Summary
Background
Testing urine improves the number of tuberculosis diagnoses made among patients in hospital with HIV. In conjunction with the two-country randomised Rapid Urine-based Screening for Tuberculosis to Reduce AIDS-related Mortality in Hospitalised Patients in Africa (STAMP) trial, we used a microsimulation model to estimate the effects on clinical outcomes and the cost-effectiveness of adding urine-based tuberculosis screening to sputum screening for hospitalised patients with HIV.
Methods
We compared two tuberculosis screening strategies used irrespective of symptoms among hospitalised patients with HIV in Malawi and South Africa: a GeneXpert assay (Cepheid, Sunnyvale, CA, USA) for Mycobacterium tuberculosis and rifampicin resistance (Xpert) in sputum samples (standard of care) versus sputum Xpert combined with a lateral flow assay for M tuberculosis lipoarabinomannan in urine (Determine TB-LAM Ag test, Abbott, Waltham, MA, USA [formerly Alere]; TB-LAM) and concentrated urine Xpert (intervention). A cohort of simulated patients was modelled using selected characteristics of participants, tuberculosis diagnostic yields, and use of hospital resources in the STAMP trial. We calibrated 2-month model outputs to the STAMP trial results and projected clinical and economic outcomes at 2 years, 5 years, and over a lifetime. We judged the intervention to be cost-effective if the incremental cost-effectiveness ratio (ICER) was less than US$750/year of life saved (YLS) in Malawi and $940/YLS in South Africa. A modified intervention of adding only TB-LAM to the standard of care was also evaluated. We did a budget impact analysis of countrywide implementation of the intervention.
Findings
The intervention increased life expectancy by 0·5–1·2 years and was cost-effective, with an ICER of $450/YLS in Malawi and $840/YLS in South Africa. The ICERs decreased over time. At lifetime horizon, the intervention remained cost-effective under nearly all modelled assumptions. The modified intervention was at least as cost-effective as the intervention (ICERs $420/YLS in Malawi and $810/YLS in South Africa). Over 5 years, the intervention would save around 51 000 years of life in Malawi and around 171 000 years of life in South Africa. Health-care expenditure for screened individuals was estimated to increase by $37 million (10·8%) and $261 million (2·8%), respectively.
Interpretation
Urine-based tuberculosis screening of all hospitalised patients with HIV could increase life expectancy and be cost-effective in resource-limited settings. Urine TB-LAM is especially attractive because of high incremental diagnostic yield and low additional cost compared with sputum Xpert, making a compelling case for expanding its use to all hospitalised patients with HIV in areas with high HIV burden and endemic tuberculosis.
Introduction
Tuberculosis is the leading cause of death among the 25 million people with HIV in sub-Saharan Africa.1 In post-mortem studies, tuberculosis accounts for approximately 40% of hospital deaths among people with HIV, but is undiagnosed before death in nearly half of these patients.2,3 Many tuberculosis tests have poor sensitivity and long turnaround times, and obtaining suitable specimens for sputum tests can be difficult. Testing urine with a lateral flow assay for Mycobacterium tuberculosis lipoarabinomannan (Determine TB-LAM Ag test, Abbott, Waltham, MA, USA [formerly Alere]; TB-LAM) or with the GeneXpert assay for M tuberculosis and rifampicin resistance (Cepheid, Sunnyvale, CA, USA; Xpert) increases tuberculosis diagnostic yield in hospitalised patients with HIV.4–6
The Rapid Urine-based Screening for Tuberculosis to Reduce AIDS-related Mortality in Hospitalised Patients in Africa (STAMP) randomised trial in Malawi and South Africa evaluated tuberculosis screening with sputum Xpert, urine TB-LAM, and concentrated urine Xpert among unselected hospitalised medical patients with HIV, irrespective of tuberculosis symptoms, compared with screening with sputum Xpert alone.7 The addition of urine testing reduced 2-month all cause mortality by 2· 8% and increased tuberculosis diagnoses by 7·3%. Among patients with CD4 counts lower than 100 cells per μzL, urine testing decreased all cause mortality by 7·1%.8 A cost-effectiveness analysis was planned with the trial.7 Weighing additional tuberculosis cases detected and deaths prevented against additional costs of widespread testing is crucial in deciding whether to scale up urine tuberculosis screening in hospitals in resource-limited settings. We used STAMP trial results in a mathematical model to project clinical and economic outcomes and cost-effectiveness of urine-based tuberculosis screening in hospitalised patients with HIV beyond the trial’s time horizon.
Methods
Study design
We adapted the Cost-Effectiveness of Preventing AIDS Complications-International (CEPAC-I) model, which is a validated microsimulation of HIV-related disease and treatment,9,10 to account for tuberculosis natural history, diagnosis, and treatment. We compared the two tuberculosis screening strategies assessed in the STAMP trial in simulated cohorts of unselected hospitalised patients with HIV in Malawi and South Africa: sputum Xpert (standard of care) versus sputum Xpert, TB-LAM, and concentrated urine Xpert (intervention). In a post-hoc analysis, we also assessed a modified intervention of sputum Xpert and TB-LAM without urine Xpert.11 To attain stable per-person results, models were run on cohorts of 1 million hospitalised patients with HIV in Malawi and South Africa. We populated the model with cohort characteristics, tuberculosis diagnostic yields, and data on use of hospital resources derived from the STAMP trial. We obtained additional natural history and treatment data from published studies to project outcomes beyond the STAMP trial’s 2-month time horizon.
The primary outcome for this analysis was the incremental cost-effectiveness ratio (ICER), which was calculated as the difference between intervention and standard of care groups in lifetime health-care costs (2017 US$) divided by the difference in life expectancy. Second-line antiretroviral therapy (ART) is featured in national HIV care guidelines for Malawi and South Africa.12,13 Therefore, to set relevant cost-effectiveness thresholds for our primary analysis, we used the CEPAC-I model to determine the ICER of a care strategy that included second-line ART (after failure of first-line ART) compared with a strategy that did not include second-line ART. This analysis yielded ICERs of $750 per year of life saved (YLS) in Malawi and $940 per YLS in South Africa.
We additionally projected all-cause mortality, life-years accrued, costs, and cost-effectiveness over 2-year and 5-year time horizons. We report undiscounted outcomes for clinical and budget evaluations and outcomes discounted by 3% per year for the cost-effectiveness analysis, as recommended by the Second Panel on Cost-Effectiveness in Health and Medicine.14
Model overview
Each simulated hospitalised patient with HIV who was entered into the model was followed up monthly from tuberculosis screening to death, counting all years of life and lifetime costs of tuberculosis and HIV care. Patients’ characteristics were created by the model by randomly selecting from the STAMP-informed characteristics (eg, CD4 cell count, tuberculosis status). Clinical outcomes were tracked as each individual transitioned through different states of tuberculosis and HIV disease progression and treatment (appendix).9,10
We assumed that tuberculosis test turnaround time and the starting of treatment for any positive result would occur within 1 month of the patient entering the model. As in STAMP and real practice, tuberculosis could also be diagnosed clinically, without microbiological confirmation, and lead to empirical treatment. Each tuberculosis treatment regimen has a probability of success, given regimen efficacy (appendix). All simulated individuals were classified as being eligible for ART12,13 and were modelled as either already taking therapy before hospitalisation or starting it within 1 month of entry into the model. ART-adherent individuals were assumed to have a decrease in HIV-related morbidity and mortality.9,10 We accounted for non-adherence after leaving hospital and loss to follow-up from care.
Model validation
To validate the outcomes of this analysis, we calibrated model-generated 2-month mortality to STAMP trial results by adjusting tuberculosis-related mortality and non-tuberculosis HIV-related mortality. The STAMP trial did not differentiate between causes of death, and we report only all-cause mortality. Published long-term cohort studies of mortality in people with HIV are largely limited to outpatients starting ART, who are generally less ill than STAMP participants. Thus, for external validation, we compared results from a modelled cohort of ambulatory people with HIV starting ART against published outcomes (appendix).
Input parameters
As in STAMP, the initial median CD4 counts in the model were set to 219 cells per μL in Malawi and 236 cells per μL in South Africa (table 1). Given the imperfect diagnostic yields of the tests, we assumed that the underlying tuberculosis prevalence at each site was 1·25 times the number of cases confirmed microbiologically by sputum Xpert, TB-LAM, or urine Xpert plus the number of clinically diagnosed cases, all in the intervention group, divided by the total number of participants in the intervention group (appendix). The estimated prevalence was 23·5% in Malawi and 28·5% in South Africa, and these values were consistent with previous reports.4–6,24 Based on rifampicin resistance data among STAMP participants who underwent Xpert testing, we assumed that among the model patients with tuberculosis, 1% in Malawi and 3% in South Africa had multidrug-resistant disease.
Table 1:
Model input parameters
| Malawi | South Africa | References | |
|---|---|---|---|
| Characteristics of patients with HIV | |||
| Median (IQR) age (years) | 38 (32–47) | 37 (30–46) | STAMP |
| Proportion of men (%) | 37% | 50% | STAMP |
| Proportion of women (%) | 63% | 50% | STAMP |
| Median (IQR) CD4 count at admission (cells per μL) | 219 (86–431) | 236 (70–445) | STAMP |
| Patients taking ART at admission | 78% | 64% | STAMP |
| Median (range*) underlying tuberculosis prevalence† | 23·5% (10–50) | 28·5% (10–50) | STAMP, 15 |
| Median (range*) number of patients able to provide sputum samples | 39% (20–100) | 75% (20–100) | STAMP |
| Median (range) probability of empirical treatment*‡ | 4% (0–40) | 10% (0–40) | STAMP |
| Monthly probability of tuberculosis infection based on age (%) | 0·4–0·8 | 0·4–0·8 | 16 |
| Screening strategy diagnostic yield for CD4 count <100 vs ≥100 cells per μL§ | |||
| Standard of care (sputum Xpert)¶ | 19% and 18% | 31% and 29% | STAMP |
| Intervention (range*) | 77% and 72% (40–90%) | 56% and 52% (40–90%) | STAMP |
| Modified intervention (sputum Xpert and TB-LAM) | 74% and 69% | 51% and 47% | STAMP |
| Resource use | |||
| Median length of hospitalisation (days) | 5 | 7 | STAMP |
| Hospital bed cost per day (US$) | $1 | $56 | 17 |
| Additional hospital resource use cost per admission (US$)∥ | $23 | $98 | STAMP, 18–20 |
| Median (range) costs per test for tuberculosis diagnostic assays (US$)** | |||
| Sputum Xpert†† | $25 ($5–35) | $15 ($5–35) | 18,19 |
| TB-LAM | $3 ($2–8) | $3 ($2–8) | 21 |
| Urine Xpert††‡‡ | $26($6–36) | $15 ($5–35) | 18,19 |
| Costs of treatment (US$)§§ | |||
| Drug-susceptible tuberculosis treatment cost per month for 6 months | $7 | $7 | 22 |
| Multidrug-resistant tuberculosis treatment per month for 24 months | $231 | $231 | 22 |
| First-line ART per month | $11 | $11 | 23 |
Values are calculated in 2017 US$. STAMP=Rapid Urine-based Screening for Tuberculosis to Reduce AIDS-related Mortality in Hospitalised Patients in Africa randomised trial. ART=antiretroviral therapy. Xpert=GeneXpert assay for Mycobacterium tuberculosis and rifampicin resistance. TB-LAM=lateral flow urine assay for M tuberculosis lipoarabinomannan.
Range values were assessed in sensitivity analysis.
Calculated as 1·25 times the number of microbiologically confirmed cases15 plus the number of clinically diagnosed cases (ie, without microbiological confirmation), divided by the study population size (all in the intervention group).
In patients diagnosed clinically without microbiological confirmation, within 1 month in the model rather than during hospitalisation in the STAMP trial.
The diagnostic yields applied in the model accounted for non-provision of sputum specimens and for concordance between test results (ie, adding a second test would increase diagnostic yield only if it detected additional tuberculosis cases not detected by the first test).
The diagnostic yield of sputum Xpert is slightly higher among patients with CD4 counts <100 cells per μL, despite lower sensitivity, because of higher sputum provision in this subgroup.
Excludes costs of tuberculosis diagnostic tests assessed in STAMP.
Diagnostic test costs include personnel time.
Xpert cost in a Malawi-specific costing study was higher than the cost reported in South African studies and by the South Africa National Health Laboratory Service19 due to different costs of maintenance and repair and different economies of scale.
Urine Xpert costs were slightly higher than those of sputum Xpert because of the centrifugation needed to concentrate the urine specimen. Cost differences are not apparent in South Africa due to rounding.
Because tuberculosis and ART drugs are imported across countries, we assumed that costs were equal across countries. Costs shown here are for drugs only.
Diagnostic yields of individual and combinations of tests, stratified by CD4 count (<100 cells per μL or ≥100 cells per μL), were based on STAMP results and our tuberculosis prevalence estimates (table 1). Overall diagnostic yields in Malawi and South Africa were 18% and 30% for the standard of care and 73% and 53% for the intervention. Because all positive results in STAMP were classified as true positives, we applied published test specificity (table 2).4,5,25–27 Informed by STAMP, in the base case in Malawi and South Africa, the probability of patients being able to provide a sputum sample was set to 39% and 75%, respectively. We assumed that all model patients in the intervention group had urine samples available. Also informed by STAMP, we assumed that 4% of model patients in Malawi and 10% of those in South Africa were diagnosed clinically and treated empirically for tuberculosis. Clinical diagnoses accounted for a greater proportion of overall tuberculosis diagnoses in the standard of care group than in the intervention group (56% vs 26%).
Table 2:
Tuberculosis diagnostic assay performance characteristics
| Sensitivity | Specificity | References | |
|---|---|---|---|
| Sputum Xpert | |||
| CD4 count <100 cells per μL | 40% (20–60)* | 99% | STAMP, 25 |
| CD4 count ≥100 cells per μL | 43% (23–63)* | 99% | STAMP, 25 |
| Urine TB-LAM | |||
| CD4 count <100 cells per μL | 53% | 96% | STAMP, 5,26,27 |
| CD4 count 100 ≥cells per μL | 42% | 98% | STAMP, 5,26,27 |
| Urine Xpert | |||
| CD4 count <100 cells per μL | 31% | 99% | STAMP, 4 |
| CD4 count ≥100 cells per μL | 13% | 99% | STAMP, 4 |
The indicated sensitivity of each test is the sensitivity among individuals in the STAMP trial who provided a specimen and is independent of other test results. In the model, diagnostic yields instead of sensitivities were used for multitest strategies to better reflect concordance between tests and incremental diagnostic yields. In multitest strategies the lowest specificity of any individual test was applied, as informed by previously published studies. Xpert=GeneXpert assay for Mycobacterium tuberculosis and rifampicin resistance. STAMP=Rapid Urine-based Screening for Tuberculosis to Reduce AIDS-related Mortality in Hospitalised Patients in Africa randomised trial. TB-LAM=lateral flow urine assay for M tuberculosis lipoarabinomannan.
Ranges (shown in brackets) were assessed in sensitivity analyses.
The parameters for tuberculosis treatment outcomes were set on the basis of previous studies (appendix). HIV treatment parameters were applied as in previous African CEPAC-I studies (appendix).9,10
Data on resource use, including number of diagnostic tests done and drugs consumed in the hospital, were collected from a subset of STAMP participants at each site.7 To approximate average hospitalisation costs, we multiplied length of stay (median 5 days in Malawi and 7 days in South Africa) by the daily cost of hospitalisation and then added the average quantities (and associated costs) of resources,28 for which we obtained costs from country-specific costing studies and national laboratory listings (appendix).18–20,22,23,29 The costs of sputum Xpert, TB-LAM, and concentrated urine Xpert were $25, $3, and $26, respectively, in Malawi and $15, $3, and $15, respectively, in South Africa (table 1). We included costs after discharge from hospital, including those of tuberculosis and HIV care (appendix).
Sensitivity and alternative scenario analyses
To assess cost-effectiveness beyond the STAMP trial and in accordance with variation reported in other settings, we assessed uncertainty with one-way and multiway deterministic sensitivity analyses.30 We used the following ranges: tuberculosis prevalence 10–50%; probability of patients being able to provide a sputum sample 20–100%; probability of empirical tuberculosis treatment 0–40%; sputum Xpert sensitivity within 20% of the base case; diagnostic yields of the intervention 40–90%; and cost of tuberculosis tests $5–35 for sputum and urine Xpert (plus urine centrifugation cost) and $2–8 for TB-LAM. We also assessed cost-effectiveness at different time horizons (2-year and 5-year horizons in addition to lifetime horizon).
We evaluated scenarios that used an alternative target population or testing strategy. First, we did a subgroup analysis (which was prespecified in STAMP) among individuals with CD4 counts less than 100 cells per μL. Second, because policy makers have been considering a strategy that adds only TB-LAM to the standard of care,11 we analysed a modified intervention that added only TB-LAM to sputum Xpert.
Budget impact analysis
We did a budget impact analysis of national implementation of the intervention or modified intervention instead of the standard of care in Malawi and South Africa for all hospitalised patients with HIV over a 2-year and 5-year period. We assumed that in Malawi and South Africa, respectively, 70 000 and 500 000 people with HIV would be admitted to hospital annually, and we applied model-generated per-person clinical and economic projections (appendix).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, writing of the report, or the decision to submit the paper for publication. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit the paper for publication.
Results
The intervention improved tuberculosis diagnostic yield compared with the standard of care, with absolute increases of 55% in Malawi and 23% in South Africa. Model-generated 2-month all-cause mortality results matched those in the STAMP trial to within 0·1% when stratified by country and study group, and when limited to patients with CD4 counts less than 100 cells per μL, also stratified by country and study group (appendix). In Malawi, model-projected all-cause mortality in the intervention group was reduced at 2 months, 2 years, and 5 years compared with the standard of care group (table 3). In South Africa, the intervention was associated with reduced mortality at all timepoints, but to a lesser degree (table 3). The intervention increased undiscounted life expectancy by 1·2 years in Malawi and 0·5 years in South Africa compared with the standard of care (table 3).
Table 3:
Clinical and economic outcomes, including cost-effectiveness, of tuberculosis screening strategies among hospitalised people with HIV
| Mortality |
Lifetime outcomes |
||||||
|---|---|---|---|---|---|---|---|
| 2 months | 2 years | 5 years | Life-years (undiscounted) | Life-years (discounted)* | Cost (US$; discounted)*† | ICER (US$/YLS; discounted)†‡ | |
| Intervention in all patients (sputum Xpert, urine TB-LAM, and concentrated urine Xpert) | |||||||
| Malawi | |||||||
| Standard of care | 24·4% | 40·7% | 50·5% | 12·5 | 8·4 | 3450 | ·· |
| Intervention | 20·9% | 35·2% | 45·8% | 13·7 | 9·1 | 3790 | 450 |
| South Africa | |||||||
| Standard of care | 17·7% | 32·0% | 42·4% | 14·1 | 9·5 | 8500 | ·· |
| Intervention | 15·5% | 29·6% | 40·4% | 14·6 | 9·8 | 8770 | 840 |
| Intervention in patients with CD4 counts <100 cells per μL (sputum Xpert, urine TB-LAM, and concentrated urine Xpert) | |||||||
| Malawi | |||||||
| Standard of care | 40·5% | 65·2% | 75·6% | 6·3 | 4·3 | 2090 | ·· |
| Intervention | 33·7% | 58·5% | 70·6% | 7·6 | 5·2 | 2500 | 490 |
| South Africa | |||||||
| Standard of care | 32·2% | 55·9% | 67·8% | 8·2 | 5·6 | 6920 | ·· |
| Intervention | 23·9% | 50·2% | 63·7% | 9·2 | 6·3 | 7630 | 1000 |
| Modified intervention in all patients (sputum Xpert and urine TB-LAM) | |||||||
| Malawi | |||||||
| Standard of care | 24·4% | 40·7% | 50·5% | 12·5 | 8·4 | 3450 | ·· |
| Modified intervention | 21·1% | 35·5% | 46·0% | 13·6 | 9·1 | 3750 | 420 |
| South Africa | |||||||
| Standard of care | 17·7% | 32·0% | 42·4% | 14·1 | 9·5 | 8500 | ·· |
| Modified intervention | 16·0% | 30·2% | 41·0% | 14·5 | 9·7 | 8690 | 810 |
| Intervention vs modified intervention in all patients | |||||||
| Malawi | |||||||
| Modified intervention | 21·1% | 35·5% | 46·0% | 13·6 | 9·1 | 3750 | ·· |
| Intervention | 20·9% | 35·2% | 45·8% | 13·7 | 9·1 | 3790 | 910 |
| South Africa | |||||||
| Modified intervention | 16·0% | 30·2% | 41·0% | 14·5 | 9·7 | 8690 | ·· |
| Intervention | 15·5% | 29·6% | 40·4% | 14·6 | 9·8 | 8770 | 930 |
Values are calculated in 2017 US$. ICER=incremental cost-effectiveness ratio. YLS=year of life saved. Xpert=GeneXpert assay for Mycobacterium tuberculosis and rifampicin resistance. TB-LAM=lateral flow urine assay for M tuberculosis lipoarabinomannan.
Discounted 3% per year.
Cost includes all health-care expenditures.
The ICER is the difference between the intervention and standard of care or between the modified intervention and standard of care in discounted costs divided by the difference in discounted life-years. The displayed life-years and costs are rounded, but the ICER was calculated with non-rounded life-years and costs. We considered the intervention or the modified intervention to be cost-effective if its ICER was less than the cost-effectiveness thresholds of $750/YLS in Malawi and $940/YLS in South Africa (the ICERs of including second-line antiretroviral therapy in these countries).
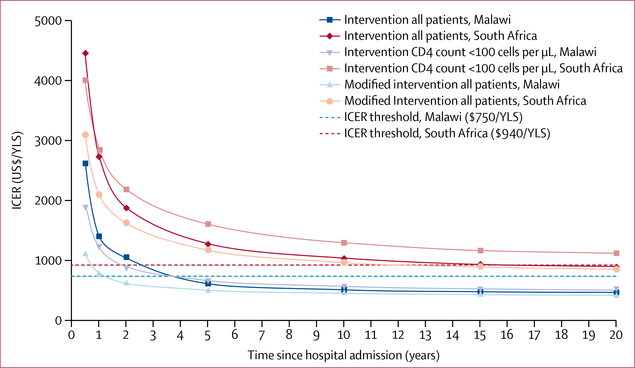
Hospitalisation costs per patient were $29 in Malawi and $491 in South Africa, including cumulative resource use and bed costs for the period in hospital, but excluding STAMP diagnostic test costs. Discounted per-person lifetime health-care costs in the standard of care and intervention groups in Malawi were $3450 and $3790, and in South Africa were $8500 and $8770. The ICER of the intervention compared with standard of care at a lifetime horizon was $450 per YLS in Malawi and $840 per YLS in South Africa, both of which were cost-effective (table 3). The intervention became cost-effective in Malawi by 4 years and in South Africa by 15 years (figure 1).
Figure 1: ICERs over time for different tuberculosis screening strategies among hospitalised people with HIV.
ICERs are calculated compared with the standard of care (sputum Xpert). The intervention consists of sputum Xpert, a lateral flow assay of urine for M tuberculosis lipoarabinomannan, and concentrated urine Xpert. The strategy “Intervention CD4 count <100 cells per μL” is compared with sputum Xpert alone also done in patients with CD4 counts <100 cells per μL. The modified intervention consists of sputum Xpert and a lateral flow assay of urine for M tuberculosis lipoarabinomannan without urine Xpert. The country-specific ICER thresholds were calculated by comparing modelled care strategies that did and did not include second-line antiretroviral therapy. ICER=incremental cost-effectiveness ratio. Xpert=GeneXpert assay for Mycobacterium tuberculosis and rifampicin resistance alone. YLS=year of life saved.
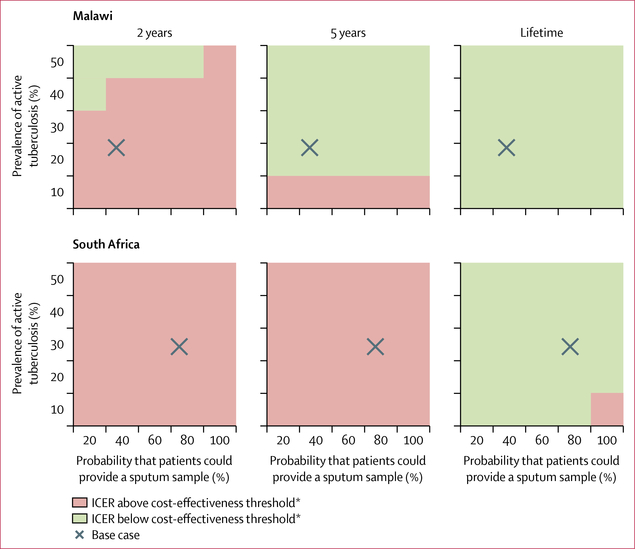
The intervention was cost-effective across nearly all parameter variations, with ICERs generally changing by less than 10% from the base case in the sensitivity analyses (appendix). The only exception was when diagnostic yield of the intervention was set to 40% in South Africa. In a sensitivity analysis that varied the time horizon, sputum provision probability, and tuberculosis prevalence, the intervention was generally not cost-effective at 2 years but was cost-effective at 5 years and over a lifetime (figure 2). With a lifetime horizon, the intervention was cost-effective across nearly all values for empirical treatment probability and tuberculosis prevalence when simultaneously varied (appendix).
Figure 2: ICERs for urine-based tuberculosis screening with varying sputum sample provision and prevalence of active tuberculosis, by different time horizons.
ICERs for the intervention are calculated compared with the standard of care. ICER=incremental cost-effectiveness ratio. *ICER threshold for Malawi is US$750 per year of life saved, and for South Africa is $940 per year of life saved. Values below the thresholds are cost-effective.
Among patients with CD4 counts less than 100 cells per μL, the intervention increased undiscounted life expectancy by 1·3 years in Malawi and 1·0 year in South Africa (table 3). The ICERs for the intervention in this subgroup were higher than those in the entire cohort, but the intervention remained cost-effective in Malawi and its ICER was slightly higher than the cost-effectiveness threshold in South Africa.
With the modified intervention, undiscounted life expectancy increased by 1·1 years in Malawi and 0·4 years in South Africa compared with the standard of care, and it was at least as cost-effective as the STAMP intervention (table 3). As in the base case, the modified intervention became more cost-effective over time in both countries (figure 1). The ICER of the intervention compared with that of the modified intervention (ie, the incremental cost-effectiveness of urine Xpert) was $910 per YLS in Malawi and $930 per YLS in South Africa (table 3, appendix). When limiting the comparison between the modified intervention and the standard of care to patients with CD4 counts of less than 100 cells per μL, ICERs were $460 per YLS in Malawi and $990 per YLS in South Africa, which were slightly higher than those among all patients (appendix).
Implementing the intervention nationally and scaled to all hospitalised patients with HIV over 5 years was associated with around 51000 YLSs in Malawi (7 · 5% increase in life-years compared with standard of care) and 171000 YLSs in South Africa (3· 2% increase). TB-LAM and urine Xpert tests themselves, not including downstream changes in health-care expenditures, added $10 million to costs in Malawi and $47 million to costs in South Africa over 5 years. In Malawi, the intervention increased cumulative health-care expenditures among screened individuals by $10 million (11·2%) over 2 years and $37 million (10· 8%) over 5 years. In South Africa,health-care expenditures increased by $73 million (2·4%) over 2 years and $261 million (2·8%) over 5 years (figure 3). In Malawi, the largest contributors to the increase in 5-year health expenditures were tuberculosis diagnostic and treatment costs (each increased by 33%), and in South Africa the largest contributor was non-tuberculosis, non-ART HIV care costs, which increased by 45% due to longer survival (figure 3). While still providing notable clinical benefit, the budget impact of the modified intervention was lower than that of the intervention (figure 3).
Figure 3: 2-year and 5-year budget impact of implementing urine-based tuberculosis screening countrywide among all hospitalised people with HIV.
SOC is sputum Xpert alone. The intervention consists of sputum Xpert, a lateral flow assay of urine for M tuberculosis lipoarabinomannan, and concentrated urine Xpert. The modified intervention consists of sputum Xpert and a lateral flow assay of urine for M tuberculosis lipoarabinomannan without urine Xpert. ART=antiretroviral therapy. SOC=standard of care. Xpert=GeneXpert assay for Mycobacterium tuberculosis and rifampicin resistance. *Includes subsequent hospitalisation.
Discussion
With use of the CEPAC-I model to simulate the STAMP trial and project long-term outcomes, we found that screening unselected hospitalised patients with HIV for tuberculosis with TB-LAM and sputum and urine Xpert increased life expectancy by 0·5–1·2 years and was cost-effective compared with sputum Xpert alone in Malawi and South Africa. This approach remained cost-effective in the sensitivity analyses intended to test generalisability beyond trial settings. Adding only TB-LAM to sputum Xpert in the modified intervention provided similar clinical benefit and was at least as cost-effective.
Access to primary STAMP trial data is a key strength of our analysis. Model structure and input parameters reflected those of the trial, and we calibrated 2-month all-cause mortality results to those of the trial. Our analysis was done in two countries with very different health-care infrastructures, costs, and annual per-capita gross domestic products ($330 in Malawi and $6090 in South Africa in 2017).31 The intervention’s clinical and economic effects and ICERs varied between Malawi and South Africa mainly because of differences in the probability of obtaining a sputum sample, diagnostic yields, and use of empirical treatment, as in the STAMP trial.8 These factors reflected differences in underlying tuberculosis prevalence and probability of clinicians starting empirical treatment based on pretest suspicion of tuberculosis. In addition, HIV care costs were lower in Malawi than in South Africa. Nonetheless, the intervention was cost-effective in both countries, signalling its generalisability as a useful strategy in countries and areas with high HIV and tuberculosis burdens and varying resource availability.
WHO conditionally recommends TB-LAM to aid diagnosis of tuberculosis in hospitalised patients with HIV with CD4 counts of 100 cells per pL or lower and symptoms of tuberculosis.11 Supporting this recommendation, a multicountry randomised trial showed that adding TB-LAM in hospitalised patients with HIV and presumed tuberculosis significantly reduced 8-week all-cause mortality.6 Some patients with HIV-associated tuberculosis present with atypical symptoms and would be missed by these criteria and the WHO testing criteria. Consistent with the STAMP trial results,8 we found a benefit in broadening the urine-based testing eligibility criteria to unselected hospitalised patients with HIV.
Acknowledging that selecting patients for tuberculosis screening by CD4 cell counts might be impractical because testing is often unavailable at the time of hospital admission, we did a subgroup comparison of the intervention with the standard of care among patients with CD4 counts less than 100 cells per μL. In the STAMP trial, the intervention significantly reduced mortality by 7·1% in this group of patients (p=0·04).8 Concordantly, in our model-based analysis, the intervention improved life expectancy to a greater degree in the subgroup of patients with low CD4 counts than among all patients. Counterintuitively, we found that the intervention was less economically attractive among those with low CD4 cell counts because the costs increased to a greater degree than among all patients. The rise in costs associated with low CD4 cell counts is multifactorial and is driven largely by costs associated with non-tuberculosis opportunistic diseases and concomitant increases in ART costs due to improved survival.
Simulation modelling provided flexibility to examine alternative strategies that were not initially assessed in the clinical trial but that might be relevant to policy. The modified intervention of adding only TB-LAM to sputum Xpert and excluding urine Xpert was at least as cost-effective as the trial intervention. Xpert costs substantially more than TB-LAM, and in the STAMP trial urine Xpert offered only modest incremental yield over sputum Xpert and TB-LAM. Being a bedside urine dipstick-type test, TB-LAM provides rapid results and the potential for starting treatment immediately.
The STAMP trial found weak evidence (p=0·07) of a 2·8% reduction in all-cause mortality at 2 months with urine-based tuberculosis screening among all patients.8 If there is truly no difference in mortality at 2 months, the cost-effectiveness of the intervention would be less robust. Even so, because of the increased diagnostic yield of the intervention, decreases in morbidity and mortality are probably realised over longer time horizons, along with reasonable cost-effectiveness.
Cost-effectiveness thresholds have often been tied to a country’s annual per-capita gross domestic product, but the appropriateness of this approach has been debated.32,33 Because care guidelines in Malawi and South Africa include second-line ART,12,13 we compared the ICERs of the modelled interventions with the ICER for this treatment. We presumed that because comparatively expensive second-line ART is endorsed and financed, interventions that are as cost-effective might be also.32,33 Urine-based screening was cost-effective and the costs of the urine assays themselves would add little to health-care budgets. New diagnostics require upfront investments and can lead to overall higher treatment costs, and those investments take time to show value.34 Our analyses highlight that the intervention would become cost-effective by 4 years in Malawi and 15 years in South Africa. Our cost-effectiveness threshold measure was based on YLSs rather than quality-adjusted life-years.35 Lacking country-specific preference-based weights for all health states (especially for Malawi) in our model, we believed it most appropriate to use YLSs for consistency in outcome measures across the two countries.
Our study is subject to limitations inherent in any model-based analysis, including uncertainties in model structure and input parameters, although these mirrored the STAMP trial. The true prevalence of active tuberculosis might differ from our estimates, but our sensitivity analyses showed that the intervention would remain cost-effective across a wide range of tuberculosis prevalence. With an effective test-and-treat strategy for HIV, the health and monetary value of tuberculosis screening could decrease, but the promise of such a strategy is far from reality, as shown by the number of STAMP trial participants without a previous HIV diagnosis or not taking ART.8,10 In our model we assumed imperfect but high specificity of Xpert and TB-LAM.4,5,25 For some tuberculosis-negative patients in the model the intervention added tuberculosis treatment costs with little effect on life expectancy. In reality, false-positive tuberculosis test results might result in a true diagnosis of another disease being missed and could have a more substantial negative effect on life expectancy. Our model did not account for tuberculosis transmission. By increasing tuberculosis diagnostic yield, the intervention would probably reduce tuberculosis transmission and be even more cost-effective at a population level. However, without data from the STAMP trial to populate this parameter, we believed that excluding it would be a conservative approach. We did not account for some fixed costs such as that of an Xpert machine or a centrifuge for urine concentration for Xpert, although we did include associated increases in staff costs. Nonetheless, in our sensitivity analyses, diagnostic test costs had little effect on our findings. The STAMP trial did not use Xpert Ultra cartridges, which might have higher sensitivity and lower specificity than the conventional Xpert cartridges and thus might have yielded different cost-effectiveness results. Our budget impact analysis did not fully account for the logistics associated with implementation and scale-up of urine testing or for logistics of increasing treatment capacity, but we did include costs of testing and treating more people for tuberculosis and higher HIV-related costs due to longer survival. Ultimately, the results of expanding urine-based tuberculosis screening with scale-up might differ from those seen in the STAMP trial and those projected by our model.
Our model analysis indicated that urine-based tuberculosis screening with TB-LAM and Xpert in hospitalised patients with HIV would increase life expectancy and be cost-effective in resource-limited settings. We suggest that care guidelines in areas with high HIV burden and endemic tuberculosis add urine-based tuberculosis screening of all hospitalised patients with HIV. In particular we recommend the use of TB-LAM because it has clinical benefit, low costs, and rapid turnaround. The scaling-up of this strategy could help to reduce the massive burden of tuberculosis mortality among people with HIV with modest budget impact and economic efficiency.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed for studies that investigated the cost-effectiveness of urine lipoarabinomannan assay (Determine TB-LAM Ag test [TB-LAM], Abbott, Waltham, MA, USA [formerly Alere]) for tuberculosis diagnosis published from Jan 1, 2000, to Jan 31, 2018. We combined the search terms “lipoarabinomannan”, “LAM”, and “urine LAM” with “tuberculosis” and with “cost-effectiveness”, “economic”, or “model”. We identified two cost-effectiveness analyses, published before the Rapid Urine-based Screening for Tuberculosis to Reduce AIDS-related Mortality in Hospitalised Patients in Africa (STAMP) randomised trial and another multicountry randomised trial of TB-LAM in hospitalised patients with HIV were completed. The analyses focused on use of TB-LAM in patients with HIV who had symptoms of tuberculosis. We found no previous cost-effectiveness analyses of TB-LAM use in unselected patients with HIV. Testing urine with the GeneXpert assay for Mycobacterium tuberculosis and rifampicin resistance (Cepheid, Sunnyvale, CA, USA; Xpert) and TB-LAM increases diagnostic yield in people with HIV, particularly those with very low CD4 cell counts, symptoms suggestive of tuberculosis, or both. Model-based studies have shown that addition of urine TB-LAM testing to standard tuberculosis diagnostic strategies in people with HIV with low CD4 cell counts and symptoms of tuberculosis is cost-effective. However, many people with HIV and tuberculosis have atypical symptoms, and CD4 cell count might not be readily available in the acute hospital setting. The STAMP trial assessed the clinical benefit of urine tuberculosis screening in unselected hospitalised patients with HIV, regardless of CD4 cell count, tuberculosis symptoms, or suspicion of tuberculosis.
The addition of TB-LAM and urine Xpert to sputum Xpert increased the number of tuberculosis diagnoses. No cost-effectiveness or budget impact analysis of such a widespread screening strategy had been done previously. Urine tests add costs and would have the greatest impact in settings with severe resource constraints. We have now used modelling to critically weigh long-term clinical benefits against costs.
Added value of this study
We adapted a mathematical model that was calibrated to STAMP trial outcomes, and validated with longer-term outcomes from other published studies, to project clinical and economic outcomes, cost-effectiveness, and budget impact of adding TB-LAM and concentrated urine Xpert to standard sputum Xpert tuberculosis screening in unselected hospitalised patients with HIV in Malawi and South Africa. The addition of urine-based screening was projected to increase life expectancy and be cost-effective across a wide range of scenarios in both countries. Urine screening countrywide could improve clinical outcomes and have modest budget impact due mostly to prolonged survival.
Implications of all the available evidence
WHO recommends restricting urine TB-LAM testing to hospitalised patients with HIV and very low CD4 cell counts, and uptake has been slow. By extending the time horizon of the STAMP trial, our results suggest that substantial clinical benefits could be achieved with economic efficiency by expanding urine-based screening to all hospitalised patients with HIV in tuberculosis-endemic settings, regardless of CD4 cell count. The consistency of our results in a low-income and a middle-income country signal generalisability across settings with varying degrees of resource availability. With these clinical benefits, rapid testing times, and little additional cost, urine TB-LAM testing is particularly attractive as a strategy to reduce the huge burden of tuberculosis-related deaths in people with HIV.
Acknowledgments
We thank Naomi Fields and Michael Girouard for technical assistance, Daniel Grint and Lingstone Chiume for statistical assistance, and Taige Hou for programming assistance. We also thank STAMP trial investigators, personnel, and participants. We have deeply missed SDL in the execution of this science in a field to which he contributed hugely. This work was supported by awards from the Medical Research Council/Department for International Development/Wellcome Trust Joint Global Health Trials scheme (STAMP trial; MR/M007375/1), the National Institute on Drug Abuse (K01 DA042687) and the National Institute of Allergy and Infectious Diseases (R37 AI093269 and R01 AI058736) of the National Institutes of Health, Royal College of Physicians (James Maxwell Grant Prophit Fellowship to AG-W), Wellcome Trust Fellowship (WT200901/Z/16/Z to ELC), and Massachusetts General Hospital (Steve and Deborah Gorlin Research Scholars Award to RPW). CRH is supported by the Providence/Boston Center for AIDS Research (P30 AI042853) and the Boston University/Rutgers Tuberculosis Research Unit (U19 AI111276). The content is solely our responsibility and does not necessarily represent the official views of the Medical Research Council, Department for International Development, Wellcome Trust, National Institutes of Health, or Massachusetts General Hospital.
Funding
UK Medical Research Council, UK Department for International Development, Wellcome Trust, US National Institutes of Health, Royal College of Physicians, Massachusetts General Hospital.
Footnotes
Declaration of interests
We declare no competing interests.
Contributor Information
Krishna P Reddy, Medical Practice Evaluation Center; Division of Pulmonary and Critical Care Medicine; Massachusetts General Hospital, Boston, MA, USA; Harvard Medical School, Boston, MA, USA.
Ankur Gupta-Wright, TB Centre, London School of Hygiene & Tropical Medicine, London, UK; Malawi-Liverpool-Wellcome Trust Clinical Research Program, Blantyre, Malawi.
Prof Katherine L Fielding, TB Centre, London School of Hygiene & Tropical Medicine, London, UK; University of the Witwatersrand, Johannesburg, South Africa.
Sydney Costantini, Medical Practice Evaluation Center.
Amy Zheng, Medical Practice Evaluation Center; Massachusetts General Hospital, Boston, MA, USA; Harvard Medical School, Boston, MA, USA.
Prof Elizabeth L Corbett, TB Centre, London School of Hygiene & Tropical Medicine, London, UK; Malawi-Liverpool-Wellcome Trust Clinical Research Program, Blantyre, Malawi.
Liyang Yu, Medical Practice Evaluation Center.
Prof Joep J van Oosterhout, Dignitas International, Zomba, Malawi; Department of Medicine, University of Malawi College of Medicine, Blantyre, Malawi.
Stephen C Resch, Department of Health Policy and Management, Harvard T H Chan School of Public Health, Boston, MA, USA.
Douglas P Wilson, Department of Internal Medicine, Edendale Hospital, University of KwaZulu-Natal, Pietermaritzburg, South Africa.
Prof C Robert Horsburgh, Jr, Department of Epidemiology; Section of Infectious Diseases, Department of Medicine.
Prof Robin Wood, Boston University School of Medicine, Boston, MA, USA; Desmond Tutu HIV Foundation, University of Cape Town, Cape Town, South Africa.
Melanie Alufandika-Moyo, Dignitas International, Zomba, Malawi.
Jurgens A Peters, TB Centre, London School of Hygiene & Tropical Medicine, London, UK.
Prof Kenneth A Freedberg, Medical Practice Evaluation Center; Division of General Internal Medicine; Division of Infectious Diseases; Massachusetts General Hospital, Boston, MA, USA; Harvard Medical School, Boston, MA, USA; Department of Health Policy and Management, Harvard T H Chan School of Public Health, Boston, MA, USA; Department of Epidemiology; Section of Infectious Diseases, Department of Medicine.
Prof Stephen D Lawn, TB Centre, London School of Hygiene & Tropical Medicine, London, UK; Boston University School of Medicine, Boston, MA, USA; Desmond Tutu HIV Foundation, University of Cape Town, Cape Town, South Africa.
Prof Rochelle P Walensky, Medical Practice Evaluation Center; Division of General Internal Medicine; Division of Infectious Diseases; Massachusetts General Hospital, Boston, MA, USA; Harvard Medical School, Boston, MA, USA.
References
- 1.WHO. HIV/AIDS: online Q&A. November, 2017. http://www.who.int/features/qa/71/en/ (accessed May 7, 2018).
- 2.Cohen T, Murray M, Wallengren K, Alvarez GG, Samuel EY, Wilson D. The prevalence and drug sensitivity of tuberculosis among patients dying in hospital in KwaZulu-Natal, South Africa: a postmortem study. PLoS Med 2010; 7: e1000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta RK, Lucas SB, Fielding KL, Lawn SD. Prevalence of tuberculosis in post-mortem studies of HIV-infected adults and children in resource-limited settings: a systematic review and meta-analysis. AIDS 2015; 29: 1987–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawn SD, Kerkhoff AD, Burton R, et al. Rapid microbiological screening for tuberculosis in HIV-positive patients on the first day of acute hospital admission by systematic testing of urine samples using Xpert MTB/RIF: a prospective cohort in South Africa. BMC Med 2015; 13: 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawn SD, Kerkhoff AD, Burton R, et al. Diagnostic accuracy, incremental yield and prognostic value of Determine TB-LAM for routine diagnostic testing for tuberculosis in HIV-infected patients requiring acute hospital admission in South Africa: a prospective cohort. BMC Med 2017; 15: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peter JG, Zijenah LS, Chanda D, et al. Effect on mortality of point-of-care, urine-based lipoarabinomannan testing to guide tuberculosis treatment initiation in HIV-positive hospital inpatients: a pragmatic, parallel-group, multicountry, open-label, randomised controlled trial. Lancet 2016; 387: 1187–97 [DOI] [PubMed] [Google Scholar]
- 7.Gupta-Wright A, Fielding KL, van Oosterhout JJ, et al. Rapid urine-based screening for tuberculosis to reduce AIDS-related mortality in hospitalized patients in Africa (the STAMP trial): study protocol for a randomised controlled trial. BMC Infect Dis 2016; 16: 501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta-Wright A, Corbett EL, van Oosterhout JJ, et al. Rapid urine-based screening for tuberculosis in HIV-positive patients admitted to hospital in Africa (STAMP): a pragmatic, multicentre, parallel-group, double-blind, randomised controlled trial. Lancet 2018; 392: 292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walensky RP, Ross EL, Kumarasamy N, et al. Cost-effectiveness of HIV treatment as prevention in serodiscordant couples. N Engl J Med 2013; 369: 1715–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walensky RP, Borre ED, Bekker L-G, et al. The anticipated clinical and economic effects of 90–90-90 in South Africa. Ann Intern Med 2016; 165: 325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO. The use of lateral flow urine lipoarabinomannan assay (LF-LAM) for the diagnosis and screening of active tuberculosis in people living with HIV: policy update. Geneva: World Health Organization, 2015. [Google Scholar]
- 12.Ministry of Health, Malawi. Malawi guidelines for clinical management of HIV in children and adults. 2016. https://aidsfree.usaid.gov/sites/default/files/malawi_art_2016.pdf (accessed May 7, 2018).
- 13.Meintjes G, Moorhouse MA, Carmona S, et al. Adult antiretroviral therapy guidelines 2017 South Afr J HIV Med 2017; 18: 776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA 2016; 316: 1093–103. [DOI] [PubMed] [Google Scholar]
- 15.Lawn SD, Kerkhoff A, Burton R, et al. Massive diagnostic yield of HIV-associated tuberculosis using rapid urine assays in South Africa. Top Antivir Med 2014; 22: 422. [Google Scholar]
- 16.Dodd PJ, Looker C, Plumb ID, et al. Age- and sex-specific social contact patterns and incidence of Mycobacterium tuberculosis infection. Am J Epidemiol 2016; 183: 156–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO. Health economics: choosing interventions that are cost effective (WHO-CHOICE). http://www.who.int/choice/en/ (accessed May 7, 2018).
- 18.Maheswaran H, Petrou S, Cohen D, et al. Economic costs and health-related quality of life outcomes of hospitalised patients with high HIV prevalence: a prospective hospital cohort study in Malawi. PLoS One 2018; 13: e0192991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Health Laboratory Service. http://www.nhls.ac.za/ (accessed May 7, 2018).
- 20.Management Sciences for Health. International medical products price guide. http://mshpriceguide.org/en/home/ (accessed May 7, 2018).
- 21.Sun D, Dorman S, Shah M, et al. Cost utility of lateral-flow urine lipoarabinomannan for tuberculosis diagnosis in HIV-infected African adults. Int J Tuberc Lung Dis 2013; 17: 552–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pooran A, Pieterson E, Davids M, Theron G, Dheda K. What is the cost of diagnosis and management of drug resistant tuberculosis in South Africa? PLoS One 2013; 8: e54587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clinton Health Access Initiative. 2016. antiretroviral (ARV) CHAI reference price list. https://clintonhealthaccess.org/content/uploads/2016/11/2016-CHAI-ARV-Reference-Price-List_FINAL.pdf (accessed May 7, 2018).
- 24.Ford N, Matteelli A, Shubber Z, et al. TB as a cause of hospitalization and in-hospital mortality among people living with HIV worldwide: a systematic review and meta-analysis. J Int AIDS Soc 2016; 19: 20714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steingart KR, Schiller I, Horne DJ, Pai M, Boehme CC, Dendukuri N. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev 2014; 1: CD009593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanifa Y, Fielding KL, Chihota VN, et al. Diagnostic accuracy of lateral flow urine LAM assay for TB screening of adults with advanced immunosuppression attending routine HIV care in South Africa. PLoS One 2016; 11: e0156866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bjerrum S, Kenu E, Lartey M, et al. Diagnostic accuracy of the rapid urine lipoarabinomannan test for pulmonary tuberculosis among HIV-infected adults in Ghana-findings from the DETECT HIV-TB study. BMC Infect Dis 2015; 15: 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO. Health economics. Health service delivery costs. http://www.who.int/choice/cost-effectiveness/inputs/health_service/en/ (accessed May 7, 2018).
- 29.Department of Health, Republic of South Africa. South African Medicine Price Registry, http://www.mpr.gov.za/ (accessed May 7, 2018).
- 30.Briggs AH, Weinstein MC, Fenwick EAL, et al. Model parameter estimation and uncertainty analysis: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force Working Group-6. Med Decis Making 2012; 32: 722–32. [DOI] [PubMed] [Google Scholar]
- 31.International Monetary Fund. World economic outlook (October 2017)—GDP per capita, current prices. http://www.imf.org/external/datamapper/NGDPDPC@WEO (accessed May 7, 2018).
- 32.Robinson LA, Hammitt JK, Chang AY, Resch S. Understanding and improving the one and three times GDP per capita cost-effectiveness thresholds. Health Policy Plan 2017; 32: 141–45. [DOI] [PubMed] [Google Scholar]
- 33.Marseille E, Larson B, Kazi DS, Kahn JG, Rosen S. Thresholds for the cost-effectiveness of interventions: alternative approaches. Bull World Health Organ 2015; 93: 118–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andrews JR, Lawn SD, Dowdy DW, Walensky RP. Challenges in evaluating the cost-effectiveness of new diagnostic tests for HIV-associated tuberculosis. Clin Infect Dis 2013; 57: 1021–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chapman RH, Berger M, Weinstein MC, Weeks JC, Goldie S, Neumann PJ. When does quality-adjusting life-years matter in cost-effectiveness analysis? Health Econ 2004; 13: 429–36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.