphatic Vessels”[Mesh] OR “Lymphatic Metastasis”[Mesh] OR “Lymphatic System”[Mesh] OR “Axilla”[Mesh] OR axilla∗) AND ((“Neoadjuvant Therapy”[Mesh] OR neoadjuvant OR preoperative∗ OR primary) AND (“Antineoplastic Agents”[Mesh] OR chemotherapy OR immunotherapy OR systemic therapy))
EMBASE search strategy∗
∗Search results were restricted to articles, reviews, articles in press, conference paper, conference review
((breast OR mamma) AND (cancer OR carcinoma OR neoplasm OR malignancy OR adenocarcinoma OR ’breast cancer’/exp OR tumor OR carcinomas OR tumors OR tumour OR tumours)) AND (('sentinel lymph node biops∗’ OR slnb OR 'sentinel node∗’ OR 'sentinel lymph node biopsy’/exp OR ’mari’:ab,ti OR (axilla∗ AND staging) OR ’targeted axillary dissection’)) AND (sensitivity OR specificity OR ’predictive value’ OR ’likelihood ratio’ OR ppv OR npv OR diagnosis OR ’false negative’ OR ’false positive’) AND ((axilla∗ OR lymph∗) AND (node∗ OR metastasis)) AND ((neoadjuvant OR primary OR preoperative∗) AND (therapy OR immunotherapy OR chemotherapy OR systemic))
Appendix 2. Forest plot of the ax-pCR rate.

ES effect size. The pooled ax-pCR is 37% (33% to 40%).
Appendix 3. Forest plot for the identification rate of SLNB.

ES. effect size. The pooled identification rate of SLNB is 89% (87% to 92%).
Footnotes
J.S. received salary from Dutch Cancer Society (KWF Kankerbestrijding). This research did not receive any further specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The authors have no competing interests to declare.
REFERENCES
- 1.van Bommel AC, Spronk PE, Vrancken Peeters MT, et al. Clinical auditing as an instrument for quality improvement in breast cancer care in the Netherlands: the National NABON Breast Cancer Audit. J Surg Oncol 2017; 115:243–249. [DOI] [PubMed] [Google Scholar]
- 2.Vugts G, Maaskant-Braat AJ, Nieuwenhuijzen GA, et al. Patterns of care in the administration of neo-adjuvant chemotherapy for breast cancer. A population-based study. Breast J 2016; 22:316–321. [DOI] [PubMed] [Google Scholar]
- 3.DICA. Annual Report of the NABON Breast Cancer Audit 2016 - Dutch Institute for Clinical Auditing. Available at: https://dica.nl/jaarrapportage-2016/nbca Accessed May 1, 2018. [Google Scholar]
- 4.Caudle AS, Bedrosian I, Milton DR, et al. Use of sentinel lymph node dissection after neoadjuvant chemotherapy in patients with node-positive breast cancer at diagnosis: practice patterns of American Society of Breast Surgeons Members. Ann Surg Oncol 2017; 24:2925–2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vugts G, Maaskant-Braat AJ, de Roos WK, et al. Management of the axilla after neoadjuvant chemotherapy for clinically node positive breast cancer: a nationwide survey study in The Netherlands. Eur J Surg Oncol 2016; 42:956–964. [DOI] [PubMed] [Google Scholar]
- 6.Caudle AS, Yang WT, Krishnamurthy S, et al. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol 2016; 34:1072–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diego EJ, McAuliffe PF, Soran A, et al. Axillary staging after neoadjuvant chemotherapy for breast cancer: a pilot study combining sentinel lymph node biopsy with radioactive seed localization of pre-treatment positive axillary lymph nodes. Ann Surg Oncol 2016; 23:1549–1553. [DOI] [PubMed] [Google Scholar]
- 8.Donker M, Straver ME, Wesseling J, et al. Marking axillary lymph nodes with radioactive iodine seeds for axillary staging after neoadjuvant systemic treatment in breast cancer patients: the MARI procedure. Ann Surg 2015; 261:378–382. [DOI] [PubMed] [Google Scholar]
- 9.Koolen BB, Donker M, Straver ME, et al. Combined PET-CT and axillary lymph node marking with radioactive iodine seeds (MARI procedure) for tailored axillary treatment in node-positive breast cancer after neoadjuvant therapy. Br J Surg 2017; 104:1188–1196. [DOI] [PubMed] [Google Scholar]
- 10.McInnes MDF, Moher D, Thombs BD, et al. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA statement. JAMA 2018; 319:388–396. [DOI] [PubMed] [Google Scholar]
- 11.Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011; 155:529–536. [DOI] [PubMed] [Google Scholar]
- 12.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health 2014; 72:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 14.Macaskill P, Gatsonis C, Deeks JJ, et al. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy. In: Deeks JJ, Bossuyt PM, Gatsonis C, eds. Version 1.0. Vol. Chapter 10: Analysing and Presenting Results The Cochrane Collaboration; 2010: 46–47. [Google Scholar]
- 15.Alvarado R, Yi M, Le-Petross H, et al. The role for sentinel lymph node dissection after neoadjuvant chemotherapy in patients who present with node-positive breast cancer. Ann Surg Oncol 2012; 19:3177–3184. [DOI] [PubMed] [Google Scholar]
- 16.Boileau JF, Poirier B, Basik M, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol 2015; 33:258–264. [DOI] [PubMed] [Google Scholar]
- 17.Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA 2013; 310:1455–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown AS, Hunt KK, Shen J, et al. Histologic changes associated with false-negative sentinel lymph nodes after preoperative chemotherapy in patients with confirmed lymph node-positive breast cancer before treatment. Cancer 2010; 116:2878–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carrera D, de la Flor M, Galera J, et al. Validation of sentinel lymph node biopsy in breast cancer women N1-N2 with complete axillary response after neoadjuvant chemotherapy. Multicentre study in Tarragona Validacion de la biopsia selectiva del ganglio centinela en mujeres con cancer de mama N1-2 con respuesta axilar completa tras la neoadyuvancia. Estudio multicentrico en la provincia de Tarragona. Rev Esp Med Nucl Imagen Mol 2016; 35:221–225. [DOI] [PubMed] [Google Scholar]
- 20.Enokido K, Watanabe C, Nakamura S, et al. Sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with an initial diagnosis of cytology-proven lymph node-positive breast cancer. Clin Breast Cancer 2016; 16:299–304. [DOI] [PubMed] [Google Scholar]
- 21.Ge WK, Yang B, Zuo WS, et al. Sentinel lymph node biopsy does not apply to all axillary lymph node-positive breast cancer patients after neoadjuvant chemotherapy. Thorac Cancer 2014; 5:550–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang E, Chung IY, Han SA, et al. Feasibility of sentinel lymph node biopsy in breast cancer patients with initial axillary lymph node metastasis after primary systemic therapy. J Breast Cancer 2011; 14:147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol 2013; 14:609–618. [DOI] [PubMed] [Google Scholar]
- 24.Ozmen V, Unal ES, Muslumanoglu ME, et al. Axillary sentinel node biopsy after neoadjuvant chemotherapy. Eur J Surg Oncol 2010; 36:23–29. [DOI] [PubMed] [Google Scholar]
- 25.Park S, Park JM, Cho JH, et al. Sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with cytologically proven node-positive breast cancer at diagnosis. Ann Surg Oncol 2013; 20:2858–2865. [DOI] [PubMed] [Google Scholar]
- 26.Pinero-Madrona A, Escudero-Barea MJ, Fernandez-Robayna F, et al. Selective sentinel lymph node biopsy after neoadjuvant chemotherapy in breast cancer: results of the GEICAM 2005-07 study. Cir Esp 2015; 93:23–29. [DOI] [PubMed] [Google Scholar]
- 27.Shen J, Gilcrease MZ, Babiera GV, et al. Feasibility and accuracy of sentinel lymph node biopsy after preoperative chemotherapy in breast cancer patients with documented axillary metastases. Cancer 2007; 109:1255–1263. [DOI] [PubMed] [Google Scholar]
- 28.Thomas S, Prakash A, Goyal V, et al. Evaluation of sentinel node biopsy in locally advanced breast cancer patients who become clinically node-negative after neoadjuvant chemotherapy: a preliminary study. Int J Breast Cancer 2011; 2011:870263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yagata H, Yamauchi H, Tsugawa K, et al. Sentinel node biopsy after neoadjuvant chemotherapy in cytologically proven node-positive breast cancer. Clin Breast Cancer 2013; 13:471–477. [DOI] [PubMed] [Google Scholar]
- 30.Yu Y, Cui N, Li HY, et al. Sentinel lymph node biopsy after neoadjuvant chemotherapy for breast cancer: retrospective comparative evaluation of clinically axillary lymph node positive and negative patients, including those with axillary lymph node metastases confirmed by fine needle aspiration. BMC Cancer 2016; 16:808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zetterlund LH, Frisell J, Zouzos A, et al. Swedish prospective multicenter trial evaluating sentinel lymph node biopsy after neoadjuvant systemic therapy in clinically node-positive breast cancer. Breast Cancer Res Treat 2017; 163:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siso C, de Torres J, Esgueva-Colmenarejo A, et al. Intraoperative ultrasound-guided excision of axillary clip in patients with node-positive breast cancer treated with neoadjuvant therapy (ILINA Trial): a new tool to guide the excision of the clipped node after neoadjuvant treatment. Ann Surg Oncol 2018; 25:784–791. [DOI] [PubMed] [Google Scholar]
- 33.Dashevsky BZ, Altman A, Abe H, et al. Lymph node wire localization post-chemotherapy: towards improving the false negative sentinel lymph node biopsy rate in breast cancer patients. Clin Imaging 2018; 48:69–73. [DOI] [PubMed] [Google Scholar]
- 34.Kim EY, Byon WS, Lee KH, et al. Feasibility of preoperative axillary lymph node marking with a clip in breast cancer patients before neoadjuvant chemotherapy: a preliminary study. World J Surg 2018; 42:582–589. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen TT, Hieken TJ, Glazebrook KN, et al. Localizing the clipped node in patients with node-positive breast cancer treated with neoadjuvant chemotherapy: early learning experience and challenges. Ann Surg Oncol 2017; 24:3011–3016. [DOI] [PubMed] [Google Scholar]
- 36.Park S, Koo JS, Kim GM, et al. Feasibility of charcoal tattooing of cytology-proven metastatic axillary lymph node at diagnosis and sentinel lymph node biopsy after neoadjuvant chemotherapy in breast cancer patients. Cancer Res Treat 2018; 50:801–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Plecha D, Bai S, Patterson H, et al. Improving the accuracy of axillary lymph node surgery in breast cancer with ultrasound-guided wire localization of biopsy proven metastatic lymph nodes. Ann Surg Oncol 2015; 22:4241–4246. [DOI] [PubMed] [Google Scholar]
- 38.Taback B, Jadeja P, Ha R. Enhanced axillary evaluation using reflector-guided sentinel lymph node biopsy: a prospective feasibility study and comparison with conventional lymphatic mapping techniques. Clin Breast Cancer 2018; e869–e874. [DOI] [PubMed] [Google Scholar]
- 39.Fontein DB, van de Water W, Mieog JS, et al. Timing of the sentinel lymph node biopsy in breast cancer patients receiving neoadjuvant therapy: recommendations for clinical guidance. Eur J Surg Oncol 2013; 39:417–424. [DOI] [PubMed] [Google Scholar]
- 40.van Nijnatten TJ, Schipper RJ, Lobbes MB, et al. The diagnostic performance of sentinel lymph node biopsy in pathologically confirmed node positive breast cancer patients after neoadjuvant systemic therapy: a systematic review and meta-analysis. Eur J Surg Oncol 2015; 41:1278–1287. [DOI] [PubMed] [Google Scholar]
- 41.NCT01901094. Comparison of Axillary Lymph Node Dissection With Axillary Radiation for Patients With Node-Positive Breast Cancer Treated With Chemotherapy. Principal Investigator: Judy Boughey. Mayo Clinic. Available at: https://clinicaltrials.gov/ct2/show/NCT01901094 Accessed February 4, 2018. [Google Scholar]
- 42.NCT01872975. Standard or Comprehensive Radiation Therapy in Treating Patients With Early-Stage Breast Cancer Previously Treated With Chemotherapy and Surgery. Principal Investigator: Norman Wolmar, MD. NSABP Foundation Inc. Available at: https://clinicaltrials.gov/ct2/show/NCT01872975 Accessed April 13, 2018. [Google Scholar]
- 43.van der Noordaa MEM, van Duijnhoven FH, Straver ME, et al. Major reduction in axillary lymph node dissections after neoadjuvant systemic therapy for node-positive breast cancer by combining PET/CT and the MARI procedure. Ann Surg Oncol 2018; 25:1512–1520. [DOI] [PubMed] [Google Scholar]
- 44.Boughey JC, Ballman KV, Le-Petross HT, et al. Identification and resection of clipped node decreases the false-negative rate of sentinel lymph node surgery in patients presenting with node-positive breast cancer (T0-T4, N1-N2) who receive neoadjuvant chemotherapy: results from ACOSOG Z1071 (Alliance). Ann Surg 2016; 263:802–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Nijnatten TJA, Simons JM, Smidt ML, et al. A novel less-invasive approach for axillary staging after neoadjuvant chemotherapy in patients with axillary node-positive breast cancer by combining radioactive iodine seed localization in the axilla with the sentinel node procedure (RISAS): a Dutch prospective multicenter validation study. Clin Breast Cancer 2017; 17:399–402. [DOI] [PubMed] [Google Scholar]
- 46.van Nijnatten TJ, Simons JM, Moossdorff M, et al. Prognosis of residual axillary disease after neoadjuvant chemotherapy in clinically node-positive breast cancer patients: isolated tumor cells and micrometastases carry a better prognosis than macrometastases. Breast Cancer Res Treat 2017; 163:159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]


