Abstract
Research on mycorrhizal interactions has traditionally developed into separate disciplines addressing different organizational levels. This separation has led to an incomplete understanding of mycorrhizal functioning. Integration of mycorrhiza research at different scales is needed to understand the mechanisms underlying the context dependency of mycorrhizal associations, and to use mycorrhizae for solving environmental issues. Here, we provide a road map for the integration of mycorrhiza research into a unique framework that spans genes to ecosystems. Using two key topics, we identify parallels in mycorrhiza research at different organizational levels. Based on two current projects, we show how scientific integration creates synergies, and discuss future directions. Only by overcoming disciplinary boundaries, we will achieve a more comprehensive understanding of the functioning of mycorrhizal associations.
Trajectories in Mycorrhiza Research
In 2000, Miller and Kling stated that ‘to succeed, the mycorrhiza (see Glossary) research community must go beyond their usual disciplinary boundaries and integrate their work with that of other researchers’ [1]. Although some advances have been made in the 18 years since, mycorrhiza research still largely develops by specializing within different disciplines of biology. During the 19th century, research into arbuscular mycorrhiza commenced with the discovery of fungal structures colonizing roots [2,3]. This early research was initially dominated by the descriptions of fungal morphological traits and potential fungal effects on plant performance. Such early research stayed at the organismal level, revealing more about the fungi, and studying effects at the individual host plant level. During the second half of the 20th century, research on mycorrhizae and their interactions developed into two main distinct directions: cellular and, later, subcellular biology on the one hand, and ecology on the other hand.
Glossary.
Arbuscule: a highly branched structure produced by AM fungi inside a root cortical cell of their host. Arbuscules are considered to be the main site of nutrient exchange between the fungal and plant symbiotic partners.
Cellular level: research that focuses on the structure and functions of plant and fungal cells involved in the symbiosis.
Common mycelial networks (CMNs): a belowground network of mycorrhizal hyphae linking roots of plants of the same or different species.
Defense priming: a process that conditions plant species for the enhanced induction of defenses, often resulting in enhanced pest and disease resistance and abiotic stress tolerance.
Discipline: field of research with a specific focus. It often focuses on a specific level of organization.
Ecosystem functioning: physical, geochemical, and biological activities and their effects within an ecosystem. They can be grouped into sizes (or stocks), such as nutrient pools, and rates of processes, and fluxes of material.
Ecosystem level: research that focuses on the whole ecosystem (i.e., a community of organisms that interact with each other and their environment).
Level of organization (organizational level): unit within a hierarchical system of biological structures and systems characterizing life. With each level, organizational complexity increases because it comprises the previous level; note that, in this paper, we refer to a system with four levels (ecosystem, plant community, plant physiological, and cellular level), which may differ from the classical ecological levels of organization.
Mycorrhiza: a symbiotic association between a soil fungus (the mycorrhizal fungus) and a plant root, in which plant photosynthates are exchanged for mineral resources acquired by the fungus from the soil.
Periarbuscular membrane: novel symbiosis-specific membrane, derived from the plant and developed at the moment of fungal penetration and arbuscule development; also known as the perifungal membrane.
Plant community level: research that focuses on interactions within a plant community (i.e., all co-occurring plants in a given time and space).
Plant physiological level: research that focuses on the internal (chemical or physical) functioning of plants and their parts that affect plant functions, such as plant nutrition and growth.
This specialization has led to many crucial discoveries in mycorrhizal biology. Focusing on cellular processes, ultrastructural studies during the 1970s led to the first detailed description of plant–fungal interactions. This included arbuscule formation, the identification of the periarbuscular membrane, as well as identification of the interface (i.e., the contact area between the interaction partners) [4]. On the ecological side, the realization that unequal benefits may be provided to different plant species [5] led to investigations of the importance of mycorrhizae in mediating plant adaptation and evolution as well as in structuring plant communities and biogeochemical cycles [6]. A milestone here was the experimental demonstration of arbuscular mycorrhizal (AM) fungal diversity effects on plant community diversity and productivity [7].
With methodological and conceptual advances, the field of mycorrhiza research has extended further following studies on the physiology of plants [8] and on ecosystem effects [7,9]. Each of the fields addresses different scales and levels of organization. Consequently, they have developed in distinct directions in terms of the questions addressed, scope, methods, and, perhaps most importantly, training and specialization of researchers. This division of the research field is illustrated by the fact that there are two main international scientific conferences on mycorrhizal research: the International Conference of Mycorrhiza (ICOM), focusing mostly on ecological research on mycorrhiza, and the International Molecular Mycorrhiza Meeting (iMMM), focusing mostly on molecular mycorrhiza research. Albeit effective in many respects, this historic partitioning into separate fields sometimes hinders a comprehensive understanding of how mycorrhizae function and influence their biotic and abiotic environment. For instance, our knowledge of the impact of mycorrhizae on ecosystem functions and services is relatively incomplete. This is due to the context-dependent nature of the symbiosis, which is driven by environmental conditions as well as the species identity of the plant and/or fungal partner. The interaction can exist along a continuum of possible outcomes for the plant, from mutualistic to detrimental [10]. Only by developing new investigation models that integrate multiple levels of organization will we be able to understand how environmental context impacts the relationships of plants with their mycorrhizal symbionts. This will allow us to make realistic predictions of the contribution of mycorrhizae to the functioning of ecosystems and to agricultural practices [11]. Thus, we argue that mycorrhiza research can reach a new level of insight by integrating the strengths of the increasingly separated research disciplines, both from the cellular to the ecosystem level and vice versa.
Here, we highlight how major topics in mycorrhiza research have been independently addressed by different disciplines across different organizational levels, and how they could complement each other. Within each topic, we focus on four organizational levels of life: the cellular and subcellular level (hereafter called the cellular level), the plant physiological level, the plant community level, and the ecosystem level. To stress the gains of integrating research across levels of organization, we present an overview of the benefits they may provide to each other. Moreover, we stress that, among the organizational levels, a consensus on research practices must be reached. Finally, we give recommendations for future directions towards integrative research networks, which aim at a more complete understanding of mycorrhizal associations and their functioning. As proof of concept, we introduce two recently developed projects in which such efforts are already underway. We focus on the two most commonly studied mycorrhizal types, namely AM and ectomycorrhizae (ECM), which are also the most common mycorrhizal symbioses in natural ecosystems. Studies of mycorrhizae at the physiological and cellular level are strongly biased towards AM compared with ECM. However, this imbalance, which is reflected in the predominance of AM compared with ECM examples in our paper, does not impede the validity of the presented framework, because this can in principle be applied to any type of mycorrhizae.
Multi-Level Connections in Mycorrhiza Research
Mycorrhiza research spans lower-organizational level processes [e.g., inorganic phosphorus (Pi) uptake and transport mechanisms, and modulation of plant immunity] to higher-organizational levels that affect plant community and ecosystem functioning (e.g., biogeochemical cycles or multitrophic interactions). While most studies focus on one or two levels of organization, mycorrhiza-related processes at one level are likely nested in and, thus, may provide mechanisms underlying the findings of, the next higher level (Figure 1, Key Figure). Here, we illustrate the potential nesting and links across the different organizational levels in mycorrhiza research by using two examples of key topics in mycorrhiza research: Pi uptake and multitrophic interactions.
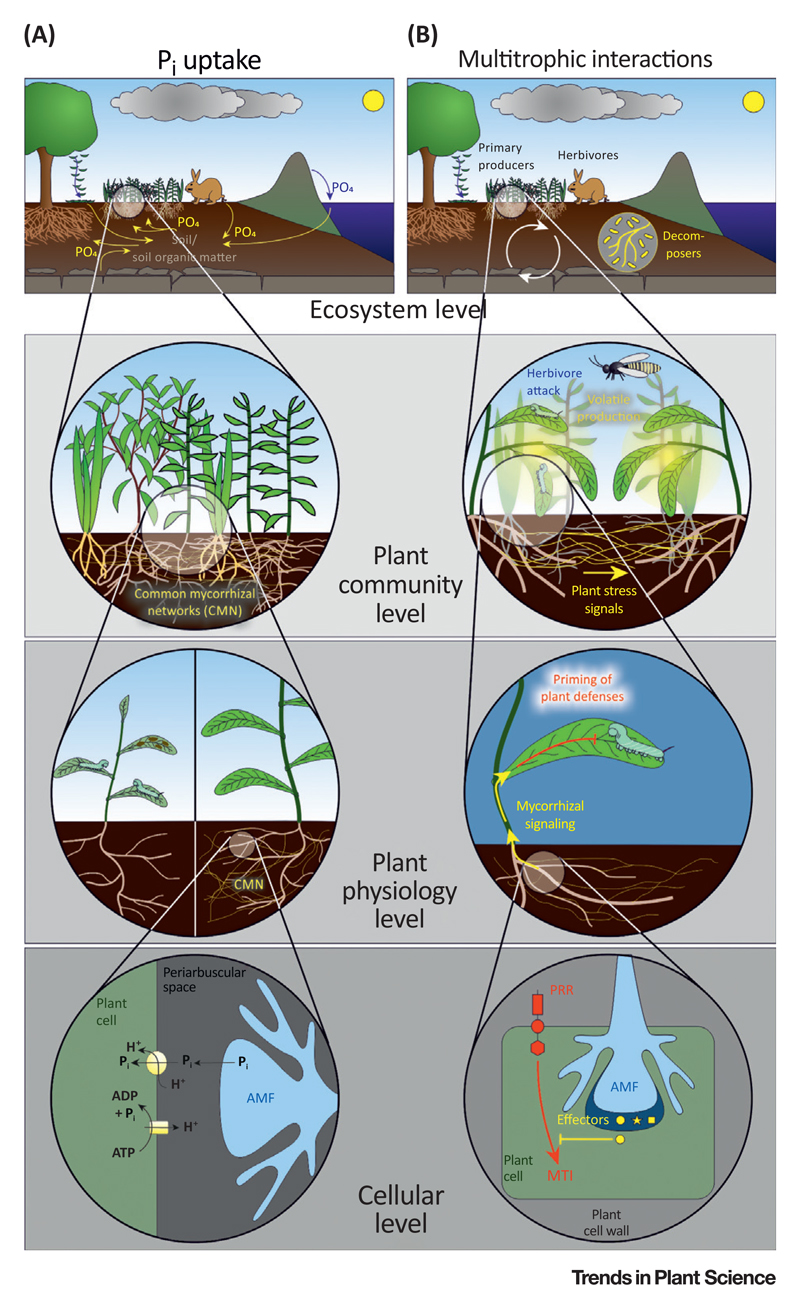
Figure 1.
Key Figure
Schematic Overview of Research at the Different Levels of Organization and the Processes Studied in Mycorrhizal Biology in the Context of (A) Inorganic Phosphorous (Pi) Uptake and (B) Multitrophic Interactions
Each drawing depicts one level of organization covering the cellular, plant physiological, plant community, and ecosystem level. Each level with its processes is nested within the next higher level, as indicated by the magnifying circles. (A) The P cycle of an ecosystem and the contribution of plant communities (top of figure). Zooming into these contributions, mycorrhizal interactions within the plant community and their diverse mycelial networks become visible. Plant physiological responses to mycorrhizal fungi, such as altered plant growth and altered susceptibility to antagonists, drive plant community responses. At the base, subcellular and cellular processes of Pi transport at the interface of the fungus and the plant root cell determine plant physiological responses. (B) Organismal interactions at the whole ecosystem level (top figure). Zooming into the interactions among mycorrhizal plants, multitrophic interactions via signaling pathways (induced by herbivore attack and subsequent parasitoid recruitment) become apparent as well as signaling of herbivore attack within the plant community via common mycelial networks. The processes by which the plant allocates defense compounds to its leaves (fostered by the association with mycorrhizae), which prevent the herbivore from feeding, become apparent at the next level. At the base, mycorrhizal fungi modulate plant immunity to establish the symbiosis, leading to a defense signaling cascade that can enhance plant immunity against herbivores. Abbreviations: AMF, arbuscular mycorrhizal fungus; MTI, microbial-associated molecular pattern (MAMP)-triggered immunity; PRR: pattern-recognition receptors.
Example 1: Effect of Mycorrhizal Associations on Plant Phosphorus Uptake
The AM symbiosis provides an effective way (the AM pathway) to scavenge Pi from large volumes of soil and rapidly deliver it to root cortical cells, thereby bypassing direct root Pi uptake. Cellular research revealed Pi transporters involved in the mycorrhizal phosphate uptake pathway. Some of these transporters show constitutive transcript levels in non-mycorrhizal roots [12], while others are mostly expressed in arbuscule-containing cells [13] (Figure 1A). These plant Pi transporters are located in the periarbuscular membrane and take up phosphate ions from the periarbuscular space, the apoplastic compartment between the plant periarbuscular membrane and fungal plasma membrane in arbuscule-containing cells. Recent molecular studies demonstrated that specific H+-ATPase proteins in the periarbuscular membrane are required to generate the proton gradient necessary for the action of Pi transporters [14]. These results are relevant for understanding the regulation of Pi acquisition in the AM symbiosis. However, without addressing the physiological level, this information is not sufficient for determining the relevance of the AM pathway with respect to the direct Pi uptake pathway. The same applies to assessing its net contribution to plant Pi uptake and, thus, plant nutrition and fitness.
Plant physiological research revealed that the AM pathway has a major role in Pi uptake and, consequently, in plant growth (Figure 1A). Experiments with single plants and plant communities showed that AM fungi contribute up to 90% of plant P levels [15–17]. However, colonization by different AM fungal species can result in different fitness responses of the plant [18]. Similarly, colonization by the same AM fungal species does not necessarily result in the same fitness response in different plant species. This indicates that there is considerable functional diversity among plant–AM fungal symbioses. Indeed, AM associations have also been shown to vary in their physiological characteristics [19]. Still, the major forces driving this functional diversity are widely unknown. Combining a cellular approach with physiological experiments is a powerful approach to illuminate mycorrhizal functions, while providing a genetic explanation for the functional variation observed. Such information can even be implemented in the development of biomarkers for the assessment of mycorrhizal functional diversity and for the selection of efficient crop–fungus combinations for increasing agricultural productivity. Moreover, the symbiotic efficiency in terms of plant Pi uptake is further influenced by fungal community composition [7]. To predict the net impact of mycorrhizae on Pi uptake, it is essential to combine physiological studies with studies at the community level, where plants are part of complex species interaction networks. To achieve this, it is crucial that techniques, such as high-throughput sequencing, transcriptomics, and metabolomics, are used in the field.
Studies at the plant community level showed that both the productivity and composition of plant communities are altered by mycorrhizal associations. Mycorrhizae indirectly cause a redistribution of soil resources among plants (Pi) by enhancing the resource acquisition and competitive abilities of some plants over others [20]. Furthermore, a greater richness of AM fungal species has been shown not only to increase net primary productivity through Pi supply, but also to maintain a more diverse plant community on which other organisms may depend [9]. Directly, Pi allocation among plants is also changed through common mycelial networks (CMNs) [16] (Figure 1A). However, our knowledge of Pi transport (and nutrients in general) among plants and carbon transfer between plants and mycorrhizal fungi in CMNs is limited [21]. Assessing the potential causes and effects of these resource fluxes on community functioning requires an understanding of the cellular and physiological processes driving this transfer, and the factors regulating these processes. Moreover, abiotic factors, such as soil nutrient availability, as well as micro- and macroclimate, can affect the net impact of mycorrhizae on plant Pi uptake and distribution in the community. The global impact of such factors can only be assessed in ecological settings.
Research at the ecosystem level revealed that mycorrhizal associations have a key role in shaping ecosystem structure and function by altering the distribution of P among species, how it is recycled between above–belowground ecosystem compartments, and ultimately retained within an ecosystem [7,22] (Figure 1A). All these processes may differ between ecosystem types and are affected by different abiotic factors, such as those mentioned above. This is typically referred to as ‘context dependency’. However, without knowledge of the physiological and plant community perspective on processes of resource distribution among plants via CMNs, it is hard to predict how changes in the processes are affected by different contexts and to identify the drivers of the changes.
Example 2: Effects of Mycorrhizal Associations on Multitrophic Interactions
Cellular biological research on multitrophic interactions with mycorrhizae has yielded two important insights. First, following the exchange of signals during the presymbiotic phase, plants initially recognize mycorrhizal fungi to a certain extent as potential invaders, triggering a mild immune response [23]. This immune response resembles the microbial-associated molecular pattern (MAMP)-triggered immunity (MTI) mounted after pathogen recognition. Such plant responses can subsequently be counteracted by the secretion of fungal effector molecules [24,25] and/or by the plant perception of MYC factors [26]. As a result of this molecular dialog between both partners, the levels of several defense-related phytohormones and other metabolites can be altered in mycorrhizal roots, and even shoots [27,28]. Second, recent studies suggest that, as a consequence of this modulation of plant immunity by mycorrhizal fungi, mycorrhizal plants can enter into a state of sensitization of the immune system. This defense-primed state allows plants to respond faster and/or stronger when they are challenged by subsequent herbivore and pathogen attack [28–30] (Figure 1B). The pathway regulated by the plant hormone jasmonic acid (JA) has a crucial role in plant defense priming [28,31]. Although more robust plant defense is usually associated with better performance in times of stress, boosting induced defense responses does not always provide an advantage to the plant [32]. The incorporation of a physiological approach is essential to understand the effects on whole-plant performance. For instance, fundamental questions on the contribution of mycorrhizal-boosted defenses to plant resistance against antagonists, or on their role in herbivore-induced changes in carbon assimilation and partitioning of assimilates, can only be addressed by integrating the mycorrhizal impact on plant defense signaling networks into whole-plant models.
Plant physiological research revealed that both AM and ECM fungi induce changes in the immune system of a plant that impact the interaction of the plant with other community members at different trophic levels [33] (Figure 1B). Given that mycorrhizal fungi prime plants for JA-signaled defenses, it was speculated that mycorrhizal colonization would predominantly negatively affect leaf-chewing herbivores and necrotrophic pathogens. Both attackers are mostly responsive to JA-mediated defenses [34]. At the same time, mycorrhizal colonization would benefit piercing and sap-sucking herbivores, and biotrophic pathogens, which are more responsive to salicylic (SA)-mediated defenses. However, studies showed high variation in the effect of mycorrhizal colonization on plant–herbivore interactions [35]. This suggests that additional factors are involved in determining the final outcome of mycorrhiza–plant–insect interactions [36]. More accurate predictions at the whole-plant level would require a better integration of cellular and physiological studies, providing insight into the mechanisms involved with studies of the ecological function of traits that are influenced by mycorrhizae. Furthermore, this combination of physiological and cell biological information has the potential to provide the foundation for the application of mycorrhizae in pest management strategies. Besides directly affecting plant antagonists, mycorrhizae might influence plant–herbivore interactions by affecting the attraction of natural enemies of insect herbivores [37]. Additionally, they can alter the competitive ability of plants within the community [38]. Finally, plants can exchange defense-related information with other plants in the community via CMNs, as shown in laboratory studies [39]. Therefore, including the community perspective in real-world scenarios is essential to predict the net impact of mycorrhizae on plant–herbivore interactions at the whole-plant level.
The effects of mycorrhization on plant physiology trickle up to influence microbial, animal, and plant community structure and ecosystem functioning [40]. Besides modifying aboveground plant productivity, mycorrhizal associations have been shown to influence the diversity of plant species as well as that of higher trophic levels, such as herbivores and parasitoids [41]. Moreover, CMNs connecting mycorrhizal plants within a community act not only as conduits for carbon and mineral nutrients, but also as an underground messaging system (‘signaling highway’) [42]. This allows neighboring plants to activate defenses before they are attacked themselves [43,44] (Figure 1B). Although this phenomenon has been exclusively studied in laboratory settings, CMNs have the potential to determine the outcome of multitrophic interactions beyond the individual plant level, by conferring information about herbivore presence within a community. Consequently, they may also change predator and parasitoid recruitment at the community level. Still, the cost and benefits of interplant signaling, and their evolutionary consequences, remain mostly unknown. Analyzing the impact of CMNs in plant- and insect-related traits at the physiological level and the main mechanisms of interplant signaling at the cellular level will help to address these questions. Typically, in nature, the outcome of such interactions is not exclusively determined by the interaction partners per se. A multitude of environmental factors that can only be measured at the ecosystem level (under field conditions) may strengthen or weaken species interactions.
Mycorrhizal fungi alter plant community performance and higher-trophic level community structure, thereby impacting element and energy cycling of the whole ecosystem (Figure 1B). For example, by changing the chemical composition of leaves and roots, mycorrhizae will likely affect the decomposition of plant litter [45], which serves as the main basal resource in the belowground food web. Changes in basal resources affect the trophic structure and multitrophic interactions of the food web, and add to plant community effects on nutrient cycling of the whole ecosystem. Patterns in multitrophic interactions observed at the ecosystem level will remain correlative if not integrated with more mechanistic studies at the plant physiological and community levels. This is also important to apply mycorrhiza research to agriculture and/or crop production. For instance, the application of fertilizers and pesticides is subject to strong political and industrial pressures. Understanding the mechanisms and processes involved in AM function could underpin the development of novel crop plant–mycorrhizal associations for optimal nutrient uptake efficiency and, simultaneously, for higher resistance against antagonists.
Opportunities and Challenges Related to the Integration of Disciplines in Mycorrhiza Research
To create a conceptual framework that transcends individual disciplines in mycorrhiza research, researchers need to learn how to appreciate the reciprocal benefits that the different disciplines offer. For example, molecular tools of model organisms can provide means for identifying genes with important roles in mycorrhizal ecology. This is essential for our understanding of the distribution and function of mycorrhizae in space and time, and for predicting how mycorrhizae respond to changes in their environment. However, mycorrhiza ecologists interested in using genomics tools are hampered by the low number of fungal and plant genomes currently available. Nevertheless, the rapidly increasing number of plant genome sequences becoming available will allow these tools to be applied more often to ecological studies. In addition, novel techniques for genetic engineering, such as CRISPR/Cas9, will allow for the elucidation of the function and ecological relevance of single or multiple genes in mycorrhizal function in non-model organisms. The application of these genetic tools, combined with knowledge at the cellular level, will be crucial for better understanding the function of mycorrhizae in the ecosystem. By contrast, cellular biologists are making important advances in understanding the molecular and cellular processes involved in mycorrhizal establishment and functioning. Nonetheless, the functions of thousands of genes identified by high-throughput genomics and transcriptomics remain poorly deciphered. Ecologists can provide guidance as to the specific contexts and situations in which to examine the functional ecology of ‘omics patterns. This can only be realized if laboratory approaches and methods are used in field settings.
Initiating and successfully maintaining crossdisciplinary research can be not only rewarding, but also challenging. One specific challenge is the difference in concepts and terminologies in the different disciplines. Mycorrhiza researchers may mean different things when considering results as ‘being proven’ [46], which is often equivalent to the ‘gain of mechanistic understanding’. For many ecologists, the term ‘mechanism’ defines a process or interaction that takes place at the organizational level beneath the level of the observation in a study. This means that this term is relative to the level of observation. For cellular biologists and physiologists, it commonly defines something that takes place at the cellular level (i.e., it is an absolute term). Further challenges for crossdisciplinary research are related to the design of collaborative projects. For instance, what constitutes a ‘control’ and ‘replicate’ can significantly differ for ecological and cellular studies on mycorrhiza functioning. In particular, ecosystem-level studies in the field lack a non-mycorrhizal control, whereas this is not the case in laboratory settings, where soils can be sterilized. In field experimental studies, replication is often based on an experimental unit that can be an individual, a species, or a plant community. However, for cellular studies conducted in laboratory settings, the whole experiment can be replicated several times to validate the results. In other words, the lack of statistical power within one experiment is counterbalanced by the consistency of results across experiments. Such a strategy is not possible in ecological settings, since variability across years, seasons, or weeks can only be counterbalanced by a robust experimental design and high replication. Therefore, a main challenge of combining multiple mycorrhiza research fields resides in integrating the ecological context dependency found in natural settings with mechanistic discoveries obtained in artificial laboratory conditions.
Concluding Remarks and Future Perspectives
Key to achieving the integration of mycorrhiza research across different organizational levels is to design and execute common projects where research questions are addressed at different levels at the same time (see Outstanding Questions). A fruitful approach is to link laboratory and field experiments through common questions, which build on each other. In the laboratory, important processes can be studied by manipulating specific biotic and abiotic parameters precisely. These findings generate hypotheses on their ecological role, which should be tested in field settings. Here, the test organisms are subject to different treatments within the context of a multitude of biotic and abiotic interactions. In turn, based on patterns that are found in real-world ecosystems, single processes and mechanisms can be disentangled using targeted laboratory experiments. Given that real-world ecosystems comprise a multitude of interacting and interwoven processes, it remains challenging to select single processes to be tested independently in the laboratory. Overall, both approaches can create positive feedback loops that fuel each other, promoting hypothesis-driven research at different levels. Another course is to incorporate laboratory approaches and methods in field experimental settings for the integration of different organizational levels. However, the main challenge is the application of highly sensitive analytical techniques designed for in vitro laboratory environments.
Outstanding Questions.
Do field-sampling conditions allow the use of highly sensitive analytical techniques as done in in vitro laboratory environments to identify cellular mechanisms in model systems?
Is it possible to identify single potentially relevant processes from general patterns in real-world ecosystems to independently test and disentangle them in targeted laboratory experiments?
How can we create a common practical base in terms of problem detection, hypothesis development, and analysis for building a full crossdisciplinary framework?
What is the common denominator regarding concepts, approaches, and definitions in experimental studies that can link all disciplines into a complete integrative research?
How can a ‘genes-to-ecosystem’ approach in mycorrhiza research be applied for solving environmental issues, such as nutrition of a growing human population?
Furthermore, it requires the collaboration of researchers from both fields to overcome the limitations of individual disciplines. To achieve this goal, it is essential, although challenging, to foster collaborations in common projects at all levels; from problem detection and hypothesis development to experimental (or observational) setup, analysis, and interpretation of findings [46]. The realization of joint workshops and conferences may lay foundations for such enterprises. Similarly, integrative MS and PhD courses will train the next generation of mycorrhizal scientists to apply both ecological and molecular approaches in their research projects.
Initial attempts to make mycorrhiza research crossdisciplinary are underway in the PhytOakmeter project. Here, clonal oaks (Quercus robur L.), which were originally developed for highly standardized molecular measurements, are now transplanted into field experiments over wide environmental gradients (Box 1). Another example is the tree diversity experiment, MyDiv, where plots consist of tree communities that are characterized by different types of mycorrhization (namely ECM and AM). MyDiv integrates part of the clonal oaks from the PhytOakmeter project, and further implements omics’ approaches (i.e., metabolomics) in the field (Box 2). These are only two example projects among several that are in the process of overcoming the specific technical and scientific boundaries of the single disciplines. Such synergistic projects can ultimately advance the comprehensive understanding of mycorrhizal associations and their functioning that spans from genes to ecosystems and how this can contribute to solving fundamental issues, such as sustainable food production while preserving our planet’s biodiversity.
Box 1. TrophinOak-PhytOakmeter: A Clone Laboratory and Field Experimental Platform.
Tree performance depends on the interplay between resource-demanding beneficial and detrimental biotrophic interactions and the own developmental program. This program is not only controlled by internal processes, but also influenced by external factors [47]. TrophinOak and PhytOakmeter are two complementary experimental systems that can be used to unravel such interplay at the laboratory and field level, respectively. They both use a clone of common oak (Quercus robur L.; DF159; Figure IA) because of its endogenous rhythmic growth (ERG) characterized by alternating growth flushes in shoot and roots paralleled by resource allocation shifts between below- and aboveground parts [48]. A Petri dish system with sterile soil and rooted microcuttings of DF159 was established to study the impact of the ECM fungus Piloderma croceum on the interplay between ERG and biotrophic interactions with above- and belowground interactors (Figure IB) [49]. Oak transcriptomic profiling using RNAseq was prompted by establishing the large specific reference library OakcontigDF159.1 [50]. The PhytOakmeter project is an extension of TrophinOak, releasing clonal DF159 oaks as phytometers in the field along European climatic, land-use intensity, and plant diversity gradients (Figure IC). Such ‘PhytOakmeters’ were integrated in the tree diversity experiment MyDiv [51] (see Box 2 in the main text). Data on PhytOakmeter development and transcriptomics will complement the analyses of physiological and cellular response analyses in relation to the diversity of AM and ECM tree neighbors manipulated in MyDiv. The use of clonal oak phytometers allows the optimal evaluation and comparison of different ecosystem functions in MyDiv. Also see http://www.ufz.de/trophinoak-phytoakmeter.
Figure I. Main Elements and Steps in the TrophinOak-PhytOakmeter Experimental Platform.
(A) In vitro propagation of the Oak DF159, (B) mycorrhization with Piloderma croceum in microcosms in the TrophinOak project, and (C) outplanting of the PhytOakmeters to a field site. Adapted from [49] (A,B). Photograph by Sylvie Herrmann (C).
Box 2. MyDiv: A Tree Diversity Experiment Manipulating Mycorrhizae.
MyDiv is a field experiment studying the relationship between tree diversity and ecosystem functioning [51]. This mostly positive relationship has been attributed to complementary use of soil resources [52]. Recent studies suggest that the strength of resource use complementarity is context dependent andthat biotic interactionswith other organism guilds, such as mycorrhizae, are crucial in this respect [53]. Given that mycorrhizae have a critical role in resource use by plants, they may be an important driver of biodiversity–ecosystem functioning relationships [54]. This hypothesis is tested explicitly in MyDiv.
The experiment comprises a tree species richness gradient as well as a mycorrhizal-type treatment using tree communities, which have AM, ECM, or both mycorrhizal types (Figure IA). MyDiv allows for investigating biodiversity–ecosystem functioning relationships in the light of physiological interactions at the interface of plant roots and mycorrhizal fungi as well as the rhizosphere. The latter are analyzed using laboratory-based chemical and ‘omics approaches. In collaboration with plant physiologists, different 15N-labeled nitrogen compound solutions are used to trace nutrient acquisition of tree species in different communities. Root exudates are further studied in situ and analyzed using metabolomic approaches (Figure IB). This yields information on the role of mycorrhizal associations for the release of labile plant resources into soil and, thus, for nutrient cycling. Also see https://www.idiv.de/en/research/platforms_and_networks/mydiv.html.
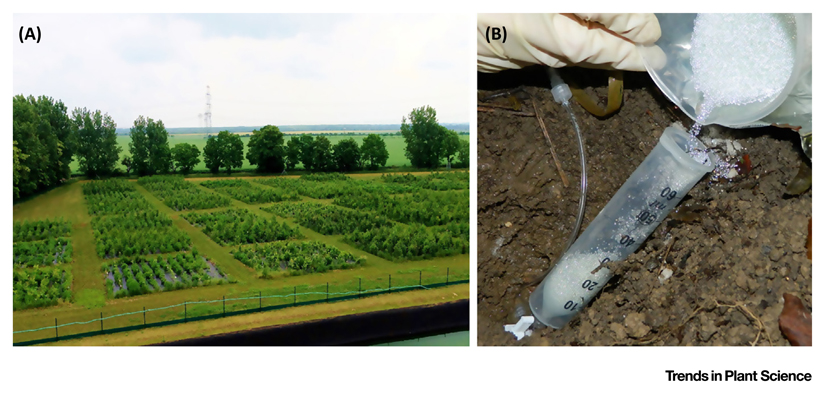
Figure I. The MyDiv Site Located in Bad Lauchstädt, Germany.
(A) Plots from an aerial perspective. (B) The preparation of the medium for exudate collection in situ from fine roots of a tree in the field. Photographs taken by Konrad Kirsch (A) and Rebecca Liese (B).
Highlights.
Mycorrhiza research has traditionally developed into distinct disciplines at different organizational levels from the cellular to ecosystem level.
This separation leads to a limited understanding of mycorrhiza functioning and its role within ecosystems.
Here, we show how the different disciplines in mycorrhiza research commonly address the same general questions and how these questions are nested in the next organizational level.
Byintegratingdifferentdisciplines, these disciplinesareable to complement each other and foster the development of a comprehensive understanding of mycorrhizal associations.
We introduce two ongoing projects as examples where the integration of disciplines in mycorrhiza research is already common practice.
Acknowledgments
All authors acknowledge funding from the German Centre for Integrative Biodiversity Research (iDiv) Halle-Jena-Leipzig funded by the German Research Foundation (DFG; FZT 118). A.M.M., A.B., M.P., S.R., and N.M.v.D. acknowledge stimulating discussions within COST Action FA1405 and funding from EU MC-ITN MiRA (grant no. 765290). I.C.M. acknowledges financial support from the DFG (grant no. ME 4156/2-1) and Volkswagen Foundation [grant no. 11-76251-99-34/13 (ZN 2928)]. M.C.R. acknowledges funding from the European Research Council (ERC, Advanced Grant ‘Gradual Change’) and BMBF funding for the project ‘Bridging in Biodiversity Science (BIBS)’. M.P. acknowledges support from MINECO (grant no. AGL2015-64990-C2-1-R). I.F. acknowledges support from iDiv Flexpool Program (grant no. RA-185/17). O.F. and N.E. acknowledge support from the ERC (European Union’s Horizon 2020 research and innovation program, grant no. 677232). S.R. acknowledges the Swiss Science Foundation (grant no. 159869). F.B., S.H., and M.T. acknowledge DFG for financial support (Grants BU 941/20-1 and TA 290/4-1). All the authors acknowledge support from the iDiv Open Science Publication Fund.
References
- 1.Miller RM, Kling M. The importance of integration and scale in the arbuscular mycorrhizal symbiosis. Plant Soil. 2000;226:295–309. [Google Scholar]
- 2.Nägeli C. Pilze im Innern von Zellen. Linnaea. 1842;16:278–285. [Google Scholar]
- 3.Frank AB. Über die auf Wurzelsymbiose beruhende Ernährung gewisser Bäume durch unterirdische Pilze. Ber Dtsch Bot Ges. 1885;3:128–145. [Google Scholar]
- 4.Bonfante P. Anatomy and morphology of VA mycorrhizae. In: Powell CL, Bagyaraj DJ, editors. VA Mycorrhizae. CRC Press; 1984. pp. 5–33. [Google Scholar]
- 5.Baylis GTS. Root hairs and phycomycetous mycorrhizas in phosphorus-deficient soil. Plant Soil. 1970;33:713–716. [Google Scholar]
- 6.Van der Heijden MGA, et al. Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytol. 2015;205:1406–1423. doi: 10.1111/nph.13288. [DOI] [PubMed] [Google Scholar]
- 7.Van der Heijden MGA, et al. Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature. 1998;396:69–72. [Google Scholar]
- 8.Koide RT, Mosse B. A history of research on arbuscular mycorrhiza. Mycorrhiza. 2004;14:145–163. doi: 10.1007/s00572-004-0307-4. [DOI] [PubMed] [Google Scholar]
- 9.Powell JR, Rillig MC. Biodiversity of arbuscular mycorrhizal fungi and ecosystem function. New Phytol. 2018 doi: 10.1111/nph.15119. Published online March 30, 2018. [DOI] [PubMed] [Google Scholar]
- 10.Chaudhary VB, et al. MycoDB, a global database of plant response to mycorrhizal fungi. Sci Data. 2016;3 doi: 10.1038/sdata.2016.28. 160028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gianinazzi S, et al. Agroecology: the key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza. 2010;20:519–530. doi: 10.1007/s00572-010-0333-3. [DOI] [PubMed] [Google Scholar]
- 12.Rausch C, Bucher M. Molecular mechanisms of phosphate transport in plants. Planta. 2002;216:23–37. doi: 10.1007/s00425-002-0921-3. [DOI] [PubMed] [Google Scholar]
- 13.Javot H, et al. Phosphate in the arbuscular mycorrhizal symbiosis: transport properties and regulatory roles. Plant Cell Environ. 2007;30:310–322. doi: 10.1111/j.1365-3040.2006.01617.x. [DOI] [PubMed] [Google Scholar]
- 14.Krajinski F, et al. The H+-ATPase HA1 of Medicago truncatula is essential for phosphate transport and plant growth during arbuscular mycorrhizal symbiosis. Plant Cell. 2014;26:1808–1817. doi: 10.1105/tpc.113.120436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jakobsen I, et al. External hyphae of vesicular-arbuscular mycorrhizal fungi associated with Trifolium subterraneum L. New Phytol. 1992;120:371–380. [Google Scholar]
- 16.Leake J, et al. Networks of power and influence: the role of mycorrhizal mycelium in controlling plant communities and agroecosystem functioning. Can J Bot. 2004;82:1016–1045. [Google Scholar]
- 17.Smith SE, Smith FA. Roles of arbuscular mycorrhizas in plant nutrition and growth: new paradigms from cellular to ecosystem scales. Annu Rev Plant Biol. 2011;62:227–250. doi: 10.1146/annurev-arplant-042110-103846. [DOI] [PubMed] [Google Scholar]
- 18.Klironomos JN. Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology. 2003;84:2292–2301. [Google Scholar]
- 19.Cavagnaro TR, et al. Functional diversity in arbuscular mycorrhizas: exploitation of soil patches with different phosphate enrichment differs among fungal species. Plant Cell Environ. 2005;28:642–650. [Google Scholar]
- 20.Collins CD, Foster BL. Community-level consequences of mycorrhizae depend on phosphorus availability. Ecology. 2009;90:2567–2576. doi: 10.1890/08-1560.1. [DOI] [PubMed] [Google Scholar]
- 21.Selosse MA, et al. Mycorrhizal networks: des liaisons dangereuses? Trends Ecol Evol. 2006;21:621–628. doi: 10.1016/j.tree.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Bender SF, et al. Mycorrhizal effects on nutrient cycling, nutrient leaching and N2O production in experimental grassland. Soil Biol Biochem. 2015;80:283–292. [Google Scholar]
- 23.Bonfante P, Genre A. Arbuscular mycorrhizal dialogues: do you speak ‘plantish’ or ‘fungish’? Trends Plant Sci. 2015;20:150–154. doi: 10.1016/j.tplants.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Kloppholz S, et al. A secreted fungal effector of Glomus intraradices promotes symbiotic biotrophy. Curr Biol. 2011;21:1204–1209. doi: 10.1016/j.cub.2011.06.044. [DOI] [PubMed] [Google Scholar]
- 25.Plett JM, et al. A secreted effector protein of Laccaria bicolor is required for symbiosis development. Curr Biol. 2011;21:1197–1203. doi: 10.1016/j.cub.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 26.Siciliano V, et al. Transcriptome analysis of arbuscular mycorrhizal roots during development of the prepenetration apparatus. Plant Physiol. 2007;144:1455–1466. doi: 10.1104/pp.107.097980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandez I, et al. Defense related phytohormones regulation in arbuscular mycorrhizal symbioses depends on the partner genotypes. J Chem Ecol. 2014;40:791–803. doi: 10.1007/s10886-014-0473-6. [DOI] [PubMed] [Google Scholar]
- 28.Kaling M, et al. Mycorrhiza-triggered transcriptomic and metabolomic networks impinge on herbivore fitness. Plant Physiol. 2018;176:2639–2656. doi: 10.1104/pp.17.01810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jung SC, et al. Mycorrhiza-induced resistance and priming of plant defenses. J Chem Ecol. 2012;38:651–664. doi: 10.1007/s10886-012-0134-6. [DOI] [PubMed] [Google Scholar]
- 30.Martinez-Medina A, et al. Recognizing plant defense priming. Trends Plant Sci. 2016;21:818–822. doi: 10.1016/j.tplants.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 31.Song YY, et al. Priming of anti-herbivore defense in tomato by arbuscular mycorrhizal fungus and involvement of the jasmonate pathway. J Chem Ecol. 2013;39:1036–1044. doi: 10.1007/s10886-013-0312-1. [DOI] [PubMed] [Google Scholar]
- 32.Douma JC, et al. When does it pay off to prime for defense? A modeling analysis. New Phytol. 2017;216:782–797. doi: 10.1111/nph.14771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pozo MJ, Azcon-Aguilar C. Unraveling mycorrhiza-induced resistance. Curr Opin Plant Biol. 2007;10:393–398. doi: 10.1016/j.pbi.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 34.Wasternack C. Action of jasmonates in plant stress responses and development-applied aspects. Biotechnol Adv. 2014;32:31–39. doi: 10.1016/j.biotechadv.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 35.Hartley SE, Gange AC. Impacts of plant symbiotic fungi on insect herbivores: mutualism in a multitrophic context. Annu Rev Entomol. 2009;54:323–342. doi: 10.1146/annurev.ento.54.110807.090614. [DOI] [PubMed] [Google Scholar]
- 36.Biere A, et al. Three-way interactions between plants, microbes and insects. Funct Ecol. 2013;27:567–573. [Google Scholar]
- 37.Schausberger P, et al. Mycorrhiza changes plant volatiles to attract spider mite enemies. Funct Ecol. 2012;26:441–449. [Google Scholar]
- 38.Wagg C, et al. Mycorrhizal fungal identity and diversity relaxes plant-plant competition. Ecology. 2011;92:1303–1313. doi: 10.1890/10-1915.1. [DOI] [PubMed] [Google Scholar]
- 39.Babikova Z, et al. Arbuscular mycorrhizal fungi and aphids interact by changing host plant quality and volatile emission. Funct Ecol. 2014;28:375–385. [Google Scholar]
- 40.Lee EH, et al. Diversity of arbuscular mycorrhizal fungi and their roles in ecosystems. Mycobiology. 2013;41:121–125. doi: 10.5941/MYCO.2013.41.3.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hempel S, et al. Specific bottom-up effects of arbuscular mycorrhizal fungi across a plant–herbivore–parasitoid system. Oecologia. 2009;160:267–277. doi: 10.1007/s00442-009-1294-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barto EK, et al. Fungal superhighways: do common mycorrhizal networks enhance below ground communication? Trends Plant Sci. 2012;17:633–637. doi: 10.1016/j.tplants.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 43.Song YY, et al. Interplant communication of tomato plants through underground common mycorrhizal networks. PLoS One. 2010;5:e13324. doi: 10.1371/journal.pone.0013324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Babikova Z, et al. Underground signals carried through common mycelial networks warn neighbouring plants of aphid attack. Ecol Lett. 2013;16:835–843. doi: 10.1111/ele.12115. [DOI] [PubMed] [Google Scholar]
- 45.Phillips RP, et al. The mycorrhizal-associated nutrient economy: a new framework for predicting carbon-nutrient couplings in temperate forests. New Phytol. 2013;199:41–51. doi: 10.1111/nph.12221. [DOI] [PubMed] [Google Scholar]
- 46.Eigenbrode SD, et al. Employing philosophical dialogue in collaborative science. BioScience. 2007;57:55–64. [Google Scholar]
- 47.Tscharntke T, Hawkins BA, editors. Multitrophic Level Interactions. Cambridge University Press; 2002. [Google Scholar]
- 48.Herrmann S, et al. Endogenous rhythmic growth in oak trees is regulated by internal clocks rather than resource availability. J Exp Bot. 2015;66:7113–7127. doi: 10.1093/jxb/erv408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herrmann S, et al. Endogenous rhythmic growth, a trait suitable for the study of interplays between multitrophic interactions and tree development. Perspect Plant Ecol Syst. 2016;19:40–48. [Google Scholar]
- 50.Tarkka MT, et al. OakContigDF159.1, a reference library for studying differential gene expression in Quercus robur during controlled biotic interactions: use for quantitative transcriptomic profiling of oak roots in ectomycorrhizal symbiosis. New Phytol. 2013;199:529–540. doi: 10.1111/nph.12317. [DOI] [PubMed] [Google Scholar]
- 51.Ferlian O, et al. Mycorrhiza in tree diversity–ecosystem function relationships: conceptual framework and experimental implementation. Ecosphere. 2018;9 doi: 10.1002/ecs2.2226. e02226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ebeling A, et al. A trait-based experimental approach to understand the mechanisms underlying biodiversity–ecosystem functioning relationships. Basic Appl Ecol. 2014;15:229–240. [Google Scholar]
- 53.Eisenhauer N. Aboveground–belowground interactions as a source of complementarity effects in biodiversity experiments. Plant Soil. 2012;351:1–22. [Google Scholar]
- 54.Wagg C, et al. Facilitation and antagonism in mycorrhizal networks. In: Horton TR, editor. Mycorrhizal Networks. Springer; 2015. pp. 203–226. [Google Scholar]