Abstract
Rationale:
Marfan syndrome (MFS) is a genetic disorder of the connective tissue. MFS has an incidence of about 2 to 3 persons per 10,000 population. MFS is characterized majorly by the involvement of the eyes, skeletal muscles, and cardiovascular system. There are limited case reports of co-existence of MFS and type 2 diabetes mellitus (T2DM).
Patient concerns:
A 16-year-old male patient who got admitted to our hospital with complaints of loss of vision from left eye for the last 3 days.
Diagnosis of MFS along with luxation of left eye lens, and T2DM were made according to the patient's symptoms, signs, biochemical results, and ultrasonography.
Interventions:
The patient received “vitrectomy (posterior approach (left eye)) + cataract extraction (left eye) + intraocular lens implantation (left eye) surgery” for luxation of left eye lens. The patient received “Bentall Operation” for MFS, and was prescribed warfarin 5 mg qod and spironolactone 20 mg bid during the follow-up period. The patient received continuous subcutaneous insulin infusion (CSII) during hospitalization, and then changed to insulin glargine preparation during the follow-up period.
Outcomes:
The vision was restored after the eye surgery and the patient also recovered well after the Bentall Operation. Additionally, there were no obvious complications during hospitalization and the follow-up period. Blood glucose levels were within normal range.
Lessons:
There is a need to improve the recognition of MFS among school and community doctors. Early detection, diagnosis, and treatment of this rare disease can improve the quality of patient's life.
Keywords: Marfan syndrome, type 2 diabetes mellitus
1. Introduction
Marfan syndrome (MFS) is an autosomal dominant multisystem disorder of the connective tissue.[1] The cardiovascular manifestations of MFS include mitral valve prolapse, dilation of the aorta, and aortic dissection. The ocular and skeletal manifestations include displacement of the lens, disproportionally long limbs, and enlarged duramater. MFS may be associated with a mutation of fibrillin and/or transforming growth factor beta receptor (TGFBR) 1 or 2. Type 2 diabetes mellitus (T2DM) is characterized initially by insulin resistance and hyperinsulinemia and it eventually results in glucose intolerance, hyperglycemia, and overt diabetes.[2] We report a case of a 16-year-old male patient diagnosed with MFS and T2DM.
2. Case report
A 16-year-old male patient was admitted to our hospital on November 27, 2017 with complaints of loss of vision from the left eye for the last 3 days. He was diagnosed with left eye lens luxation in our hospital. Additionally, the patient was diagnosed with MFS as he had an aortic root Z-score of 4.81 and his mother was also a known case of MFS. During hospitalization in the Ophthalmology Department, the patient's fasting plasma glucose (FPG) levels were between 12.1 and 15.4 mmol/L and his 2-hour postprandial glucose (PPG) was between 13.4 and 17.8 mmol/L; however, he neither felt intense thirst nor had complaint of polyuria. He received “vitrectomy + posterior approach (left eye) + cataract extraction (left eye) + intraocular lens implantation (left eye) surgery” on November 28, 2017 and was then transferred to the Endocrinology Department on November 30, 2017.
The patient characteristics are mentioned in Table 1. Physical examination revealed the presence of arachnodactyly (Figs. 1 and 2); abnormally long fingers and toes as compared to the palm of hands and the arch of toes) and dolichostenomelia (abnormally long limbs), as well as a positive Walker Murdoch wrist sign. The patient had no family history of DM. The protein-creatinine ratio, levels of pituitary hormones, body mass index, HBV markers, thyroid function, and renal functions of the patient were within the normal range. The islet cell antibody (ICA), glutamic acid decarboxylase antibody (GAD), insulin autoantibodies (IAA), tyrosine phosphatase antibody (IA-2A), and zinc transporter-8 levels were negative. The lateral whole spine radiograph revealed a slight angulation deformity, while cervical and cerebral Doppler ultrasonography demonstrated normal results. The patient was diagnosed with T2DM and was started on a continuous subcutaneous insulin infusion (CSII) on the first day of his transfer to the Endocrinology department. After 8 days of CSII administration, the FPG levels were 8.1 to 9.4 mmol/L, and the 2-hour PPG levels were 8.7 to 10.3 mmol/L. The patient was transferred to the Cardiac Surgery Department with an insulin pump on December 8, 2017. He underwent “Bentall Operation” to replace the aortic root on December 18, 2017. Over the next 20 days, the patient recovered from the surgery and on January 14, 2018, insulin glargine (Lantus) injection was administered instead of CSII. The resulting FPG levels were 5.1 to 6.0 mmol/L and the 2-hour PPG levels were in the range of 6.2 to 7.3 mmol/L between January 14 and February 15, 2018. The patient was discharged from the hospital on February 15, 2018. During the first follow-up, that is, 15 days following his discharge, the patients’ glucose parameters (FPG and PPG) were within the normal range and he was in a healthy condition. He was taking warfarin sodium 5 mg qod, spironolactone tablets 20 mg bid, and insulin glargine (Lantus) 35 U qd subcutaneous injection during the follow-up.
Table 1.
Patient characteristics.
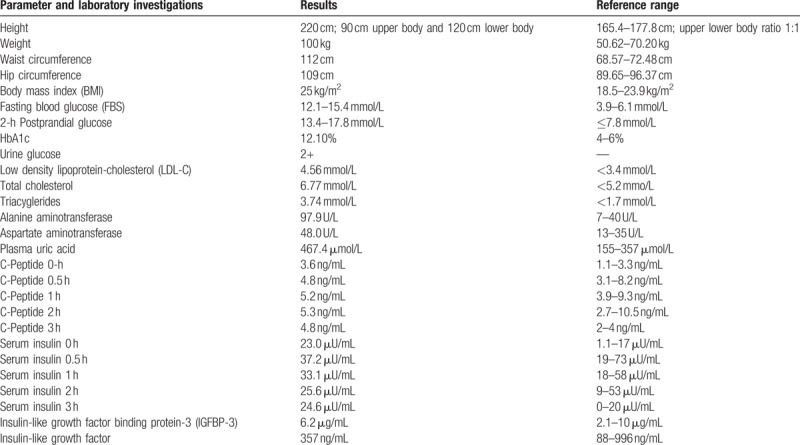
Figure 1.
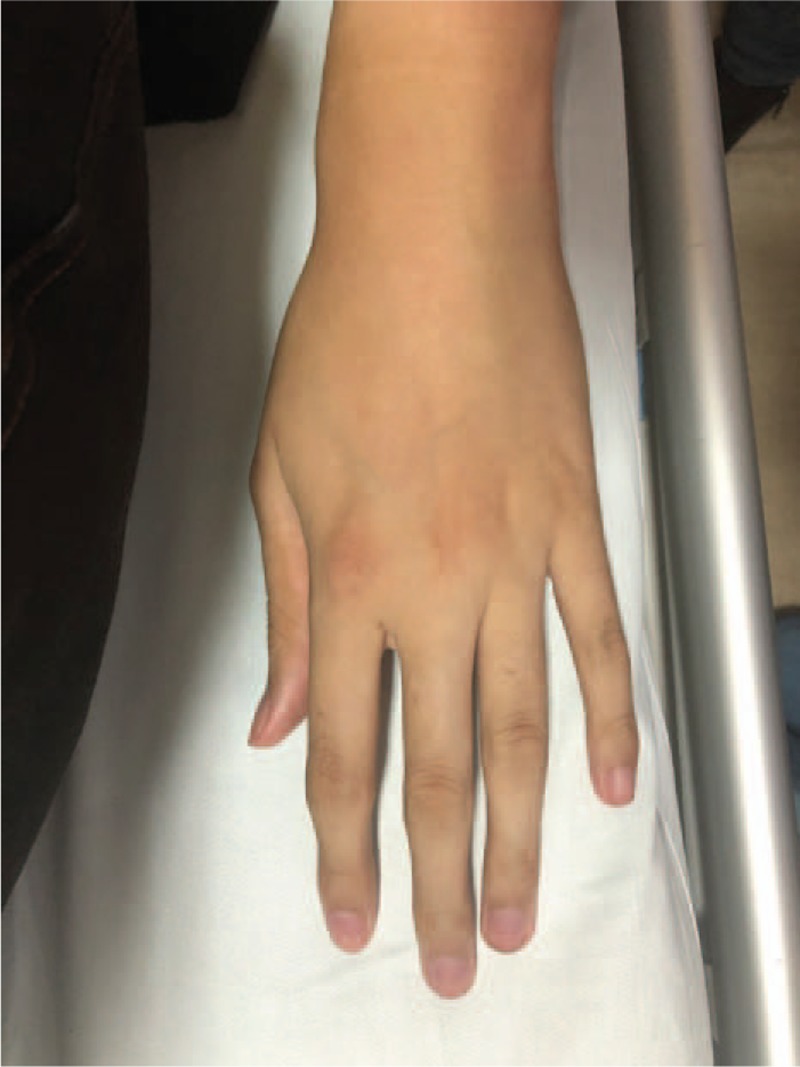
Presence of arachnodactyly: Image displaying the patient's abnormally long fingers.
Figure 2.
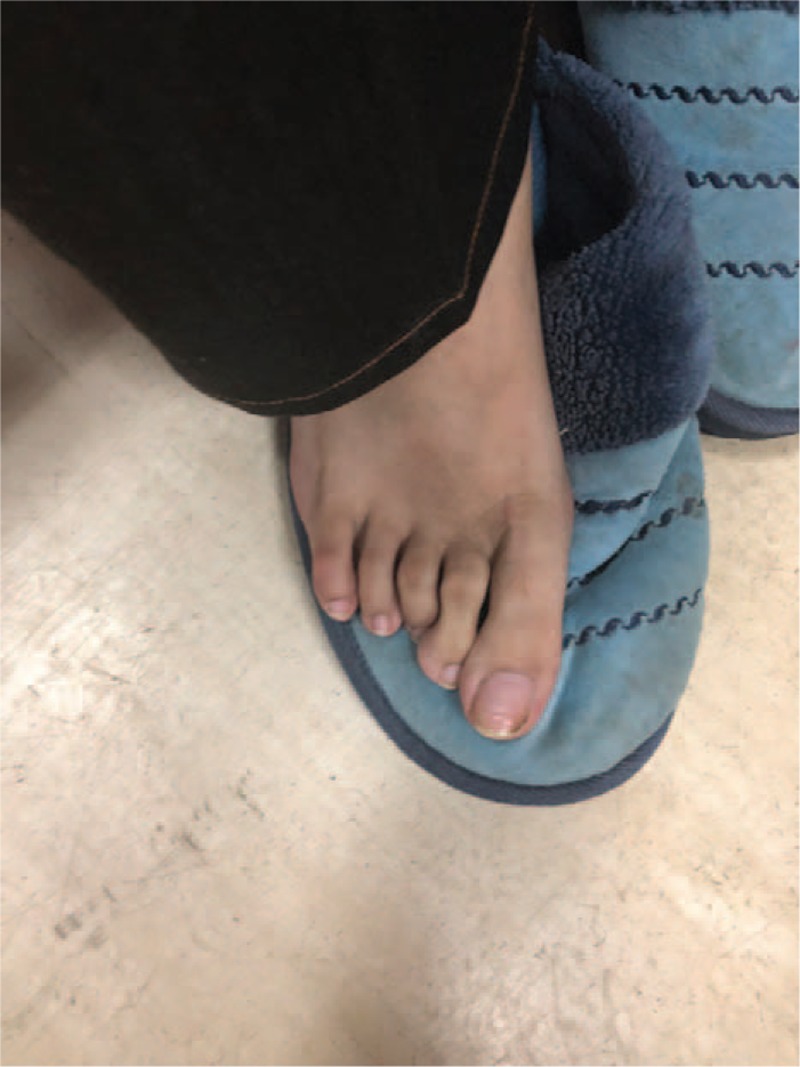
Presence of arachnodactyly: Image displaying the patient's abnormally long toes.
The ethical approval was not necessary for this case report. Informed consent was obtained from the patient.
3. Discussion
MFS was first described in 1896 by a French pediatrician.[3] Due to the involvement of the cardiovascular system, the skeletal system, and the eye, MFS can limit the quality of life of patients. MFS is associated with a mutation of the FBN1 gene, which encodes the matrix protein, fibrillin, a major component of the extracellular microfibrils of the connective tissue.[4] Additionally, there is a dysregulation of cytokine-transforming growth factor beta (TGF-β) signaling.[5] The possible role of a mutation of the gene encoding TGFBR has widened the scope of the etiopathogenesis of MFS. The Ghent II diagnostic criteria has added the fibrillin mutation as an additional factor in the diagnosis of MFS, in addition to the clinical symptoms that are used in the Berlin criteria.[3]
Worldwide, the incidence of MFS ranges from 4 to 20/100,000. Sun et al reported a MFS prevalence of 17.2/100,000 among the Chinese population.[6] There is no effect of race or gender on the incidence or prevalence of MFS. In addition to the known characteristic symptoms of arachnodactyly, ectopia lentis and dilated aortic root, patients with MFS may have elongation of intracranial arteries, cerebral aneurysm, as well as increased length and tortuosity of the basilar artery, which might lead to neurovascular compression.[5] Additionally, case reports of concomitant diseases with MFS have also been reported. There are a limited number of cases of MFS in which hemifacial spasm and trigeminal neuralgia were observed.[5] A literature search revealed several case reports demonstrating the concomitant occurrence of MFS and DM. The first case of the coexistence of MFS and DM from Japan was reported in 1992. The patient was described as a case of T1DM, who was later diagnosed with MFS at the age of 15. The observed clinical features included systolic murmur, mitral valve prolapse, and arachnodactyly.[7] Another case of the coexistence of diabetic retinopathy and MFS was reported in a 32 year male patient.[8] The patient had a family history of T2DM and MFS in his mother and sister. Additionally, a case of coexistence of MFS and T1DM in a young 17-year-old male patient has been reported from India.[9] Furthermore, there was an additional case reporting the coexistence of MFS, Banti's syndrome, and DM.[10]
The association between MFS and DM is not clear, however, the mutation of TGF-β might play a critical role. Various vascular pathologies are involved in both MFS and DM. TGF-β plays an important role in both of these diseases. TGF-β1 is a polypeptide member of the transforming growth factor beta superfamily of cytokines. A decrease in fibrillin-1 is associated with increase in TGF-β, which may lead to the inflammatory manifestations of MFS.[8] TGF-β1 is an important factor in the control of the immune system, and it has multiple actions on different cell types at different developmental stages. The majority of immune cells (or leukocytes) secrete TGF-β1.[11] In T2DM, TGF-β can mediate the inflammatory reaction and the immune response, which can further lead to endothelial dysfunction and may be involved in arteriosclerosis.[12] Fibrillin-1[11] and TGF-β also participate in the development and progression of diabetic nephropathy and retinopathy. Although further evaluation is required, till now it appears that TGF-β may be the link between MFS and T2DM. There may be a plausible role of matrix metalloproteinases (MMPs) in the pathogenesis of MFS and diabetic retinopathy. The expression of MMPs is increased in both DM and MFS. MMPs are proteases that are responsible for the degradation of extracellular matrix proteins, and increased levels of proteases are associated with tissue destruction.[12]
In patients with both MFS and T2DM, there should be more focus on vascular complications, early diagnosis, and proper glycemic control. The current case of the coexistence of MFS and T2DM adds evidence to the limited data that is available regarding the coexistence of these 2 disease states. Future research and reporting of cases will help to increase our understanding of the occurrence of T2DM among patients with MFS.
Author contributions
Conceptualization: Bimin Shi, Ke Chen.
Formal analysis: Chao Chen, Ke Chen.
Funding acquisition: Xuan Du, Chao Chen.
Investigation: Yingyi Zhou, Xuan Du, Chao Chen, Bimin Shi, Ke Chen.
Methodology: Chao Chen.
Project administration: Xuan Du, Bimin Shi.
Resources: Xuan Du.
Software: Xuan Du, Chao Chen, Bimin Shi.
Supervision: Bimin Shi, Ke Chen.
Validation: Yingyi Zhou.
Visualization: Yingyi Zhou.
Writing – original draft: Yingyi Zhou.
Writing – review & editing: Yingyi Zhou.
Footnotes
Abbreviations: CSII = continuous subcutaneous insulin infusion, FPG = fasting plasma glucose, GAD = glutamic acid decarboxylase antibody, IA-2A = tyrosine phosphatase antibody, IAA = insulin autoantibodies, ICA = islet cell antibody, MFS = Marfan syndrome, PPG = postprandial glucose, T2DM = type 2 diabetes mellitus, TGFBR = transforming growth factor beta receptor.
The authors have no conflicts of interest to disclose.
References
- [1].Pyeritz RE, McKusick VA. The Marfan syndrome: diagnosis and management. N Engl J Med 1979;300:772–7. [DOI] [PubMed] [Google Scholar]
- [2].Rossi G. American Diabetes Association. [Diagnosis and classification of diabetes mellitus]. Recenti Prog Med 2010;101:274–6. [PubMed] [Google Scholar]
- [3].Judge DP, Dietz HC. Marfan's syndrome. Lancet 2005;366:1965–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Adlakha A, Dhar KL. Marfanoid hypermobility syndrome. J Indian Med Assoc 1988;86:103–5. [PubMed] [Google Scholar]
- [5].Sakakura K, Akutsu H, Yamamoto T, et al. Trigeminal neuralgia in a patient with Marfan syndrome: case report. Neurol Med Chir (Tokyo) 2015;55:101–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Groth KA, Hove H, Kyhl K, et al. Prevalence, incidence, and age at diagnosis in Marfan syndrome. Orphanet J Rare Dis 2015;10:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yamamoto T, Inoue F, Matsumura A, et al. Report of a Japanese girl with Marfan syndrome associated with insulin-dependent diabetes mellitus. Acta Paediatr Jpn 1992;34:551–3. [DOI] [PubMed] [Google Scholar]
- [8].Ka PY, Cristina AM, Adel MB, et al. Marfan syndrome and early-onset diabetic retinopathy: a case report. Int J Endocrinol Metab 2009;1:41–5. [Google Scholar]
- [9].Kumar KH, Chandrasekhar P. Marfan syndrome with type-1 diabetes and pulmonary tuberculosis—a rare case. Indian Heart J 2015;67:S119. [Google Scholar]
- [10].Yamamoto K, Ito K, Wakasugi H, et al. [A case of Marfan's syndrome with Banti's syndrome and diabetes mellitus]. Nihon Naika Gakkai Zasshi 1966;55:185–9. [DOI] [PubMed] [Google Scholar]
- [11].Hartner A, Schaefer L, Porst M, et al. Role of fibrillin-1 in hypertensive and diabetic glomerular disease. Am J Physiol Renal Physiol 2006;290:F1329–36. [DOI] [PubMed] [Google Scholar]
- [12].Sorsa T, Tjaderhane L, Salo T. Matrix metalloproteinases (MMPs) in oral diseases. Oral Dis 2004;10:311–8. [DOI] [PubMed] [Google Scholar]


