Abstract
Rationale:
Ovarian mucinous tumor with malignant mural nodule is exceedingly rare. We report a case of ovarian mucinous cystic tumor associated with sarcomatous mural nodule and benign Brenner tumor and accompanied by nodular histiocytic aggregates in the greater omentum.
Patient concerns:
A 60-year-old postmenopausal woman was presented with a history of one month of lower abdominal discomfort, abdominal distension, nausea, and vomiting. A physical examination revealed a hard, palpable mass with mild tenderness in her right lower abdomen.
Diagnoses:
The mucinous tumor was solid and cystic and contained benign, borderline, and malignant elements. Within the solid areas, two nodules representing pleomorphic undifferentiated sarcoma and benign Brenner tumor were identified. The diagnosis of malignant mural nodule was based on vascular invasion and marked nuclear atypia, including atypical mitoses and mitotic activity.
Interventions:
Bilateral salpingo-oophorectomy and partial omentectomy were performed. Malignant cells were not found on cytologic examination of the peritoneal washing fluid. The patient underwent three cycles of chemotherapy with 210 mg paclitaxel liposome via an intravenous drip, 20 mg nedaplatin via an intravenous drip, and 80 mg nedaplatin via intraperitoneal perfusion.
Outcomes:
The patient has been followed up for 3 years without evidence of tumor recurrence and metastasis.
Lessons:
Careful classification of a mural nodule is important to triage patients in need of aggressive adjuvant treatment.
Keywords: Brenner tumor, mucinous cystic tumor, mural nodule, ovarian tumor, pleomorphic undifferentiated sarcoma
1. Introduction
Ovarian mucinous cystic tumors are commonly associated with other types of ovarian neoplasms, for example, Brenner tumor.[1] However, ovarian mucinous tumors with mural nodules are rare. Most such nodules are reactive or benign, although there have been sporadic reports of malignant mural nodules, such as anaplastic carcinoma, clear cell carcinoma, and neuroendocrine carcinoma, giant-cell carcinoma, carcinosarcoma, and sarcoma. The most common type of mural nodular malignancy is anaplastic carcinoma; however, a few cases of sarcoma have also been reported.[2–4] Cases of pleomorphic undifferentiated sarcoma as a primary ovarian tumor[5] or as a component of a teratoma[6] are rare, and to our knowledge, pleomorphic undifferentiated sarcoma as a malignant mural nodule in an ovarian mucinous neoplasm has never been reported. Herein, we describe the case of a postmenopausal woman with an ovarian intermixed mass composed of a mucinous cystic tumor and mural nodules of pleomorphic undifferentiated sarcoma and benign Brenner tumor and associated with multifocal nodular histiocytic aggregates on the surface of the greater omentum. The clinicopathological characteristics in this patient are discussed.
2. Case presentation
A 60-year-old postmenopausal woman (gravida 4, para 1) was presented with a history of one month of lower abdominal discomfort, abdominal distension, nausea, and vomiting. A physical examination revealed a hard, palpable mass with mild tenderness in her right lower abdomen. An ultrasound scan and a computed tomography scan of the abdomen revealed a large oval cystic and solid mass measuring 9.4 cm × 8.4 cm × 8.3 cm with irregular separation. The preoperative serum levels of cancer antigen (CA)-125, CA19-9, CA72-4, human epididymis protein 4, carcinoembryonic antigen, and alpha-fetoprotein were all within the normal range. A laparotomy revealed a large right ovarian cystic mass with an old surface rupture (1.0 cm × 1.0 cm) and proliferation of granulation tissue. Bilateral salpingo-oophorectomy and partial omentectomy were performed. Malignant cells were not found on cytologic examination of the peritoneal washing fluid. The tumor was staged as International Federation of Gynecology and Obstetrics (FIGO) grade IC. The postoperative course was uneventful. The patient underwent three cycles of chemotherapy with 210 mg paclitaxel liposome via an intravenous drip, 20 mg nedaplatin via an intravenous drip, and 80 mg nedaplatin via intraperitoneal perfusion. She has been followed up for 3 years without evidence of tumor recurrence and metastasis.
Macroscopic examination of the right ovary showed a brownish mass with a smooth outer surface. The mass was composed of multilocular cysts filled with turbid tan fluid or clear mucinous material. Yellowish papillary structures were found projecting into the cystic cavities. The septa of these cysts had uneven thickness, from 0.2 cm to 0.5 cm. The mass contained two distinct solid nodules. The larger one measured 3.0 cm × 2.8 cm × 2.5 cm, protruded into the cystic lumen, and was well-circumscribed. It was gray-brown, with a medium consistency and visible hemorrhage and necrosis. The smaller nodule measured 1.6 cm in diameter and was gray and hard. The right fallopian tube was found adherent to the capsule of the tumor. The left ovary and fallopian tubes were unremarkable. The surface of the greater omentum showed numerous small sallow nodules of various sizes, from 0.2 to 0.4 cm in diameter.
Microscopic examination showed that the right ovarian tumor was composed of three distinct components. The cystic component was preponderant, and the cysts were lined largely by low columnar or cuboidal well-differentiated mucinous epithelium, indicating a benign tumor with focal borderline changes and intraepithelial carcinoma (Fig. 1A). The second component was mesenchymal. The larger mural nodule underneath the mucinous epithelium consisted of ovoid mononucleated cells and numerous multinucleated osteoclast-like giant cells (Fig. 1B). The mononucleated cells contained abundant eosinophilic cytoplasm with ill-defined borders and showed marked nuclear pleomorphism and atypia with vesicular hyperchromatic nuclei and prominent nucleoli. Multinucleated giant cells were distributed evenly between the mononuclear cells. Foci of extensive or focal necrosis and hemorrhage presented throughout the tumor, but only a sparse inflammatory infiltrate was observed. Local hypocellular areas formed spongy-appearing foci, which were filled with diffusate or erythrocytes. Mitotic figures were frequent and sometimes atypical (Fig. 1C), and approximately 25 mitoses per 10 high-power fields were recorded. Vascular invasion was also observed in this mural nodule (Fig. 1D). The larger nodule was sharply demarcated from the epithelial elements. As the third component, the smaller nodule consisted of nests of transitional epithelium, which embedded in a dense fibromatous stroma (Fig. 1E). Occasional transitional nests with central dilation were present in the transition areas of mucinous tumor and Brenner tumor. The greater omental lesions consisted of little nests or sheets of cohesive polygonal-to-oval epithelioid cells with distinct cell borders, abundant cytoplasm, and inconspicuous nucleoli (Fig. 1F).
Figure 1.
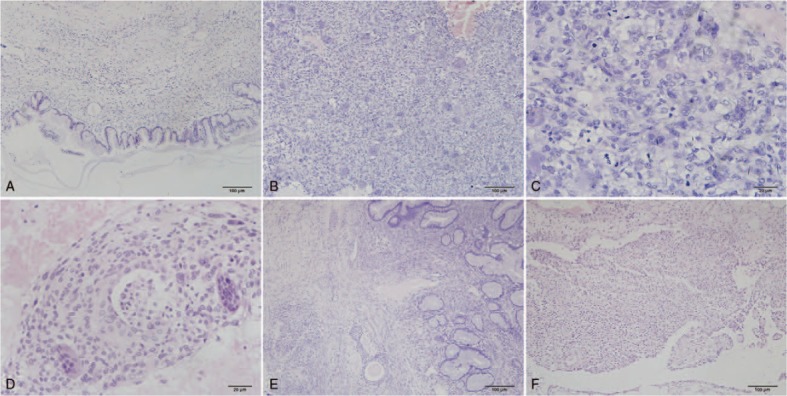
Microscopic appearance of ovarian tumor and greater omental lesion (hematoxylin-eosin staining). A: A mucinous cystadenoma is the main part of the epithelial elements (×100). B: The sarcomatous mural nodule consists of ovoid mononucleated cells and numerous multinucleated osteoclast-like giant cells (×100). C: Nine mitotic figures are observed in one high-power field in the mural nodule consisting of pleomorphic undifferentiated sarcoma (×400). D: Vascular invasion is observed in the lager mural nodule (×200). E: Benign Brenner tumor is composed of nests of transitional epithelium (×100). F: The greater omental lesion shows nodular histiocytic aggregates.
Immunohistochemical staining for two mural nodules was performed using the Ventana BenchMark XT system (Roche Ltd., Basel, Switzerland). The mononucleated and multinucleated cells in the sarcomatous mural nodule were positive for vimentin (Fig. 2A), cluster of differentiation (CD)56, CD68 (Fig. 2B), and human alpha1 antichymotrypsin, but negative for pan-cytokeratin (CK), CK7, CK20, desmin, α-smooth muscle actin, calretinin, CD10, and P63. Some mononucleated cells showed positive staining for S-100 protein. Staining with CD34 revealed intravascular tumor emboli and vascular invasion (Fig. 2C). MIB-1 staining indicated that the mononucleated cells had very high proliferative activity (approximately 35%). The mononuclear cells on the surface of the greater omentum showed diffuse and strong reactivity against vimentin (Fig. 3A) and CD68 (Fig. 3B). There were a few scattered mesothelial cells among the mononuclear cells that stained positive for calretinin (Fig. 3C) and CK5/6. MIB-1 staining indicated that these cells had very low proliferative activity (approximately 3%).
Figure 2.
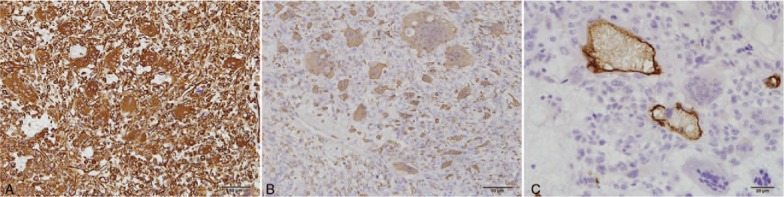
Immunohistochemical features of mural nodule of pleomorphic undifferentiated sarcoma. Mononucleated and multinucleated cells are positive for vimentin (A, ×200) and CD68 (B, ×200). CD34 staining shows tumor cells infiltrating capillary vessels (C, ×400).
Figure 3.
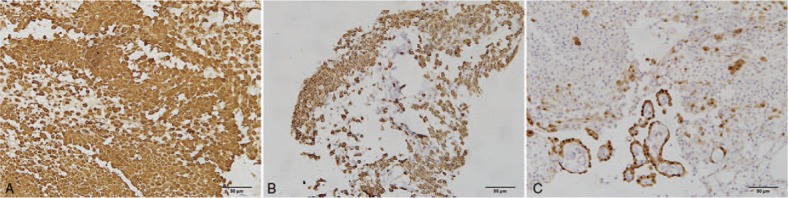
Immunohistochemical features of nodular histiocytic aggregates in the greater omentum (×200). The mononuclear cells on the surface of the greater omentum show diffuse and strong reactivity against vimentin (A), CD68 (B), and scattered positivity for calretinin (C).
The final diagnosis was ovarian mucinous cystic tumor (including mucinous cystadenoma, mucinous cystadenoma of borderline malignancy, and intraepithelial carcinoma) associated with mural nodules of pleomorphic undifferentiated sarcoma and benign Brenner tumor, and accompanied by nodular histiocytic aggregates in the greater omentum.
3. Discussion
Mural nodules of different origins have been described in ovarian mucinous tumors in a number of reports.[2,3,7] Sarcoma-like mural nodules are the most common ones and have a favorable prognosis. Malignant mural nodules are infrequent, including foci of anaplastic carcinoma and sarcomatous or carcinosarcomatous mural nodules. Although the relationship between ovarian mucinous tumor and Brenner tumor is well known, the coexistence of three types of ovarian tumors has not yet been reported. This report describes an ovarian intermixed tumor composed of mucinous cystic tumor, mural nodules of pleomorphic undifferentiated sarcoma and benign Brenner tumor, and accompanied by nodular histiocytic aggregates in the greater omentum.
This tumor was composed of malignant epithelial and mesenchymal elements, which distinguished it from a malignant mixed mesodermal tumor. Although the sarcomatous nodule was observed within the ovarian mucinous cystic tumor, the nodule was sharply demarcated from the epithelial component without an intimate admixture of neoplastic epithelium and sarcoma. In addition, the epithelial component showed mucinous cystadenoma of borderline malignancy with focal intraepithelial carcinoma, which is different from the high-grade adenocarcinoma of malignant mixed mesodermal tumor. The immunoprofile of the sarcomatous component in this case failed to assign the nodule to any of the other sarcomas due to its lack of immunoreactivity for markers of smooth muscle, skeletal muscle, and endothelial differentiation, in addition to its negativity for epithelial and mesothelial markers. Therefore, it was interpreted as a pleomorphic undifferentiated sarcoma, not otherwise specified.
Mural nodules of pleomorphic undifferentiated sarcoma have similar histomorphological features with sarcoma-like mural nodules, including a composition of mononucleated cells and multinucleated giant cells, cellular pleomorphism and atypia, numerous mitotic figures, and extensive necrosis; therefore, their differential diagnosis is very difficult. Prat and Scully reported seven cases of sarcoma-like nodules in mucinous tumors.[7] Upon reviewing the clinicopathological data of the seven cases, they presented evidence that these nodular proliferations were not sarcomatous although they had been generally regarded as such. It is generally considered that vascular invasion is the most reliable evidence for confirming the diagnosis of sarcomatous mural nodule, ill demarcation, marked nuclear atypia, and numerous atypical mitotic figures are helpful to distinguish sarcomatous nodules from sarcoma-like nodules. In addition, cytokeratin staining is crucial to distinguish ovarian mucinous tumors with sarcomatous mural nodules from anaplastic carcinomas in problematic cases. The former usually show negative or focally positive staining, while the latter exhibit strong positivity.
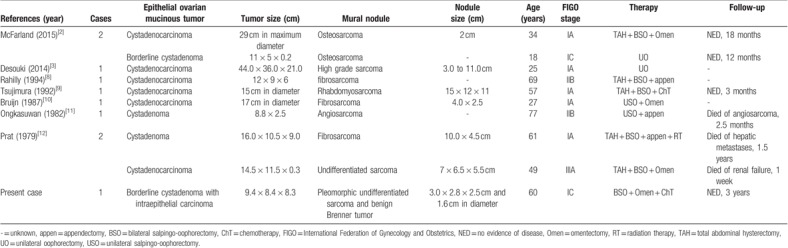
Ovarian mucinous tumor associated with a sarcomatous mural nodule is an exceedingly rare neoplasm, and an extensive review of the English literature showed only nine well-documented cases (Table 1).[2,3,8–12] The reported occurrences involve patients with a wide age range, from 18 to 77 years old, with a median age of 53 years. Clinically, patients usually present with lower abdominal pain or abdominal discomfort. Although most patients are in the early clinical stage, they usually present with a poor prognosis. The mortality rate is up to 43% within 1.5 years, although the follow-up period of most reported cases was no more than 1.5 years. Based on a small number of cases, this finding raises the possibility that elderly patients have a worse prognosis. The present case had the longest follow-up period, and the patient had no evidence of tumor recurrence and metastasis. Careful classification of a mural nodule is important to triage patients in need of aggressive adjuvant treatment. The outcome is potentially favorable in patients with a small mural nodule who are in the early clinical stage and undergo appropriate treatments.
Table 1.
Summary of cases of ovarian mucinous cystic tumor with sarcomatous mural nodules.
Although ovarian mucinous tumors are rarely associated with transitional metaplasia, Brenner tumors tend to be related with mucinous tumors in a significant number of cases. Roma et al reported that up to 27% of Brenner tumors were associated with mucinous tumors, and mucinous epithelium was seen in 47% of all Brenner tumors.[13] Many morphological, immunohistochemical, and molecular studies suggest a common origin, at least when these tumors occur within the same ovary.[1,13,14] The transitional epithelium of the Brenner tumor undergoes cell lineage reprogramming to mucinous epithelium through metaplasia, and the latter then proliferates and gives rise to a mucinous tumor.[13,14] As in our case, Brenner tumor associated with mucinous tumor usually develops in an older patient, and the mucinous component accounts for most of the tumor size.[15]
Nodular histiocytic aggregate in the omentum is a rare benign proliferative process composed predominantly of histiocytes with scattered mesothelial cells. Similar cases of borderline mucinous cystadenoma with nodular histiocytic aggregates have been reported.[16] The majority of cells in this lesion are histiocytes. This focal aggregation of histiocytes might result from irritation by the tumor and inflammation.
In conclusion, we have described an ovarian mucinous cystic tumor associated with mural nodules of pleomorphic undifferentiated sarcoma and benign Brenner tumor and accompanied by nodular histiocytic aggregates in the greater omentum. The sarcomatous nodule with pleomorphic and atypical mononucleated and multinucleated cells is likely to behave aggressively. Numerous atypical mitotic figures, extensive or focal necrosis, and vascular invasion were found. The patient received chemotherapy and is still alive after 3 years. This is the first report of such a complex tumor arising in an ovarian mucinous cystic tumor.
Author contributions
Methodology: Shaolong Yang, Li Wang.
Resources: Shaolong Yang, Li Wang.
Writing – original draft: Shaolong Yang, Kai Sun.
Footnotes
Abbreviations: CD = cluster of differentiation, CK = cytokeratin, FIGO = International Federation of Gynecology and Obstetrics.
SY and LW contributed equally to this work.
Consent for publication: Informed consent for publication was obtained from the patient.
Funding: This study was supported by The Science and Technology Innovation Team Project of Zhengzhou Railway Vocational and Technical College (17KJCXTD01).
The authors have no conflicts of interest to disclose.
References
- [1].Tafe LJ, Muller KE, Ananda G, et al. Molecular genetic analysis of ovarian Brenner tumors and associated mucinous epithelial neoplasms: high variant concordance and identification of mutually exclusive RAS driver mutations and MYC amplification. Am J Pathol 2016;186:671–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].McFarland M, Dina R, Fisher C, et al. Osteosarcoma as malignant mural nodule in ovarian mucinous neoplasms of intestinal type: report of 2 cases. Int J Gynecol Pathol 2015;34:369–73. [DOI] [PubMed] [Google Scholar]
- [3].Desouki MM, Fadare O, Kanbour A, et al. Immunophenotype and K-RAS mutation in mucinous ovarian adenocarcinoma with mural nodule of high-grade sarcoma: case report. Int J Gynecol Pathol 2014;33:186–90. [DOI] [PubMed] [Google Scholar]
- [4].Zhang Y, Yuan Z, Sun K, et al. Ultrasonic and pathological characteristics of ovarian mucinous cystic tumors with malignant mural nodules: two cases report. Medicine (Baltimore) 2017;96:e8636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kurtoglu E, Celik H, Kokcu A, et al. Undifferentiated pleomorphic sarcoma with focally rhabdomyosarcomatous differentiation of the ovary. Eur J Gynaecol Oncol 2016;37:401–3. [PubMed] [Google Scholar]
- [6].Savitchi E, Rao S. Squamous cell carcinoma and pleomorphic sarcoma (MFH) arising in a mature cystic teratoma of the ovary. Int J Gynecol Pathol 2012;31:443–6. [DOI] [PubMed] [Google Scholar]
- [7].Prat J, Scully RE. Ovarian mucinous tumors with sarcoma-like mural nodules: a report of seven cases. Cancer 1979;44:1332–44. [DOI] [PubMed] [Google Scholar]
- [8].Rahilly MA, Candlish W, Al-Nafussi A. Fibrosarcoma arising in an ovarian mucinous tumor: a case report. Int J Gynecol Cancer 1994;4:211–4. [DOI] [PubMed] [Google Scholar]
- [9].Tsujimura T, Kawano K. Rhabdomyosarcoma coexistent with ovarian mucinous cystadenocarcinoma: a case report. Int J Gynecol Pathol 1992;11:58–62. [DOI] [PubMed] [Google Scholar]
- [10].Bruijn JA, Smit VT, Que DG, et al. Immunohistology of a sarcomatous mural nodule in an ovarian mucinous cystadenocarcinoma. Int J Gynecol Pathol 1987;6:287–93. [DOI] [PubMed] [Google Scholar]
- [11].Ongkasuwan C, Taylor JE, Tang CK, et al. Angiosarcomas of the uterus and ovary: clinicopathologic report. Cancer 1982;49:1469–75. [DOI] [PubMed] [Google Scholar]
- [12].Prat J, Scully RE. Sarcomas in ovarian mucinous tumors: a report of two cases. Cancer 1979;44:1327–31. [DOI] [PubMed] [Google Scholar]
- [13].Roma AA, Masand RP. Different staining patterns of ovarian Brenner tumor and the associated mucinous tumor. Ann Diagn Pathol 2015;19:29–32. [DOI] [PubMed] [Google Scholar]
- [14].Wang Y, Wu RC, Shwartz LE, et al. Clonality analysis of combined Brenner and mucinous tumours of the ovary reveals their monoclonal origin. J Pathol 2015;237:146–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Abbas AM, Amin MT. Brenner's tumor associated with ovarian mucinous cystadenoma reaching a huge size in postmenopausal woman. J Cancer Res Ther 2015;11:1030. [DOI] [PubMed] [Google Scholar]
- [16].Lv Y, Li P, Zheng J, et al. Nodular histiocytic aggregates in the greater omentum of patients with ovarian cancer. Int J Surg Pathol 2012;20:178–84. [DOI] [PubMed] [Google Scholar]


