Abstract
Background and Aims
Diagnosis and surveillance of Barrett’s esophagus (BE) and eosinophilic esophagitis (EoE) have become emerging public health issues. Cytosponge® is a novel, minimally invasive esophageal cell collection device. We aimed to assess the data on safety and acceptability of this device.
Methods
We performed a patient-level review of 5 prospective trials assessing Cytosponge® performance in patients with reflux disease, BE and EoE in primary and secondary care. Acceptability of Cytosponge® and subsequent endoscopy were recorded with visual analogue scale (VAS), wherein 0 and 10 denoted lowest and highest acceptability. Median VAS scores were compared using a Mann-Whitney test. The number of attempts, failures in swallowing the device and occurrence of adverse events were analyzed. Risk factors for failure in swallowing were analyzed using a multivariate regression model.
Results
In total 2,672 Cytosponge® procedures were performed in 2,418 individuals between 2008 and 2017. There were two adverse events related to the device: a minor pharyngeal bleed and one case of detachment (<1:2,000). The median acceptability score for the Cytosponge® was 6.0 (IQR 5.0–8.0), which was higher than endoscopy without sedation (median 5.0, IQR 3.0–7.0; p<0.001) and lower than endoscopy with sedation (median 8.0, IQR 5.0–9.0; p<0.001). Nearly all patients (91.1%) successfully swallowed the Cytosponge® and most (90.1%) were achieved with the first swallow attempt. Failure to swallow the device was more likely to occur in secondary care (OR= 5.13, 95%CI 1.48–17.79, P<0.01).
Conslusions
The Cytosponge® test is a safe procedure with good acceptability ratings in a variety of health care settings.
Keywords: Medical device, Acceptability of Healthcare, Safety
INTRODUCTION
Two chronic esophageal diseases - Barrett’s esophagus (BE) and eosinophilic esophagitis (EoE) - have become emerging issues in the public health over the last several decades1,2,3.
BE develops on the background of long-standing gastro-esophageal reflux disease (GERD) and is defined as a metaplastic change in the esophageal lining, from a squamous-type epithelium to a specialized columnar epithelium. The estimated population prevalence of BE is 1– 2%4. BE is a major risk factor for esophageal adenocarcinoma (EAC) - a cancer with rapidly increasing incidence in the Western world5. Patients with chronic GERD and other risk factors (male sex, age of ≥ 50 years, white race, family history of BE or EAC) may be offered endoscopic screening for the presence of BE6, however most BE cases remain undiagnosed. Patients with the benefit of a BE diagnosis undergo endoscopic surveillance with the aim to identify neoplastic changes within BE segment at the earliest possible stage7,8,9. Such patients are candidates for endoscopic treatment with either endoscopic mucosal resection (EMR) or radiofrequency ablation (RFA)10,11 with excellent survival results for intra-mucosal disease12.
EoE, on the other hand, is a relatively newly defined immune-mediated disease characterized by predominant eosinophilic inflammation of the esophagus (a peak count of ≥ 15 eosinophil per high-power field of biopsy tissue)13. EoE is seen predominantly in younger men, however it affects all age groups and both sexes14,15. It is one of the most common condition in adult patients leading to food bolus impaction. As with BE, most cases of EoE are undiagnosed, and its incidence rate is reaching up to 12.8 /100,000 / year in some regions of the US16. The aim of diagnosis and treatment is to control the symptoms, resolve esophageal eosinophilia, and reduce complications.
Although the nature of these two entities is highly disparate, both require long-term, endoscopic monitoring and repeated collection of mucosal samples to optimize and monitor the treatment. To perform systematic screening and surveillance for these conditions would constitute a huge burden on health care systems. A survey study analyzing trends in endoscopic volume in the US showed that there was a 54% increase in upper GI endoscopy between 2000 and 2009, with an estimated number of 6.9 million of these procedures performed in 200917. The rising incidence of BE and EoE may have contributed to these numbers. Patients with EoE alone have an estimated annual health-care cost of as much as $1.4 billion in the US18.
While diagnostic esophagogastroduodenoscopy (EGD) is considered to be a safe procedure, it is not devoid of complications. The overall mortality rates for EGD are ranging from none to 1 in 2,000 in various studies19. Perforation, a potentially life-threatening complication, is reported to occur from 1 in every 2,500 to 1 in every 11,000 procedures20,21. Moreover, many of the EGDs in the US and Europe are performed under sedation, exposing patients to additional risks. These include cardiopulmonary complications, which account for as much as 60% of endoscopy adverse events and an incidence ranging between 1 in 170 and 1 in 10,00022.
Therefore, new, less invasive methods of esophageal mucosal sampling are being investigated. Cytosponge® is a novel, minimally invasive cell collection device that consists of a 30-mm polyurethane sponge, contained within a capsule attached to a string. When withdrawn, the device collects esophageal cells for analysis (Figure 1A). The procedure requires minimal training and can be safely administered by a nurse in a primary care setting. Cytosponge® has already been successfully used in several studies to identify BE and EoE23,24,25. The cells retrieved from the sponge are spun down and embedded to produce a pseudo-biopsy suitable for routine laboratory analysis (Figure 1B-D). To aid the identification of BE, the histopathological analysis is coupled with a diagnostic biomarker, Trefoil Factor-3 (TFF-3); Figure 1C. Of note, the utility of the Cytosponge® goes beyond the confines of BE and EoE diagnosis since a range of pathologies affecting the esophagus and proximal stomach, such as esophageal candidiasis, esophageal ulcers, H.pylori infection, intestinal metaplasia at the cardia and viral esophagitis can also be diagnosed26.
Figure 1.
A. Cytosponge® in gelatin capsule (right) and expanded (left).
B, C. Haematoxylin and eosin (B) and trefoil-factor 3 (C) staining (20×) from patient with Barrett’s oesophagus showing columnar lined epithelium with goblet cells (arrowheads) (courtesy of dr Maria O’Donovan)
D. Haematoxylin and eosin staining (200×) from patient with eosinophilic oesophagitis showing squamous epithelium with admixed eosinophils (arrowheads)
The aim of this study was to combine data from 5 large trials on Cytosponge® performed in patients with chronic GERD, BE and EoE in 3 different countries (UK, USA and Australia) to assess the overall safety and acceptability of this test.
METHODS
Study design and study participants
This was a retrospective, patient-level technical review of prospectively collected data. Studies included in the analyses were the Barrett’s ESophagus Trial 1 (BEST1)24, BEST225, BEST-Australia, the ongoing BEST2-RFA study (ClinicalTrials.gov number NCT02106910) and Cytosponge® Eosinophilic Esophagitis study (EoE Study, NCT02114606)23. Principal investigators of each trial shared the original trial databases. All studies were conducted with the use of Cytosponge® approved by the UK Medicines and Healthcare Products Regulatory Agency (MHRA).
Briefly, the setting and patients’ eligibility criteria of each study were as follows:
BEST1: individuals with chronic GERD managed in primary care with long-term PPI (>3 months).
BEST2: patients with previously diagnosed BE (cases) and patients with GERD without BE (control group) referred to the secondary care unit for endoscopy.
BEST-Australia: patients with chronic GERD symptoms referred for endoscopy in a secondary care unit.
BEST2-RFA: patients with BE with low-grade dysplasia (LGD) or high-grade dysplasia (HGD), who received radiofrequency ablation (RFA) or are under surveillance following ablative treatment.
EoE study: patients with EoE referred for the secondary care unit to undergo clinically indicated endoscopy.
Exclusion criteria were generally consistent between studies and included bleeding disorders, known cirrhosis +/− varices, history of esophageal surgery, dysphagia and esophageal stricture. An overview of study characteristics is presented in Table 1.
Table 1.
Characteristics of Cytosponge® studies included in the analysis
| Study 1 (BEST1) |
Study 2 (BEST2) |
Study 3 (BEST-Australia) |
Study 4 (BEST2-RFA) |
Study 5 (EoE) |
|
|---|---|---|---|---|---|
| Country: | UK | UK | Australia | USA | USA |
| Disease: | GERD | GERD and BE | GERD | BE after RFA treatment | EoE |
| No. of patients (%): | 518 (21.4%) | 1,498 (62.0%) | 224 (9.3%) | 76 (3.1%) | 102 (4.2%) |
| No. of Cytosponge® procedures (%): |
518 (19.4%) | 1,752 (65.6%) | 224 (8.4%) | 76 (2.8%) | 102 (3.8%) |
| Time of recruitment: | May 2008 – Dec 2009 |
July 2011 – Dec 2013 |
May 2010 – August 2014 |
October
2014 –present (ongoing) |
December 2012– present (ongoing) |
| Inclusion criteria: | • 50 – 70 yrs. • Prescription of acid suppressants for>3 months |
• Cases: BE under
surveillance • Controls: GERD referred for endoscopy |
• 50 – 70 yrs. • Prescription of acid suppressants for>3 months |
• 18 –
80 yrs. • BE with LGD / HGD after successful RFA treatment |
• 18 – 65 yrs. • EoE undergoing endoscopy |
| Setting: | Primary care (12 general practices) | Secondary care (11 hospitals) | Secondary care (1 hospital) |
Secondary care (1 hospital) | Secondary care (2 hospitals) |
| Time between Cytosponge® and endoscopy | Up to 3 weeks | Same day (within an hour) |
Same day | Same day | Same day (2 hours prior to endoscopy) |
BE, Barrett’s esophagus; EoE, eosinophilic esophagitis; GERD, gastro-esophageal reflux disease; HGD, high-grade dysplasia; LGD, low-grade dysplasia; RFA, radiofrequency ablation
Cytosponge® Procedure
The Cytosponge® was administered in a similar fashion in each trial by trained research nurses. After swallowing the device in sitting position, the capsule coating disintegrates within 5 minutes upon reaching the stomach, revealing a 3-cm diameter spherical mesh that is withdrawn by pulling the string. Following its retrieval, the string is cut, the Cytosponge® is then immersed in SurePath Preservative Fluid (TriPath Imaging, Burlington, North Carolina, USA) and kept at 4oC until transported to the laboratory for processing. Hematoxilin Eosin (H&E) staining and immunohistochemistry for TFF-3 is then performed on paraffin-embedded Cytosponge® specimens by adhering to standard H&E and TFF3 protocols on a BOND-MAX autostainer (Leica Biosystems, Newcastle Upon Tyne, UK).
Outcome measures
Acceptability of the Cytosponge® and subsequent endoscopy (regardless of sedation) was recorded using a visual analogue scale (VAS), wherein 10 indicated the best and 0 the worst experience. Patients in secondary care studies (BEST2, BEST-Australia, EoE Study, BEST2-RFA) underwent the Cytosponge® and endoscopy on the same day, whereas patients from BEST1 (primary care) had their endoscopy scheduled within three weeks and the acceptability score for endoscopy was not recorded. Number of swallow attempts and failure in swallowing the Cytosponge® were noted. ‘Failure to swallow’ was stated when the device could not be swallowed despite three attempts. Patients in BEST2 and EoE study had repeated Cytosponge® tests. All serious adverse events (SAE) were reported in accordance to the Good Clinical Practice guidelines. Minor events, such as sore throat, were not systematically recorded.
Cytosponge® abrasions grading system
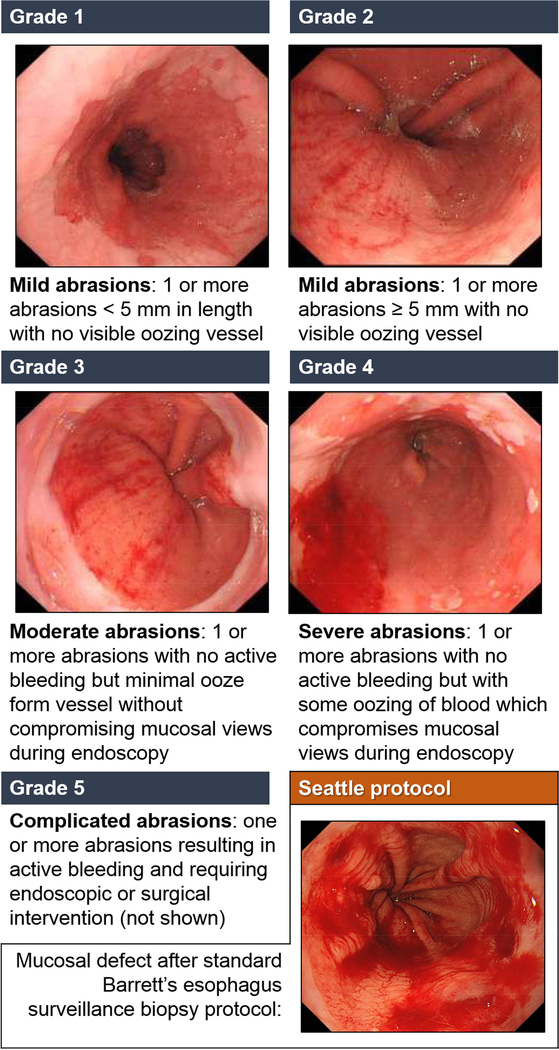
An abrasion grading system was introduced to categorize the severity of abrasions following the Cytosponge® procedure. The presence and degree of abrasions were recorded during subsequent EGD. Abrasions provide useful information on the most distal passage of the device (important for diagnosing BE) as well as a comparator with biopsies for the bleeding risk. The grading system is presented in Figure 2.
Figure 2.
The abrasion grading system after Cytosponge®
Statistical Analysis
Statistics for continuous variables were expressed as medians and interquartile ranges (IQRs). The Mann-Whitney test was used to compare continuous variables between groups. The association between failure in swallowing the Cytosponge® and risk factors was analyzed using multivariable regression model. We reported odds ratios (OR) and 95% confidence intervals (CI) adjusted for patient’s sex, study setting, BMI and indication. All statistical tests were two-sided. For all analyses, P value of less than 0.05 was considered statistically significant. All analyses were performed using R Statistics version 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Patient Demographics
In total, data on 2,418 patients from 5 studies between May 2008 and August 2017 were analyzed. Eighty-four patients were unable to swallow the Cytosponge® (3.5%) and 50 were withdrawn due to study eligibility (2.1%), leaving 2,284 patients who successfully underwent the Cytosponge® test. The study cohort comprised of 518 BEST1 patients (21.4%), 1,498 BEST2 patients (62.0%), 224 BEST-Australia patients (9.3%), 76 BEST2-RFA patients (3.1%), and 102 EoE study patients (4.2%).
There were 1,329 patients with GERD (56.7%), 987 patients with previously diagnosed BE (40.8%; 911 from BEST2 and 76 from BEST2-RFA) and 102 patients with EoE (4.2%). The median age was 62 years (IQR 54–68) and the male to female ratio was 1.7:1.0. The median body mass index (BMI) was 28.2 kg/m2 (IQR 25.1–31.5), indicating that most patients were overweight. The median waist-to-hip ratio for females was 0.86 (IQR 0.81–0.91) and for males it was 0.96 (IQR 0.92–0.99). Smoking status was recorded for 1,971 patients. Of these, 809 were reported as lifetime non-smokers (41.0%), 971 as former smokers (49.2%) and 191 as active smokers (9.7%). Majority of patients who underwent endoscopy had been diagnosed with hiatus hernia (53.7%). Combined demographic data is presented in Table 2.
Table 2.
Demographic characteristics of patients from Cytosponge® studies. Values are numbers (percentages) unless stated otherwise
| Characteristics | All participants* | Men** | Women** |
|---|---|---|---|
| Age (years) - median (IQR) | 62 (54–68) | 63 (54–69) | 61 (54–67) |
| Missing data | 153 (6.3) | 119 (12.8) | 36 (2.4) |
| Number of participants | |||
| All studies | 2,418 (100) | 1,486 (61.5) | 932 (38.5) |
| Study 1 (BEST1 Study) | 518 (21.4) | 240 (46.3) | 278 (56.7) |
| Study 2 (BEST2 Study) | 1,498 (62.0) | 1,035 (69.1) | 463 (30.9) |
| Study 3 (BEST Study Australia) | 224 (9.3) | 95 (42.4) | 129 (57.6) |
| Study 4 (POST-RFA Study) | 76 (3.1) | 58 (76.3) | 18 (23.7) |
| Study 5 (EoE Study) | 102 (4.2) | 58 (56.9) | 44 (43.1) |
| Indication to Cytosponge® | |||
| GERD | 1,329 (55.0) | 632 (47.6) | 697 (52.4) |
| BE | 987 (40.8) | 796 (80.6) | 191 (19.4) |
| EoE | 102 (4.2) | 58 (56.9) | 44 (43.1) |
| Body Mass Index (BMI, kg/m2) | |||
| Median (IQR) | 28.2 (25.1–31.5) | 28.1 (25.6–31.0) | 28.6 (24.8–33.1) |
| Underweight (<18.5) | 14 (0.6) | 12 (85.7) | 2 (14.3) |
| Normal (18.5 to 24.9) | 447 (18.5) | 185 (41.4) | 262 (58.6) |
| Overweight (25.0 to 29.9) | 853 (35.3) | 236 (27.7) | 617 (72.3) |
| Obese (≥30.0) | 739 (30.6) | 313 (42.4) | 426 (57.6) |
| Missing data | 365 (15.0) | 186 (51.0) | 179 (49.0) |
| Waist to Hip Ratio*** | |||
| Median (IQR) | 0.93 (0.87–0.98) | 0.96 (0.92–0.99) | 0.86 (0.81–0.91) |
| Low Risk | 786 (32.5) | 622 (79.1) | 164 (20.9) |
| Moderate Risk | 558 (23.1) | 379 (67.9) | 179 (32.1) |
| High Risk | 626 (25.9) | 244 (39.0) | 382 (61.0) |
| Missing data | 448 (18.5) | 241 (53.8) | 207 (46.2) |
| Smoking Status | |||
| Never | 809 (33.5) | 466 (57.6) | 343 (42.4) |
| Former | 971 (40.2) | 630 (64.9) | 341 (35.1) |
| Active | 191 (7.9) | 133 (69.6) | 58 (30.4) |
| Missing data | 447 (18.5) | 257 (57.5) | 190 (42.5) |
| Hiatus hernia | |||
| Present | 1,191 (49.3) | 825 (69.3) | 366 (30.7) |
| Absent | 1,025 (42.4) | 538 (52.5) | 487 (47.5) |
| Missing data | 202 (8.3) | 123 (60.9) | 79 (39.1) |
| Previous endoscopic treatment (EMR, RFA, PDT) | |||
| Yes | 243 (10.0) | 204 (84.0) | 39 (16.0) |
| No | 2,175 (90.0) | 1,282 (58.9) | 893 (41.1) |
EMR, endoscopic mucosal resection; PDT, photo-dynamic therapy; RFA, radiofrequency ablation
The proportion (%) of patients from each group in the first column refers to the total participant number
The proportion (%) of male and female patients refers to the number of participants from each group (first row), not the total participant number
Waist to hip ratio was considered low risk for male <0.95 and female <0.80, moderate risk for male 0.95–1, female 0.81–0.85 and high risk for male >1, female >0.85
Cytosponge® Acceptability
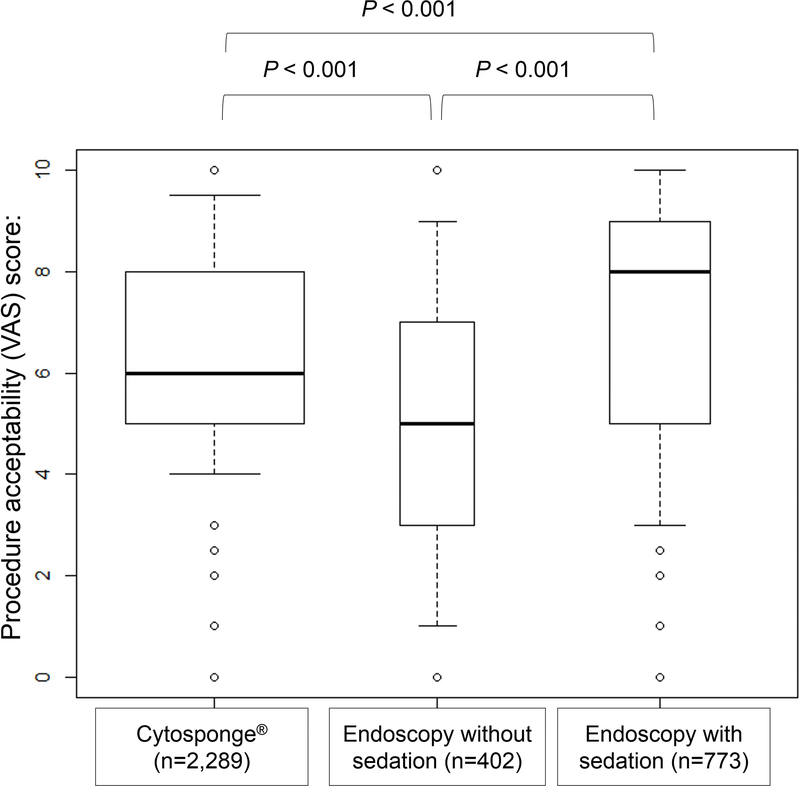
Overall, 2,672 Cytosponge® were performed, of which 2,289 had acceptability score recorded (85.7%). The endoscopy acceptability score was recorded for 1,406 procedures in 1,221 patients. Of these, 1,175 endoscopies included data on sedation (96.2%), indicating that 402 EGD’s were performed without sedation (34.2%) and 773 with sedation (65.8%).
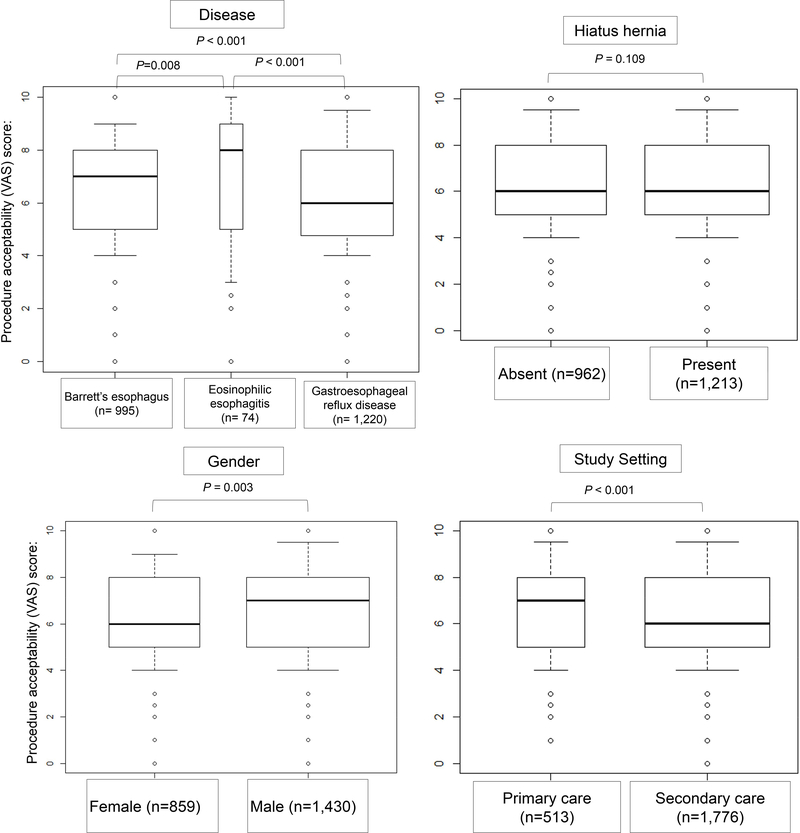
The overall acceptability for the Cytosponge® was satisfactory, with a median score of 6.0 (IQR 5.0–8.0). This was significantly higher when compared to endoscopy without sedation with median VAS score of 5.0 (IQR 3.0–7.0) (P<0.001), but still comparatively lower than endoscopy with sedation (VAS 8.0, IQR 5.0–9.0)(P<0.001); see Figure 3. EoE patients had the highest acceptability for the test (VAS 8.0, IQR 5.1–9.0), as compared to patients with BE [VAS 7.0 (IQR 5.0–8.0)] and GERD [VAS 6.0 (IQR 4.9–8.0)]; P<0.001 for both comparisons. The presence of hiatus hernia did not influence the acceptability score (P=0.109). Males had higher acceptability than females [median 7.0 (IQR 5.0–8.0) vs 6.0 (IQR 5.0–8.0), P=0.003], as did patients in primary care setting, when compared to patients in secondary care (7.0 [IQR 5.0–8.0] vs. 6.0 [IQR 5.0–8.0], P<0.001). See Figure 4.
Figure 3.
Cytosponge® and endoscopy acceptability (per-procedure)
Figure 4.
Acceptability scores for the Cytosponge® in different groups of patients (per-procedure).
Failure to swallow the Cytosponge®
Eighty-four patients failed to swallow the Cytosponge® (3.5%). The proportion of patients who were unable to swallow the device was over 2-times higher within BE patients than in GERD patients (5.7% vs 2.1%) and slightly higher within males as compared to females (3.9% vs 2.7%). All EoE patients successfully swallowed the device. The majority of successful tests were achieved with the first swallow attempt (90.1%). Using a multivariable regression model, we found that patients examined in secondary care setting were over 5-times more likely to fail swallowing the device as compared to primary care setting (OR= 5.13, 95% CI 1.48–17.79, P<0.01). High BMI and gender were not associated with rates of failure in swallowing. Patients with previously diagnosed chronic diseases (BE and EoE combined) had a similar risk of swallow failure, when compared to patients with GERD. Supplementary table 1 presents the multivariable regression model results.
Cytosponge® adverse events
Overall, of the 2,672 Cytosponge® tests performed, there were 12 SAE reported, of which only 2 could be directly attributed to the Cytosponge® (<1: 2,000). These included one detachment of the sponge and one pharyngeal bleeding after Cytosponge® withdrawal. The others were related to endoscopic therapy performed immediately after the Cytosponge® test (see Supplementary table 2). As sore throat is a frequent event following endoscopy, we did not consider it an AE and the data was not collected systematically across all studies. No late AE, such as strictures have been reported.
Cytosponge® detachment occurred in a 76-year-old male patient with BE in the BEST2-RFA study at the University of North Carolina. The patient did not report any discomfort when the device was retained. Since the Cytosponge® test was performed in the secondary care setting, it was retrieved endoscopically on the same day. The detached device was found in the pylorus and was successfully retrieved with a Roth net without further adverse consequences for the patient.
There was one case of mild pharyngeal bleeding in a patient from BEST2 study. The patient was on warfarin for atrial fibrillation, that was stopped prior to the procedure (INR was 1.2). The bleeding resolved spontaneously and there was no drop in Haemoglobin levels. He was hospitalized as a precautionary measure and was discharged home the next day.
Cytosponge® abrasions
A Cytosponge® abrasions grading system was devised in November 2011. It categorizes abrasions into five categories based on visual appearance of abrasions during endoscopy. This grading system was used in BEST2, BEST2-RFA and EoE Study. Overall, 1,075 Cytosponge® procedures were followed by an endoscopy with abrasion score assessment. In most of the cases (85.5%, 919/1075) Cytosponge® caused no or only mild abrasions (grade 0–2). There were only 24 cases (2.2%) of severe post-Cytosponge® abrasions (Grade 4) and no cases of grade 5 abrasions that required endoscopic or surgical intervention. Of note, Cytosponge® abrasions, even at the highest grade of 4, appear less severe when compared to current standard of care (quadrantic biopsies obtained every 2 cm - Seattle protocol27), as pictured in Figure 2.
DISCUSSION
This technical review of five large prospective studies on the performance of the Cytosponge® showed that it is a safe procedure with good acceptability ratings. The test can be safely performed by a nurse in both the primary and secondary care setting, with minimal risk of AE. The Cytosponge® test was feasible when used for screening purposes (GERD patients with high-risk for BE), as well as for surveillance (EoE and BE after endoscopic treatment).
Safety is paramount for any procedure especially when being performed in the primary care setting. Our review showed that of 2,672 Cytosponge® procedures there were only two SAE that could be directly attributed to the device (<1: 2,000) and both resolved without any ill-effects for the patient. The detachment is the most concerning risk factor to both clinicians and patients28. However, a retained sponge in the stomach would not be expected to cause any symptoms as was the case in the patient reported here. Since objects greater than 2–2.5cm in diameter do not pass through the pylorus29, we expect the expanded sponge (which has a diameter of 3 cm) to stay in the stomach after detachment and since endoscopy is widely available, retrievable should be easily arranged in case of this unlikely event.
In a recent perspective article, it was reported that the Cytosponge® had been recalled due to two cases of detachment in the CASE1 study (FDA Recall Z-2123–2016)30. We would like to emphasize that the above article refers to an alternative prototype device developed by Covidien GI Solutions (now Medtronic), not the original prototype patented by the Medical Research Council (MRC) UK, which was used in all the studies reported here. FDA and CE marking of the original device is underway [Cytosponge® received 510(k) clearance from the FDA on November 26, 2014 (K142695)].
Previous interview-based, quality study on 33 participants with GERD showed that Cytosponge® is acceptable for most participants, as well as being preferred to endoscopy28. In our study, most patients (79.3%) scored their experience as at least “neutral” (VAS≥5) and the median VAS score was 6.0 (IQR 5.0–8.0). This was significantly higher when compared to endoscopy without sedation (VAS 5.0, IQR 3.0–7.0), however lower than endoscopy with sedation (VAS 8.0, IQR 5.0–9.0, P<0.001 for both comparisons). It must be stressed, that the Cytosponge® has other advantages as a screening tool, when compared to the latter. Endoscopy with sedation is an invasive, time-consuming procedure (usually several hours including recovery time), that requires the patient to avoid work and operating machinery for the subsequent 24 hours. Cytosponge® can be performed in 5–7 minutes, within a primary care office, and (usually) does not involve any restrictions for the remaining part of the day.
Our review shows that patients with previously diagnosed chronic esophageal conditions (BE and EoE) have a higher acceptability rating for Cytosponge® as compared to patients with GERD (P<0.001). Supposably, these patients are more aware of the importance of undergoing regular monitoring and are more used to repeated endoscopic examinations, which might explain the higher degree of acceptability. Patients examined in the primary care setting (n= 518), had markedly higher acceptance, as compared to patients examined in the secondary care (n=2,154). The unequal size of the groups could be a confounding factor. Nevertheless, we postulate that the more patient-friendly environment and individual approach of a primary care setting benefits the overall acceptability of the test. These results are promising, since the Cytosponge® was developed to be a minimally invasive test for use in a primary-care offices.
Prior to implementation in clinical practice, randomized trial data is required to fully evaluate the diagnostic yield of Cytosponge® and further evaluate its safety, acceptability and health economic outcomes. This is currently underway in the Barrett’s ESophagus Trial 3 [(BEST3); trial ID ISRCTN68382401], a 10,000-patient cluster randomized controlled trial which is being conducted in multiple UK primary care surgeries (more information: https://www.best3trial.org/the-best3-trial, funded by Cancer Research UK).
The main strength of the study is the direct access to original dataset to minimize missing data and ensure high quality of the statistical analyses. The studies were undertaken in several countries, for different indications and in different health care settings. This study does have some limitations. There were comparatively fewer acceptability scores recorded for endoscopy than the Cytosponge®. This was because patients enrolled onto the BEST1 trial did not have the acceptability score recorded following endoscopy. Furthermore, the VAS scale is a crude measure of acceptability and further quantitative and qualitative interviews will be required to fully understand the patient experience. Some of the studies included in this analysis had more complex tools to measure patients’ experience, such as Impact Event Score or Spielberger state trait anxiety inventory, however we did not include it in this analysis since they were not used in all the studies.
CONCLUSIONS
In conclusion, in this first review of clinical data on safety and acceptability of Cytosponge® we have demonstrated that this device has a favourable safety and acceptability profile. The relative ease of administration and the higher safety profile as compared to endoscopy makes it a promising tool to be used in the primary care setting as a screening and surveillance test for esophageal disorders such as BE or EoE. Results from the ongoing BEST3 randomized trial will be critical prior to implementing the Cytosponge® test for widespread use.
Supplementary Material
Supplementary Table 1. Multivariate analysis model for failure of swallowing the Cytosponge®
Supplementary Table 2. Combined adverse events from all studies included in the analysis
Acknowledgement
We would like to thank Natasha Di Costanzo, Ashan Green and Alberto Stella for help in data extraction and Paulina Wieszczy and Sarah Killcoyne and for help with statistics analyses.
Grant support: The BEST1 study was funded by the MRC gap fund. The BEST2 study was funded by Cancer Research UK. The BEST Australia study was supported by Cancer Australia.
Abbreviations
- AE
adverse events
- BE
Barrett’s esophagus
- EAC
esophageal adenocarcinoma
- EGD
esophagogastroduodenoscopy
- EMR
endoscopic mucosal resection
- EoE
eosinophilic esophagitis
- GERD
gastro-esophageal reflux disease
- HGD
high-grade dysplasia
- LGD
low-grade dysplasia
- RFA
radiofrequency ablation
- SAE
serious adverse events
- VAS
visual analogue scale
Footnotes
Conflict of Interest: The data presented herein all relate to the in-house (design and local manufacture) produced Cytosponge®. The Cytosponge® is licensed by the Medical Research Council (MRC) to Covidien GI Solutions (now owned by Medtronic) and they are in the process of developing an FDA approved and CE marked device. Medtronic are not privy to the contents of this manuscript prior to publication. Rebecca Fitzgerald, Sudarshan Kadri and Pierre Lao-Sirieix are named inventors on patents related to the Cytosponge®. Evan S. Dellon has received research funding from NIH, ACG, AGA, Adare, Meritage, Miraca, Nutricia, Celgene/Receptos, Regeneron, Shire, CURED, UNC/NC TraCS, consulting fees from Adare, Alivio, Banner, Enumeral, GSK, Celegene/Receptos, Regeneron, Robarts Clnical Trials, Shire and educational grant from Banner, Holoclara. All of the remaining authors disclose no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coleman HG, Bhat S, Murray LJ, et al. Increasing incidence of Barrett’s oesophagus: a population-based study. European journal of epidemiology 2011;26:739–745. [DOI] [PubMed] [Google Scholar]
- 2.van Soest EM, Dieleman JP, Siersema PD, et al. Increasing incidence of Barrett’s oesophagus in the general population. Gut 2005;54:1062–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dellon ES, Hirano I. Epidemiology and Natural History of Eosinophilic Esophagitis. Gastroenterology 2018;154:319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ronkainen J, Aro P, Storskrubb T, et al. Prevalence of Barrett’s esophagus in the general population: an endoscopic study. Gastroenterology 2005;129:1825–31. [DOI] [PubMed] [Google Scholar]
- 5.Coleman HG, Xie Sh, Lagergren J The Epidemiology of Esophageal Adenocarcinoma. Gastroenterology 2018;154:390–405. [DOI] [PubMed] [Google Scholar]
- 6.Fitzgerald RC, di Pietro M, Ragunath K, et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut 2014;63:7–42 [DOI] [PubMed] [Google Scholar]
- 7.Weusten B, Bisschops R, Coron E, et al. Endoscopic management of Barrett’s esophagus: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 2017;49:191–198. [DOI] [PubMed] [Google Scholar]
- 8.di Pietro M, Fitzgerald RC; BSG Barrett’s guidelines working group. Revised British Society of Gastroenterology recommendation on the diagnosis and management of Barrett’s oesophagus with low-grade dysplasia. Gut 2018;67:392–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaheen NJ, Falk GW, Iyer PG, et al. American College of Gastroenterology. ACG Clinical Guideline: Diagnosis and Management of Barrett’s Esophagus. Am J Gastroenterol 2016;111:30–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. The New England journal of medicine 2009;360:2277–2288. [DOI] [PubMed] [Google Scholar]
- 11.Phoa KN, van Vilsteren FG, Weusten BL, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA 2014;311:1209–17. [DOI] [PubMed] [Google Scholar]
- 12.Vaughan TL, Fitzgerald RC. Precision prevention of oesophageal adenocarcinoma. Nat Rev Gastroenterol Hepatol 2015;12:243–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dellon ES, Hirano I. Epidemiology and Natural History of Eosinophilic Esophagitis. Gastroenterology 2018;154:319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kapel RC, Miller JK, Torres C, et al. Eosinophilic esophagitis: a prevalent disease in the United States that affects all age groups. Gastroenterology 2008;134:1316–21. [DOI] [PubMed] [Google Scholar]
- 15.Furuta GT, Katzka DA. Eosinophilic Esophagitis. N Engl J Med 2015;373:1640–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noel RJ, Putnam PE, Rothenberg ME. Eosinophilic esophagitis. N Engl J Med 2004;351:940–941. [DOI] [PubMed] [Google Scholar]
- 17.Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 2012;143:1179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jensen ET, Kappelman MD, Martin CF, Dellon ES. Health-care utilization, costs, and the burden of disease related to eosinophilic esophagitis in the United States. Am J Gastroenterol 2015;110:626–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ben-Menachem T, Decker GA, Early DS, et al. Adverse events of upper GI endoscopy. Gastrointest Endosc 2012;76:707–18. [DOI] [PubMed] [Google Scholar]
- 20.Sieg A, Hachmoeller-Eisenbach U, Eisenbach T. Prospective evaluation of adverse events in outpatient GI endoscopy: a survey among German gastroenterologists. Gastrointest Endosc 2001;53:620e7. [DOI] [PubMed] [Google Scholar]
- 21.Quine MA, Bell GD, McLoy RF, et al. Prospective audit of perforation rates following upper gastrointestinal endoscopy in two regions of England. Br J Surg 1995;82:530e3. [DOI] [PubMed] [Google Scholar]
- 22.Agostoni M, Fanti L, Gemma M, et al. Adverse events during monitored anesthesia care for GI endoscopy: an 8-year experience. Gastrointest Endosc 2011;74:266–75. [DOI] [PubMed] [Google Scholar]
- 23.Katzka DA, Smyrk TC, Alexander JA, et al. Accuracy and Safety of the Cytosponge for Assessing Histologic Activity in Eosinophilic Esophagitis: A Two-Center Study. Am J Gastroenterol 2017;112:1538–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kadri SR, Lao-Sirieix P, O’Donovan M, et al. Acceptability and accuracy of a non-endoscopic screening test for Barrett’s oesophagus in primary care: cohort study. Bmj 2010;341:c4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ross-Innes CS, Debiram-Beecham I, O’Donovan M, et al. Evaluation of a minimally invasive cell sampling device coupled with assessment of trefoil factor 3 expression for diagnosing Barrett’s esophagus: a multi-center case–control study. PLoS medicine 2015;12:e1001780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paterson AL, Lao-Sirieix P, O’donovan M, et al. Range of pathologies diagnosed using a minimally invasive capsule sponge to evaluate patients with reflux symptoms. Histopathology 2017;70:203–210. [DOI] [PubMed] [Google Scholar]
- 27.Levine DS, Haggitt RC, Blount PL, et al. An endoscopic biopsy protocol can differentiate high-grade dysplasia from early adenocarcinoma in Barrett’s esophagus. Gastroenterology 1993;105:40–50. [DOI] [PubMed] [Google Scholar]
- 28.Freeman M, Offman J, Walter FM, et al. Acceptability of the Cytosponge procedure for detecting Barrett’s oesophagus: a qualitative study. BMJ Open. 2017;7:e013901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Birk M, Bauerfeind P, Deprez PH, et al. Removal of foreign bodies in the upper gastrointestinal tract in adults: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2016;48:489–96. [DOI] [PubMed] [Google Scholar]
- 30.Reid BJ. Genomics, Endoscopy, and Control of Gastroesophageal Cancers: A Perspective. Cellular and Molecular Gastroenterology and Hepatology 2017;3:359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Multivariate analysis model for failure of swallowing the Cytosponge®
Supplementary Table 2. Combined adverse events from all studies included in the analysis