Abstract
Rationale:
Lung cancer is a series of gene-driven disease. EGFR, ALK, and ROS1 are 3 major driver genes that play an important role in lung cancer development and precision management. Additionally, rare genetic alterations continue to be discovered and may become novel targets for therapy. The RET gene is one of such rare genetic alteration of non-small cell lung cancer (NSCLC). In this report, we present a RET-positive case that benefited from cabozantinib treatment.
Patient concern:
A 50-year-old male patient was diagnosed with lung adenocarcinoma 2 years ago, at that time he received palliative surgery of pulmonary carcinoma and completed 4 cycles of chemotherapy with gemcitabine and cisplatin. Six months later, he was hospitalized in our cancer center due to the disease recurrence, presenting with pleural metastasis.
Diagnosis:
Gene alteration was examined using the intraoperative specimen by PCR method, and KIF5B/RET gene fusion was detected. Therefore, the patient was diagnosed with late-stage lung adenocarcinoma with RET gene mutation.
Interventions:
The patient received treatment with cabozantinib from June 2017.
Outcomes:
Cabozantinib was administered (140 mg orally, once daily) for approximate 9 months, and his disease achieved stable disease (SD). During that period, there were no severe adverse events (AE), except for a grade II rash (CTCAE 4.0).
Lessons:
We found that the RET fusion gene is a novel driver molecular of lung adenocarcinoma in patients without common mutations in such genes as EGFR, ALK, and ROS1. This case report supports a rationale for the treatment of lung adenocarcinoma patients with a RET fusion and provides alternative treatment options for these types of NSCLC patients.
Keywords: cabozantinib, non-small-cell lung cancer, RET gene, targeted therapy
1. Introduction
Lung cancer is a series of gene-driven disease. EGFR, ALK, and ROS1 are 3 major driver genes that play an important role in lung cancer development and precision management.[1] Additionally, a rare genetic alteration, which is called RET rearrangement, is detected in 1% to 2% of non-small cell lung cancer (NSCLC).[2] On the other hand, Gene rearrangements involving RET, have been characterized most extensively in papillary thyroid carcinomas, and later have been observed in other cancers, especially lung cancer.[3] Cabozantinib (XL184) is a small-molecule kinase inhibitor with activity toward MET and VEGFR2, as well as RET, KIT and FLT3.[4,5] It could inhibit tumor angiogenesis, invasiveness, metastasis, and tumor progression.[5] A phase II study had reported the response to cabozantinib in patients with RET fusion-positive lung adenocarcinoma, and the partial response with a 66% decrease was observed after 4 to 12 weeks of treatment.[3] The final outcomes of this study reported the overall response to cabozantinib in patients with RET fusion-positive lung adenocarcinoma could achieve 28%.[6] Basing on this rationale, cabozantinib has been suggested as a novel targeted therapy according to the guideline of National Comprehensive Cancer Network (NCCN). However, there is still no data about progression-free survival (PFS) or overall survival (OS) of cabozantinib used in RET fusion-positive lung cancer. Furthermore, related clinical trials and reports about the duration of effectiveness with cabozantinib in NSCLC are limited. In this report, we present a RET-positive case that benefited from cabozantinib treatment in the real world, from the response to PFS, and still from the effectiveness to adverse events (AEs).
2. Case report
The patient was a 50-year-old male presenting with a cough, sputum, and left chest pain that persisted for 20 days; he sought medical attention from a doctor on July 20, 2016. A chest CT scan (performed July 12, 2016) showed a lesion measuring approximately 2.5 × 2.0 cm in the left lung and the absence of enlarged lymph nodes in the mediastinum. No metastases were found in other areas of the body, which supported an initial clinical diagnosis of left lung upper lobe cancer (cT1bN0M0 Stage IA) before surgery. The patient underwent resection and lymphadenectomy for the left lung upper lobe cancer on July 25, 2016. Postoperative pathology showed a left lung upper lobe middle differentiated adenocarcinoma measuring 2.5 × 2 × 1.8 cm. Para-aortic lymph nodes in the mediastinum and multiple visceral pleural metastases were found. Immunohistochemistry analyses of the tumor showed TTF-1+, Syn-, Ki-67 (20%), CD31+, CK+, EGFR- and ALK-. Following postoperative pathology, a new diagnosis was made of left lung upper lobe adenocarcinoma (pT1N2M1, Stage IV, EGFR-, ALK-). Subsequently, the patient received 4 cycles of chemotherapy with gemcitabine and cisplatin from August 2016 to December 2016; stable disease (SD) was observed during this period by chest CT (Fig. 1A). Chemotherapy could not continue due to the poor tolerance of this patient. Six months later, he felt pain in his left chest. Multiple pleural metastases were revealed by chest CT (May 31, 2017) (Fig. 1B), indicating progressed disease (PD). He did not accept second-line chemotherapy due to the obvious adverse effects. Furthermore, we performed genetic testing of the patient's resected tumor tissue and identified the presence of the KIF5B/RET fusion gene; other mutations in EGFR, KRAS, ALK and MET were all negative. According to NCCN guidelines, the patient began treatment with cabozantinib (140 mg orally, once daily), which is a receptor tyrosine kinase inhibitor of RET. Subsequently, the patient's left chest pain was alleviated and disappeared after 1 month of targeted therapy. Chest CTs were performed every 2 months, and SD continued (Fig. 1C) until March 2018 (Fig. 1D). At this point, RET inhibitor (cabozantinib) therapy was stopped. The complete PFS was more than 9 months. No severe AEs were observed in this process, except rash (grade II).
Figure 1.
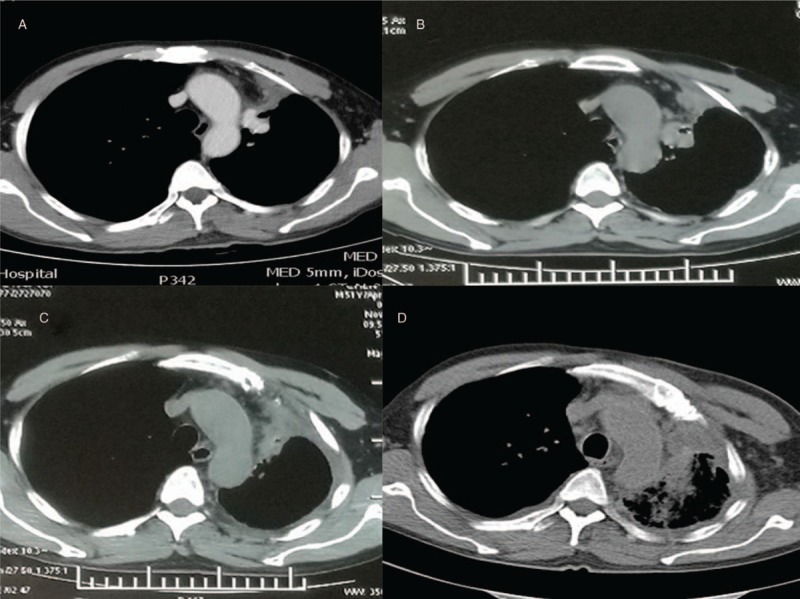
Chest CT. (A) shows SD after 4 cycles of chemotherapy on December 15, 2016. (B) shows multiple pleural metastases on May 31, 2017. (C) shows SD after 5 months cabozantinib on November 9, 2017. (D) shows disease progression after 9 months cabozantinib in March 2017.
3. Discussion
The RET gene is a novel driver of lung cancer differing from other major driver genes, such as EGFR, ALK, and ROS1. RET is an oncogene located on chromosome 10q11.2 initially identified from the NIH3T3 cells of transformed cultured mice by Takahashi et al in 1985.[7,8] The RET gene encodes the RET receptor protein, one of the first receptor tyrosine kinases (RTKs) found to play a role in neoplasia.[9] RTKs consist of 3 domains:
-
1.
an extracellular domain (containing 4 cadherin-like repeats, a calcium binding site and a cysteine-rich region),
-
2.
a transmembrane domain, and
- 3.
The ligands of the RET receptor belong to the glial cell line-derived neurotrophic factor (GDNF) family of proteins, which includes GDNF, neurturin (NRTN), artemin (ARTN), and persephin (PSPN). The RET receptor and its ligand form a multimeric complex that can activate the kinase domain, resulting in autophosphorylation of the intracellular domain. Activation of RET protein can activate several signaling pathways, including those of mitogen activated protein kinase (MAPK), phosphoinositide 3-kinase (PI3K)/AKT, Rac/c-jun NH, kinase (JNK), phospholipase C-γ, and Ras/mitogen-activated protein (MAP) kinase (also known as ERK).[9,10] Normally, RET is essential for the development of the enteric nervous system, kidney and embryogenesis of mammals, and its expression level in normal lung tissue is notably low.[11,12] The mechanisms of the relationship between RET and distinct neoplastic diseases remain largely unknown. The first association of RET with tumorigenesis was its discovery in papillary thyroid carcinoma (PTC) in 1987.[13]RET gene rearrangements are frequently found in papillary thyroid carcinomas.[14] Furthermore, increased RET gene expression was identified in different diseases, including multiple endocrine neoplasia type 2 (MEN2)[14,15] and Hirschsprung disease.[16] Recently, the RET gene has been reported in lung cancer development.
The RET gene was first identified in lung adenocarcinoma patients in 2012 by Kohno et al[2] Several studies reported that the RET gene, accounting for approximately 1% to 2% of non-small-cell lung carcinomas (NSCLCs) worldwide,[2,17–19] is almost observed in lung adenocarcinoma patients, especially in younger individuals, females, and/or never/light-smokers.[20] Meanwhile, RET is often regarded as an independent factor that does not co-occur with such mutations as KRAS, EGFR, BRAF, MEK1, HER2, and ALK fusions.[2,6,20,21]
The RET gene combines with a partner gene to form a fusion gene, activating the RET tyrosine kinase, subsequently evading regulation by ligands and activating tyrosine kinase by autophosphorylation.[2,12,22,23] Multiple RET fusion genes were identified in lung adenocarcinoma patients. To date, at least 7 RET fusion partner genes involving KIF5B, CCDC6, CUX1, TRIM33, NCOA4, KIAA1468 and KIAA1217 have been identified in lung adenocarcinoma.[24,25]KIF5B, presented in the case report, is the most common fusion partner gene accounting for approximately 70% to 80% of the rearrangements followed by CCDC6-RET and NCOA4-RET.[3,18,19,22]KIF5B-RET is a fusion gene between KIF5B and the RET proto-oncogene caused by a pericentric inversion of 10p11.22–q11.21. This fusion was first revealed by whole-genome and transcriptome sequencing by Ju et al[12] The fusion occurred between the 16th exon of KIF5B and the 12th exon of RET.[12] The fusion kinase consists of 638 N-terminal residues of KIF5B and 402 C-terminal residues of RET kinase. The fusion protein contains a coiled-coil domain of KIF5B and a tyrosine kinase unit from RET; the coiled-coil domain induces dimerization of the fusion kinase, which activates the fusion oncogene.[12]
As emerging targeted agents, cabozantinib and vandetanib have been recommended by NCCN guidelines (which are based on a series of clinical trials) for non-small-cell lung cancer with RET fusion. As a receptor tyrosine kinase inhibitor with activity against MET, VEGFR2, FLT3, c-KIT, and RET, cabozantinib could decrease metastasis potential and tumor invasiveness.[4] Cabozantinib was first approved by the Food and Drug Administration (FDA) for medullary thyroid carcinoma in 2012. In 2015, based on a prospective phase II trial (NCT01639508), cabozantinib was recommended by the NCCN guidelines for RET rearrangements in patients with non-small-cell lung cancer. This trial (NCT01639508)[6] in patients with advanced RET-rearranged lung adenocarcinoma reported an overall cabozantinib response rate of 28%. Treatment-related adverse events were predominantly grade 1 or grade 2, and the most common treatment-related adverse events of any grade were increased alanine aminotransferase, increased aspartate aminotransferase, hypothyroidism, diarrhea, palmar plantar erythrodysesthesia, and skin hypopigmentation. The most common grade 3 treatment-related adverse events were lipase elevation (15%), increased alanine aminotransferase (8%), increased aspartate aminotransferase (8%), decreased platelet count (8%) and hypophosphataemia (8%).[6] In addition, a recent global multicenter prospective trial[19] collected and analyzed 165 NSCLC patients with a RET-rearranged gene from 29 centers across Europe, Asia and the United States from April 2016. Of those patients, the rate of any complete or partial response to cabozantinib (21 patients), vandetanib (11 patients), and sunitinib (10 patients) were 37%, 18%, and 22%, respectively.
In this case report, we found that the NSCLC patient with the KIF5B-RET fusion gene benefited from cabozantinib with a PFS of greater than 9 months and no apparent severe AEs. Given these results, we have identified the RET gene as a new alternative target for lung adenocarcinoma patients without common mutations, such as EGFR, ALK, and ROS1. This case report also provided a useful reference for the treatment of lung adenocarcinoma patients with RET fusions and may provide support for an alternative therapy for this category of NSCLC patient. However, the mechanisms involved in RET-rearranged lung cancers should be further investigated, and larger population samples are needed to verify the effects of therapy targeting RET.
Author contributions
Data curation: Yinghui Xu, Kewei Ma.
Formal analysis: Yucong Wang, Yinghui Xu.
Funding acquisition: Yinghui Xu.
Investigation: Yucong Wang, Yinghui Xu.
Methodology: Yucong Wang, Yinghui Xu.
Project administration: Xu Wang.
Resources: Xu Wang, Chao Sun, Ye Guo, Shi Qiu, Kewei Ma.
Supervision: Chao Sun, Ye Guo, Shi Qiu, Kewei Ma.
Validation: Chao Sun, Ye Guo, Shi Qiu.
Writing – original draft: Yucong Wang.
Writing – review & editing: Yucong Wang, Yinghui Xu, Xu Wang, Guoguang Shao, Zhiguang Yang, Kewei Ma.
Yucong Wang orcid: 0000-0002-9878-9833.
Footnotes
Abbreviations: AEs = adverse events, GDNF = glial cell line–derived neurotrophic factor, NCCN = National Comprehensive Cancer Network, NSCLC = non-small-cell lung cancer, ORR = overall response rate, OS = overall survival, PD = progressed disease, PFS = progression-free survival, RTKs = receptor tyrosine kinases, SD = stable disease.
YW and YX contributed equally to this work.
Informed written consent was obtained from the patient for publication of this case report and accompanying images.
The Ethics Committee of the “The Jilin University First Hospital” has approved this report.
Xu Wang was supported by The National Natural Science Foundation of China (Grant ID: 81501962).
Yinghui Xu was supported by Youth Foundation of The First Hospital of Jilin University (Grant ID: JDYY82017020).
Yinghui Xu was also supported by Xisike Clinical Oncology Research Foundation (CSCO-Haosen) (Grant ID: Y-HS2017-062).
The authors declare no conflict of interest.
References
- [1].Testa U, Castelli G, Pelosi E. Lung cancer: molecular characterization, clonal heterogeneity and evolution, and cancer stem cells. Cancers 2018;10:8doi: 10.3390/cancers10080248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kohno T, Ichikawa H, Totoki Y, et al. KIF5B-RET fusions in lung adenocarcinoma. Nat Med 2012;18:375–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Drilon A, Wang L, Hasanovic A, et al. Response to cabozantinib in patients with RET fusion-positive lung adenocarcinomas. Cancer Discov 2013;3:630–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Grüllich C. Cabozantinib: A MET, RET, and VEGFR2 Tyrosine Kinase Inhibitor. Small Molecules in Oncology. 2014;Berlin Heidelberg: Springer, 201: 207-214. [DOI] [PubMed] [Google Scholar]
- [5].Yakes FM, Chen J, Tan J, et al. Cabozantinib (XL184), a Novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther 2011;10:2298–308. [DOI] [PubMed] [Google Scholar]
- [6].Drilon A, Rekhtman N, Arcila M, et al. Cabozantinib in patients with advanced RET-rearranged non-small-cell lung cancer: an open-label, single-centre, phase 2, single-arm trial. Lancet Oncol 2016;17:1653–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Takahashi M, Ritz J, Cooper GM. Activation of a novel human transforming gene, ret, by DNA rearrangement. Cell 1985;42:581–8. [DOI] [PubMed] [Google Scholar]
- [8].Ishizaka Y, Itoh F, Tahira T, et al. Human ret proto-oncogene mapped to chromosome 10q11.2. Oncogene 1989;4:1519–21. [PubMed] [Google Scholar]
- [9].Phay JE, Shah MH. Targeting RET receptor tyrosine kinase activation in cancer. Clin Cancer Res 2010;16:5936–41. [DOI] [PubMed] [Google Scholar]
- [10].Califano D, Rizzo C, D’Alessio A, et al. Signaling through Ras is essential for ret oncogene-induced cell differentiation in PC12 cells. J Biol Chem 2000;275:19297–305. [DOI] [PubMed] [Google Scholar]
- [11].Durbec P, Marcosgutierrez CV, Kilkenny C, et al. GDNF signalling through the Ret receptor tyrosine kinase. Nature 1996;381:789–93. [DOI] [PubMed] [Google Scholar]
- [12].Ju YS, Lee WC, Shin JY, et al. A transforming KIF5B and RET gene fusion in lung adenocarcinoma revealed from whole-genome and transcriptome sequencing. Gen Res 2012;22:436–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fusco A, Grieco M, Santoro M, et al. A new oncogene in human thyroid papillary carcinomas and their lymph-nodal metastases. Nature 1987;328:170–2. [DOI] [PubMed] [Google Scholar]
- [14].Jhiang SM. The RET proto-oncogene in human cancers. Oncogene 2000;19:5590–7. [DOI] [PubMed] [Google Scholar]
- [15].Krampitz GW, Norton JA. RET gene mutations (genotype and phenotype) of multiple endocrine neoplasia type 2 and familial medullary thyroid carcinoma. Cancer 2014;120:1920–31. [DOI] [PubMed] [Google Scholar]
- [16].Bolk S, Pelet A, Angrist M, et al. A human model for multigenic inheritance: phenotypic expression in hirschsprung disease requires both the RET gene and a new 9q31 Locus. Proc Natl Acad Sci US A 2000;97:268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wang R, Hu H, Pan Y, et al. RET fusions define a unique molecular and clinicopathologic subtype of non–small-cell lung cancer. J Clin Oncol 2012;30:4352–9. [DOI] [PubMed] [Google Scholar]
- [18].Cai W, Su C, Li X, et al. KIF5B-RET fusions in Chinese patients with non-small cell lung cancer. Cancer 2013;119:1486–94. [DOI] [PubMed] [Google Scholar]
- [19].Gautschi O, Milia J, Filleron T, et al. Targeting RET in patients with RET-rearranged lung cancers: results from the global, multicenter RET registry. J Clin Oncol 2017;35:1403–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kohno T, Nakaoku T, Tsuta K, et al. Beyond ALK-RET, ROS1 and other oncogene fusions in lung cancer. Transl Lung Cancer Res 2015;4:156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Suehara Y, Arcila M, Wang L, et al. Identification of KIF5B-RET and GOPC-ROS1 fusions in lung adenocarcinomas through a comprehensive mRNA-based screen for tyrosine kinase fusions. Clin Cancer Res 2012;18:6599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Raue F, Frank-Raue K. Genotype-phenotype relationship in multiple endocrine neoplasia type 2. Implications for clinical management. Hormones (Athens) 2009;8:23–8. [DOI] [PubMed] [Google Scholar]
- [23].Borrello MG, Ardini E, Locati LD, et al. RET inhibition: implications in cancer therapy. Expert Opin Ther Targets 2013;17:403–19. [DOI] [PubMed] [Google Scholar]
- [24].Lira ME, Choi YL, Sun ML, et al. A single-tube multiplexed assay for detecting ALK, ROS1, and RET, fusions in lung cancer. J Mol Diagnos 2014;16:229–43. [DOI] [PubMed] [Google Scholar]
- [25].Arai S, Kita K, Tanimoto A, et al. In vitro and in vivo anti-tumor activity of alectinib in tumor cells with NCOA4-RET. Oncotarget 2017;8:73766–73. [DOI] [PMC free article] [PubMed] [Google Scholar]


