Abstract
Background:
The prognostic value of pretreatment systemic immune-inflammation index (SII) in lung cancer has yet to be fully established.
Methods:
Relevant articles were obtained by performing a systematic search. Hazard ratios (HRs) and 95% confidence intervals (95% CIs) were used to assess the relationship between SII index and overall survival (OS) in lung cancer; the OS was calculated from the time of cancer diagnosis to the date of death due to any cause or to the last date of follow-up.
Results:
In total, 2786 patients with lung cancer from 7 studies were included in this meta-analysis. The median thresholds to define high SII was 640 (range 395.4–1600) in the analyzed studies. The pooled HR for OS was 1.77 (95% CI: 1.54–2.00, P < .001), suggesting that the patients with a high SII score had a worse OS. In addition, results from subgroup meta-analysis showed the significant prognostic significance of SII in lung cancer. Especially, the predictive value of SII was significant in the multivariable model for NSCLC (HR: 1.97, 95% CI: 1.69–2.25, P < .001; 5 studies, 1746 patients), and SCLC (HR: 1.38, 95% CI: 1.02–1.85, P < .001; 1 study, 919 patients).
Conclusion:
Our data suggest that high SII index indicates poor survival rate in lung cancer. Further researches are warranted to verify the significance of SII index in clinical practice.
Keywords: lung cancer, meta-analysis, prognosis, systemic immune-inflammation index
1. Introduction
Lung cancer is an aggressive disease with high mortality and incidence worldwide. Nonsmall-cell lung cancer (NSCLC) and small cell lung cancer (SCLC) were the 2 pathological types.[1,2] Due to the characteristic of rapid growth and metastases, most of the patients with lung cancer had been in an advanced stage when diagnosed, and the median survival rate for such disease is low and unfavorable.[3–5] Predictive indicators that are closely related to cancer survival could assist clinicians to adopt preventive and therapeutic strategies for patients. Therefore, identifying novel prognostic factors that could be conveniently detected and cheaply available is in need of clinical practice.
Systemic immune inflammation (SII) index, as a novel inflammation-related index, is a comprehensive combination based on the peripheral lymphocyte, neutrophil, and platelet counts. It was calculated as follows: SII = platelet count × neutrophil/lymphocyte count.[6]
SII has been investigated as a marker to predict cancer survival in various tumors, such as hepatocellular carcinoma, gastric cancer, germ-cell tumor, and prostate cancer.[6–12] With regard to lung cancer, the prognostic value of SII was reported in several studies, and most of them showed that SII index could be a biological prognostic biomarker for lung cancer[13–18]; however, Chen et al [19] found that there was no significant association between SII index and survival time in lung cancer. Therefore, owing to the inconsistent results and the limited number of individual studies, we aimed to perform a meta-analysis based on all relevant data to assess the relationship between pretreatment SII index in lung cancer and prognosis.
2. Materials and methods
2.1. Search strategy and study selection
Because the present study is a meta-analysis, the ethical approval is not required. We searched the PubMed, Web of Science, Embase, Chinese National Knowledge Infrastructure (CNKI) ,and Wanfang databases to identify the relevant articles according to the following terms: “systemic-immune-inflammation index” or “neutrophil × platelets/ lymphocyte” or “SII” or “platelet count × NLR,” “lung cancer” or “NSCLC” or “lung tumor” or “SCLC.” The literature search was conducted up to June 1, 2018, and the published language was limited to English or Chinese.
2.2. Inclusion and exclusion criteria
The studies were considered eligible if they met the following criteria:
-
(1)
Studies reported the association between SII index and prognosis in patient with lung cancer.
-
(2)
A definite cutoff-value of pretreatment SII was given.
-
(3)
The hazard ratios (HRs) with 95% confidence intervals (95% CIs) for cancer survival were available.
Articles were excluded if they were reviews or meta-analysis, or not for lung cancer, or involved animal experiments.
2.3. Data extraction and quality assessment
In this meta-analysis, all included studies reported HRs and 95% CIs for OS; among them, 6 studies [13–18] reported the HRs and 95% CIs in multivariate survival analysis for OS, so the HRs and 95% CIs were extracted directly from the studies. And 1 study provided the Kaplan–Meier curve for OS[19]; thus, the Engauge Digitizer version 4.1 (http://digitizer.source forge.net/) was applied to retrieve the HRs and 95% CIs. For progression-free survival (PFS), we did not perform combined analysis in this report for only 1 study reported the relationship between SII and PFS in lung cancer. The Newcastle–Ottawa Scale (NOS) was used to assess the quality of the study, and a study with cumulative scores ≥ 6 was assigned as high-quality.
2.4. Statistical analysis
All the synthesis analyses were carried out using the STATA statistics software (Version 12.0, College Station, Texas, USA). The HRs with its 95% CIs were used to evaluate the prognostic value of SII in lung cancer. Cochran Q test and Higgins I2 statistic were performed for evaluating heterogeneity among studies. Studies with a Phet >.1 and/or I2 < 50% were considered to have low heterogeneity and then the fixed-effects model was applied.
Egger and Begg tests were conducted to assess the publication bias, and the robustness of the results was assessed by sensitivity analysis. Differences were considered statistically significant when P < .05.
3. Results
The detailed selection procedure is listed in Fig. 1. After excluding duplication and reading the texts for further examination, finally, a total of 7 studies [13–19] were proved to be within the scope of the study and all followed an observational design.
Figure 1.
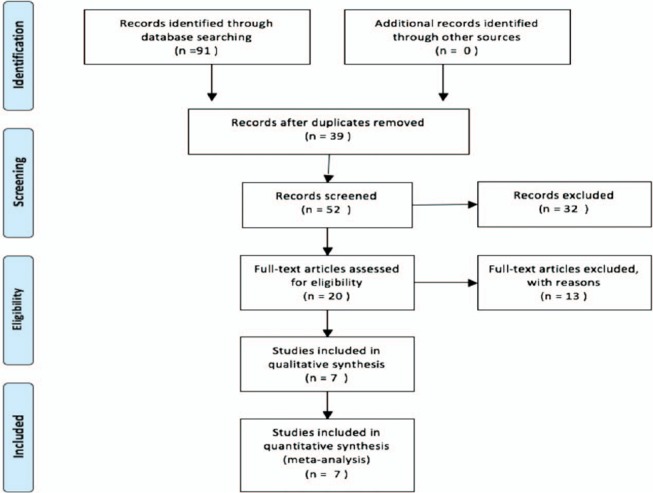
Flow diagram of included studies for this meta-analysis.
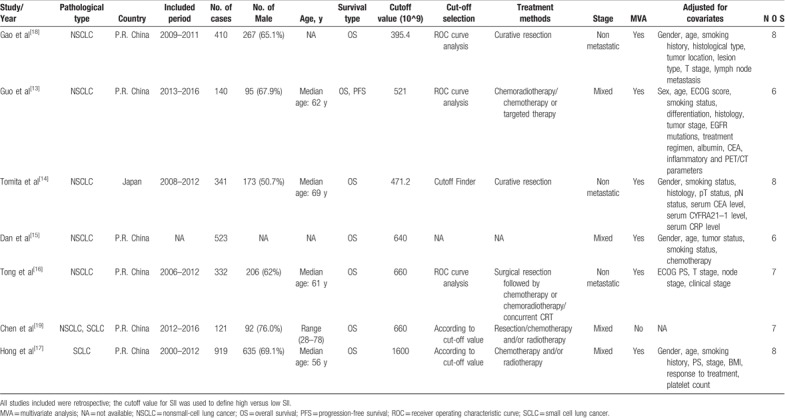
A total of 2786 cases were included in this meta-analysis. With respect to prognostic outcomes, 7 studies reported overall survival (OS) and 1 covered PFS. For the relationship between SII index and OS, 5 studies focused on NSCLC,[13–16,18] and 1 study focused on SCLC,[17] and another one focused on both NSCLC and SCLC.[19] All included studies were from Asian countries (China and Japan). The cut-off value of high SII varies in those studies, ranging from 395.4 to 1600 with the median of 640. The main characteristics of the studies included are summarized in Table 1.
Table 1.
Main characteristics of all included studies.
3.1. SII and OS in lung cancer
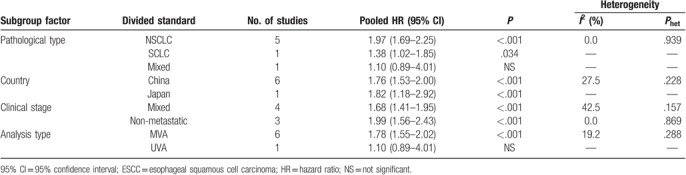
There were 7 studies with a total of 2786 cases exploring the relationship between SII and OS of lung cancer; the combined findings are shown in Fig. 2. Our meta-analysis revealed that a high SII index was significantly correlated with a poor OS in patients with lung cancer (HR: 1.77, 95% CI: 1.54–2.00, P < .001). The result suggested that SII index could predict prognosis of lung cancer. Furthermore, subgroup meta-analysis stratified by the pathological type, country, clinical stage, and analysis type also confirmed the prognostic role of SII in lung cancer (Fig. 3A–D, Table 2).
Figure 2.
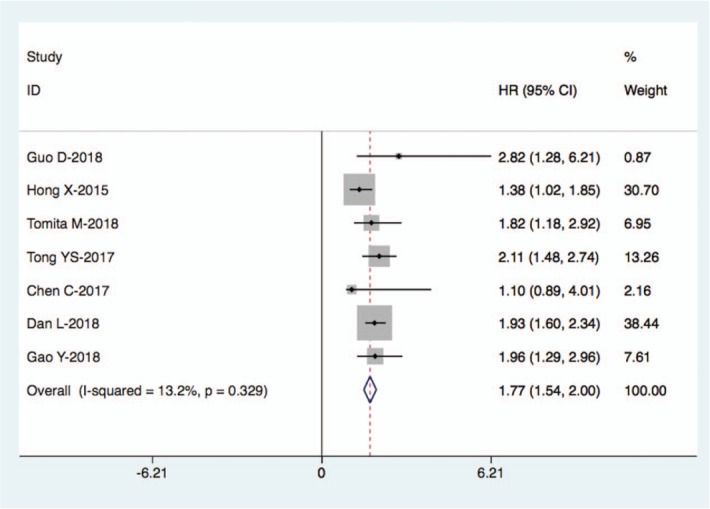
Forest plot for the association between SII and OS in lung cancer.
Figure 3.
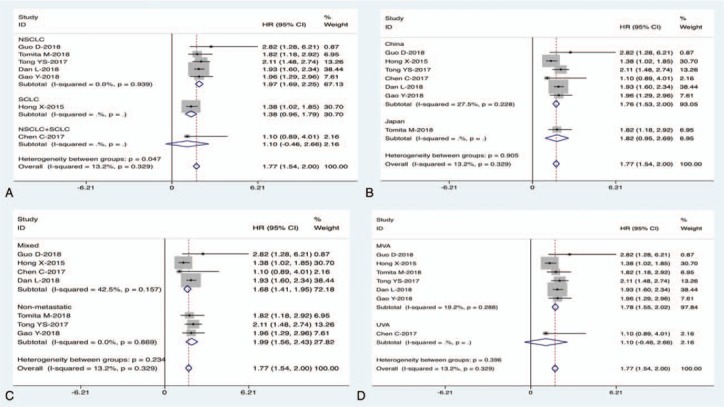
Subgroup meta-analysis of the association between SII and OS in lung cancer.
Table 2.
Subgroup analysis of the relationship between SII and OS in lung cancer.
3.2. Publication bias
The Begg test and Egger test were carried out to assess the potential publication bias; the Begg test (Pr continuitycorrected > |z| = 1.000) and Egger test (P>|t| = 0.822) (Fig. 4) all showed that there was no possibility of publication bias among studies.
Figure 4.
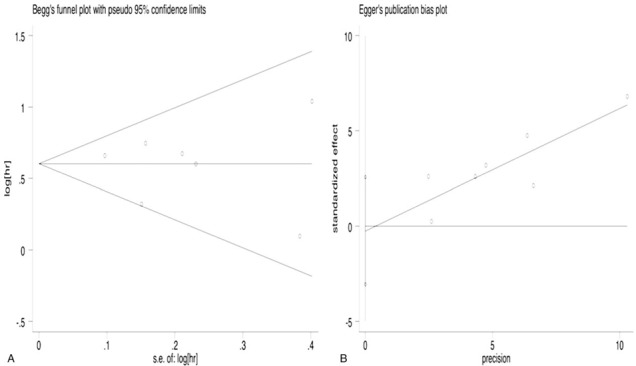
Publication bias assessment. (A) Begg funnel plot. (B) Egger publication bias plot.
3.3. Sensitivity analysis
The sensitivity analysis was performed (Fig. 5), which showed that the pooled results did not change after the removal of any one study.
Figure 5.
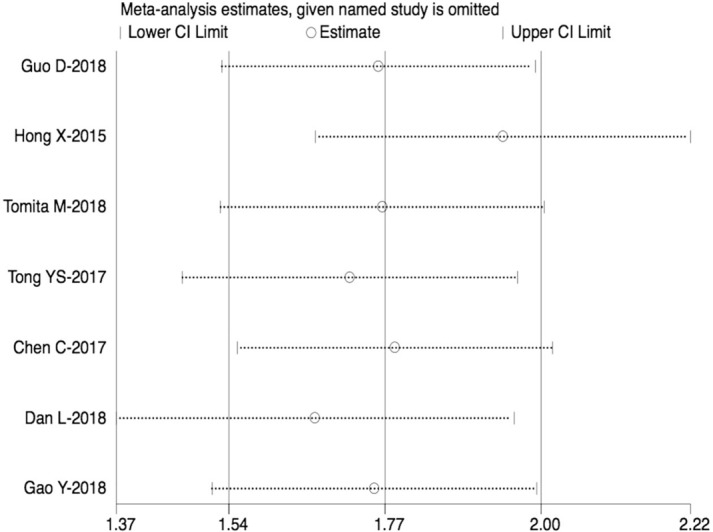
Sensitivity analysis.
4. Discussion
Numerous researches have reported the link between inflammation and cancer and found that cancer-related inflammation is an indispensable component of the tumor microenvironment.[20,21] Inflammatory cycling cells, such as neutrophils, lymphocytes, and platelets, play important roles in the oncogenesis and cancer development.[22–25] These cells could contribute to tumor cell invasion and distant metastasis.[26,27] In recent years, several inflammation-related biomarkers based on the systemic inflammatory cells, such as the platelet-to-lymphocyte ratio (PLR), the lymphocyte-to-monocyte ratio (LMR), and the neutrophil-to-lymphocyte ratio (NLR), have been shown to be of prognostic value in various cancers, including lung cancer.[28–33] However, these predictors are typically based on 2 inflammatory cells, and systemic immune-inflammation index (SII), a novel noninvasive biomarker, which is based on 3 peripheral blood parameters (platelet, neutrophil, and lymphocyte counts), could comprehensively reflect the balance of host immune and inflammatory status and has shown to be a more objective marker with a better predictive reliability for prognosis.[16–18]
To our knowledge, this is the first meta-analysis to investigate the impact of SII index on the survival of patients with lung cancer. On this topic, a total of 7 studies with up to 2786 cases were enrolled after a comprehensive search of literature. From the pooled results, we found that there was a significant association between SII index and prognosis in lung cancer. The patients with high SII index had a shorter OS when compared with the group in low SII index, suggesting that SII index could serve as a promising prognostic factor for patients with lung cancer.
Our meta-analysis has several limitations. First, the studies included were relatively small both in number and overall patients. There was an insufficient number of studies to perform more subgroups analysis for OS. In addition, only 1 study reported the association between SII index and PFS, which showed that SII was an independent factor for PFS in NSCLC; the prognostic value of SII index for PFS still needs to be confirmed by more studies. Third, all included studies were retrospectively designed and all patients from these studies were Asians from China and Japan; more studies conducted in other countries are needed. Fourth, low heterogeneity was observed for OS for the reported HR-adjusted different factors or diverse therapies utilized. In addition, although the SII was significant in the multivariable model for NSCLC and SCLC, the various individual studies were not adjusted for the same outcome-related covariates. At last, the thresholds used by the 7 studies to define high SII were different.
In conclusion, the present meta-analysis identified pretreatment SII index as a prognostic factor for OS in patients with lung cancer. SII index might be a cost-effective and significant biological marker for monitoring of survival in lung cancer. In future, larger multicenter researches should be required to further validate the prognostic value of SII index for lung cancer in clinical practice.
Author contributions
Conceptualization: Xianjin Yang.
Data curation: Yi Zhang, Bo Chen.
Formal analysis: Yi Zhang, Bo Chen.
Investigation: Yi Zhang, Bo Chen, Lijuan Wang, Rong Wang.
Methodology: Yi Zhang, Bo Chen, Lijuan Wang, Rong Wang.
Software: Lijuan Wang, Rong Wang.
Supervision: Xianjin Yang.
Writing – original draft: Yi Zhang, Bo Chen.
Writing – review & editing: Xianjin Yang
Footnotes
Abbreviations: 95% CIs = 95% confidence intervals, CNKI = Chinese National Knowledge Infrastructure, HRs = hazard ratios, LMR = lymphocyte-to-monocyte ratio, MVA = multivariate analysis, NA = not available, NLR = neutrophil-to-lymphocyte ratio, NSCLC = non-small-cell lung cancer, OS = overall survival, PFS = progression-free survival, PLR = platelet-to-lymphocyte ratio, ROC = receiver operating characteristic curve, SCLC = small cell lung cancer, SII = systemic immune-inflammation index.
YZ and BC contributed equally to this paper, they are co-first authors.
The author (s) of this work have nothing to disclose and the authors declare that there are no competing interests regarding the publication of this paper.
References
- [1].Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol 2016;893:1–9. [DOI] [PubMed] [Google Scholar]
- [2].Bareschino MA, Schettino C, Rossi A, et al. Treatment of advanced non small cell lung cancer. J Thorac Dis 2011;3:122–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Moro-Sibilot D, Smit E, de Castro Carpeño J, et al. Outcomes and resource use of non-small cell lung cancer (NSCLC) patients treated with first-line platinum-based chemotherapy across Europe: FRAME prospective observational study. Lung Cancer 2015;88:215–22. [DOI] [PubMed] [Google Scholar]
- [4].Lu H, Jiang Z. Advances in antibody therapeutics targeting small-cell lung cancer. Adv Clin Exp Med 2018;27:1317–23. [DOI] [PubMed] [Google Scholar]
- [5].Govindan R, Bogart J, Vokes EE. Locally advanced non-small cell lung cancer: the past, present, and future. J Thorac Oncol 2008;3:917–28. [DOI] [PubMed] [Google Scholar]
- [6].Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res 2014;20:6212–22. [DOI] [PubMed] [Google Scholar]
- [7].Wang K, Diao F, Ye Z, et al. Prognostic value of systemic immune-inflammation index in patients with gastric cancer. Chin J Cancer 2017;36:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Albany C. Systemic immune-inflammation index in germ-cell tumours: search for a biological prognostic biomarker. Br J Cancer 2018;118:761–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chovanec M, Cierna Z, Miskovska V, et al. Systemic immune-inflammation index in germ-cell tumours. Br J Cancer 2018;118:831–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fan L, Wang R, Chi C, et al. Systemic immune-inflammation index predicts the combined clinical outcome after sequential therapy with abiraterone and docetaxel for metastatic castration-resistant prostate cancer patients. Prostate 2018;78:250–6. [DOI] [PubMed] [Google Scholar]
- [11].Zhong JH, Huang DH, Chen ZY. Prognostic role of systemic immune-inflammation index in solid tumors: a systematic review and meta-analysis. Oncotarget 2017;8:75381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tang JN, Goyal H, Yu SS, et al. Prognostic value of systemic immune-inflammation index (SII) in cancers: a systematic review and meta-analysis. J Lab Precis Med 2018;3:29. [Google Scholar]
- [13].Guo D, Zhang J, Jing W, et al. Prognostic value of systemic immune-inflammation index in patients with advanced non-small-cell lung cancer. Future Oncol 2018;14:2643–50. [DOI] [PubMed] [Google Scholar]
- [14].Tomita M, Ayabe T, Maeda R, et al. Systemic immune-inflammation index predicts survival of patients after curative resection for non-small cell lung cancer. In Vivo 2018;32:663–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dan L, Xia Y, He Y, et al. Prognostic value of systemic immune inflammation index in advanced non small cell lung cancer. Am J Respir Crit Care Med 2018;197:A7331. [Google Scholar]
- [16].Tong YS, Tan J, Zhou XL, et al. Systemic immune-inflammation index predicting chemoradiation resistance and poor outcome in patients with stage III non-small cell lung cancer. J Transl Med 2017;15:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hong X, Cui B, Wang M, et al. Systemic immune-inflammation index, based on platelet counts and neutrophil-lymphocyte ratio, is useful for predicting prognosis in small cell lung cancer. Tohoku J Exp Med 2015;236:297–304. [DOI] [PubMed] [Google Scholar]
- [18].Gao Y, Zhang H, Li Y, et al. Preoperative increased systemic immune-inflammation index predicts poor prognosis in patients with operable non-small cell lung cancer. Clin Chim Acta 2018;484:272–7. [DOI] [PubMed] [Google Scholar]
- [19].Chen C, Ge P, Bai YP, et al. CRP/Alb ratio in the prognosis of lung cancer. Lab Med 2017;32:173–7. [Google Scholar]
- [20].Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001;357:539–45. [DOI] [PubMed] [Google Scholar]
- [21].Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature 2008;454:436–44. [DOI] [PubMed] [Google Scholar]
- [22].Lerman I, Hammes SR. Neutrophil elastase in the tumor microenvironment. Steroids 2018;133:96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Vidal AC, Howard LE, de Hoedt A, et al. Neutrophil, lymphocyte and platelet counts, and risk of prostate cancer outcomes in white and black men: results from the SEARCH database. Cancer Causes Control 2018;29:581–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chou C, Li MO. Re (de)fining innate lymphocyte lineages in the face of cancer. Cancer Immunol Res 2018;6:372–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Leblanc R, Houssin A, Peyruchaud O. Platelets, autotaxin and lysophosphatidic acid signaling: win-win factors for cancer metastasis. Br J Pharmacol 2018;175:3100–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Grivennikov SI, Greten FR, Karin M. Immunity, inflammation and cancer. Cell 2010;140:883–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Contursi A, Sacco A, Grande R, et al. Platelets as crucial partners for tumor metastasis: from mechanistic aspects to pharmacological targeting. Cell Mol Life Sci 2017;74:3491–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Minami S, Ihara S, Kim SH, et al. Lymphocyte to monocyte ratio and modified Glasgow prognostic score predict prognosis of lung adenocarcinoma without driver mutation. World J Oncol 2018;9:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Liu D, Jin J, Zhang L, et al. The neutrophil to lymphocyte ratio may predict benefit from chemotherapy in lung cancer. Cell Physiol Biochem 2018;46:1595–605. [DOI] [PubMed] [Google Scholar]
- [30].Toda M, Tsukioka T, Izumi N, et al. Platelet-to-lymphocyte ratio predicts the prognosis of patients with non-small cell lung cancer treated with surgery and postoperative adjuvant chemotherapy. Thorac Cancer 2018;9:112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Xia H, Sun Z, Deng L, et al. Prognostic significance of the preoperative lymphocyte to monocyte ratio in patients with stage I non-small cell lung cancer undergoing complete resection. Cancer Invest 2016;34:378–84. [DOI] [PubMed] [Google Scholar]
- [32].Kang KH, Efird JT, Sharma N, et al. Prognostic potential of neutrophil-to-lymphocyte ratio and lymphocyte nadir in stage III non-small-cell lung cancer. Future Oncol 2017;13:1405–14. [DOI] [PubMed] [Google Scholar]
- [33].Käsmann L, Bolm L, Schild SE, et al. Neutrophil-to-Lymphocyte ratio predicts outcome in limited disease small-cell lung cancer. Lung 2017;195:217–24. [DOI] [PubMed] [Google Scholar]


