Abstract
Background:
Preoperative neutrophil-to-lymphocyte ratio (NLR) has been suggested as a useful predictive factor for prognosis in patients with various cancers. However, the prognostic value of NLR in patients with colorectal cancer (CRC) remains controversial. Therefore, the goal of this study was to perform a meta-analysis to evaluate the prognostic value of NLR in patients with CRC undergoing curative surgery.
Methods:
PubMed, EMBASE and Cochrane Library databases were searched to screen the relevant studies. Pooled hazard ratio (HR) with 95% confidence interval (CI) was used to assess the associations of preoperative NLR and overall survival (OS), disease-free survival (DFS), recurrence free survival (RFS) and disease specific survival (DSS) by STATA 13.0 software.
Results:
Sixteen studies involving 5897 patients were included in our meta-analysis. Our pooled results demonstrated that high NLR was associated with poor OS (HR: 1.66, 95%CI: 1.36–2.02, P < .001), DFS (HR = 1.54, 95%CI: 1.18–2.02, P = .002), RFS (HR = 2.31, 95%CI: 1.68–3.17, P < .001) and DSS (HR = 2.27; 95% CI: 1.75–2.96, P < .001). When the patients were stratified according to country, sample size, NLR cut-off, follow up and postoperative chemotherapy, high NLR was still significantly correlated with OS. The limitation was that the majority of enrolled studies were retrospective.
Conclusion:
Preoperative NLR may be an effective predictive biomarker for prognosis in patients with CRC. Detection of NLR may be beneficial to identify the high-risk patients who need other antitumor therapies in addition to surgery.
Keywords: colorectal cancer, meta-analysis, neutrophil-to-lymphocyte ratio, prognosis, survival
1. Introduction
Colorectal cancer (CRC), including colon and rectum cancer, is the most common gastrointestinal malignant tumor, with an estimated 135,430 new cases diagnosed and 50,260 death in 2017 in the United States.[1] Surgery resection is considered as the cornerstone of curative therapy for CRC and may provide a good prognostic outcome compared with unresectable patients.[2] However, clinical studies indicate the prognosis following surgery differs substantially in different patients. Therefore, it is essential to identify preoperative biomarkers that are beneficial for assessment of treatment efficacy of patients and thus provide reference for schedules of the follow-up medical treatment program.
Conventionally, prognosis of patients with CRC can be predicted by histopathological parameters, including tumor-node-metastasis (TNM) staging, cell differentiation, tumor grade, Dukes’ stage or tumor type.[3,4] Nevertheless, it is reported that heterogeneous prognostic outcomes still exist in patients with the same stage and tumor grade,[5] indicating their inaccuracy for predicting the risk of patient’ mortality. Therefore, more effective, alternative biomarkers need to be identified for prognostic prediction of CRC patients.
Recently, accumulating evidence suggests that inflammation may play important roles in the development and progression of CRC.[6,7] Elevated inflammation promotes proliferation, migration, invasiveness of malignant CRC cells, while silencing of cytokines [interleukin (IL)-21, IL-8 or IL-32] reversed these effects.[8,9] Thus, systemic immune cells (such as neutrophils and lymphocytes, both of which can be used to calculate the neutrophil-lymphocyte ratio, NLR) and their released inflammatory cytokines may be potential predictors for prognosis of CRC patients.[9,10] This hypothesis has been demonstrated by several studies. For example, Li et al. demonstrated that CRC patients with a higher NLR had a lower overall survival (OS; HR: 1.846, 95% confidence intervals [CI]: 1.159–2.941, P = .01) and DFS (HR: 1.855, 95% CI: 1.164–2.954, P = .009).[11] Similarly, Ishizuka et al proved that the higher level of NLR was associated with poorer OS (HR: 1.811, 95% CI: 1.229–2.669, P = .003).[12] However, the inconsistent results were also reported, with the study of Wei et al as an example in which Cox regression model showed NLR was not an independent prognostic factor for OS (P = .457) and DFS (P = .856). Therefore, the prognostic value of NLR in CRC remains controversial and it is necessary to further evaluate the prognostic significance of NLR in patients with CRC by performing a meta-analysis that can comprehensively analyze all related articles and may achieve a more convinced conclusion.
Although previous studies have investigated the prognostic value of NLR for survival in patients with CRC, all of them focused on various treatment methods,[13–15] not only on patients undergoing surgical resection, which was the goal of this study.
2. Materials and methods
2.1. Literature search strategy
A systematic literature search was performed by using PubMed, EMBASE and Cochrane Library databases to evaluate the prognostic value of NLR in patients with CRC. The key words used were the combinations of the following search terms: (“neutrophil to lymphocyte ratio” OR “neutrophil-to-lymphocyte ratio” OR “neutrophil lymphocyte ratio” or “NLR”) AND (“colon cancer” OR “CRC” OR “rectal cancer” OR “CRC”) AND (“surgery”) or (“resection”). The deadline of our primary search was April 2018. Furthermore, the reference lists of identified publications were also manually scanned to further screen potential related articles. The protocol adhered to the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) Guidelines.[16] Ethics approval was not necessary as this is a meta-analytic study.
2.2. Inclusion and exclusion criteria
Studies were considered eligible if they met the following inclusion criteria:
-
1.
CRC was diagnosed by pathological examination;
-
2.
curative surgery was performed for CRC patients;
-
3.
NLR was measured preoperatively;
-
4.
the NLR was measured by blood-based methods;
-
5.
the associations between NLR and prognosis related outcomes (OS, DFS, recurrence-free survival [RFS] and disease-specific survival [DSS]) were investigated;
-
6.
HR, 95%CI could be obtained by multivariate Cox regression analysis; and
-
7.
only English publication languages.
The exclusion criteria were:
-
1.
abstracts, letters, reviews, case reports, comments or nonhuman studies;
-
2.
insufficient prognosis data to estimate HR and 95%CI;
-
3.
failed to provide the cut-off value;
-
4.
adjuvant chemoradiotherapy was received preoperatively;
-
5.
surgery was not performed;
-
6.
NLR was tested after surgery;
-
7.
combined with other cancers; and
-
8.
literature written in other language.
2.3. Data extraction
Two authors (HCL and YZ) independently screened eligible studies from the databases and extracted the following data: author name, publication year, country, sampling time, sample size, patients’ sex, age, pathological stage, postoperative treatment, cut-off level, follow-up, and HRs and 95% CIs for NLR in multivariable analysis and prognosis (OS, DFS, RFS and DSS). During study identification and data abstraction, discrepancy was resolved through discussion or the third researcher (FYZ).
The quality of the included studies was assessed using the Newcastle–Ottawa Scale (NOS)[17] that consists of 3 domains: patients selection (4 items: representativeness of the exposed cohort; selection of the nonexposed cohort; assessment of exposure; and outcome not present at start of study), comparability (2 items, comparability of cohorts on the basis of the design; or analysis), and outcome assessment (3 items: assessment of outcome; follow-up long enough for outcomes; and adequacy of follow-up). A positive result on any 1 of them was counted as 1 point. Studies with the scores greater than or equal to 6 were considered to be of high-quality.
2.4. Statistical analysis
Heterogeneity between the trials was tested by using Cochrane's Q and I2 statistic. A significant heterogeneity was defined as P < .10 and I2 > 50%, after which a random-effects model was chosen to pool the study results; P ≥ .10 and I2 ≤ 50% were considered the values that indicated homogeneity, and thus a fixed-effects model was subsequently applied. HRs with 95% CI for NLR in multivariable analysis were extracted from each study to generate a pooled HR. Egger's linear regression test and funnel plots were used to evaluate publication bias.[18] The influence of publication bias on the overall effect was assessed by the “trim and fill” method.[19] Sensitivity analysis was performed by omitting 1 study in each turn to investigate the influence of a single study on the overall HR estimates. In addition, a subgroup analysis was also performed according to stratification of country, sample size, NLR cut-off point, follow up time and postoperative chemotherapy. All statistical analyses were performed using STATA 13.0 (STATA Corporation, College Station, TX). P < .05 was considered to be statistically significant.
3. Results
3.1. Description of the included studies
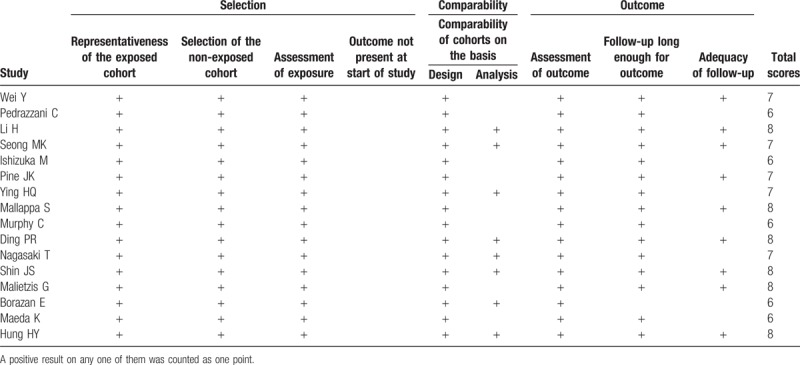
A flowchart of the literature search is shown in Figure 1. The initial search identified 1011 studies. After removal of duplicates, 809 studies were excluded. Of the remaining 202 studies, 105 were further excluded by reading titles and abstracts: surgery not performed (n = 6), no prognosis information (n = 33), not CRC (n = 17), NLR not evaluated (n = 11), drug therapy assessed (n = 1), combined with other cancers (n = 3), review (n = 11), descriptive study (n = 1), comments (n = 4), non-English (n = 14), non-preoperative NLR (n = 1), case (n = 1) and animal studies (n = 2). Ninety-seven full-text articles were then downloaded to assess their eligibility, in which 81 were excluded because non-effective data could be collected (n = 45), cut-off value was not recorded (n = 8) and adjuvant chemoradiotherapy was received preoperatively (n = 28). Ultimately, 16 studies [5897 patients/2385 (40.4%) females] published between 2010 and 2018 were included for this meta-analysis.[11,12,20–33] The characteristics of the included studies are summarized in Table 1. Five studies were performed in China, 3 in Japan, 2 in Korea, 3 in the UK, one in Australia, 1 in Turkey and 1 in USA. The NLR was calculated on the basis of preoperative laboratory data using the white blood cell (WBC) differential counts with dividing the neutrophil count by the lymphocyte count. All studies used multivariate analysis results to pool HR and 95%CI. The cut-off value for NLR was <3 in 4 studies, ≥3 in 7 studies, ≥4 in 1 study and ≥5 in 4 studies. According to the NOS score, all the studies were considered to be in high-quality, ranging from 6 to 8 (Table 2).
Figure 1.
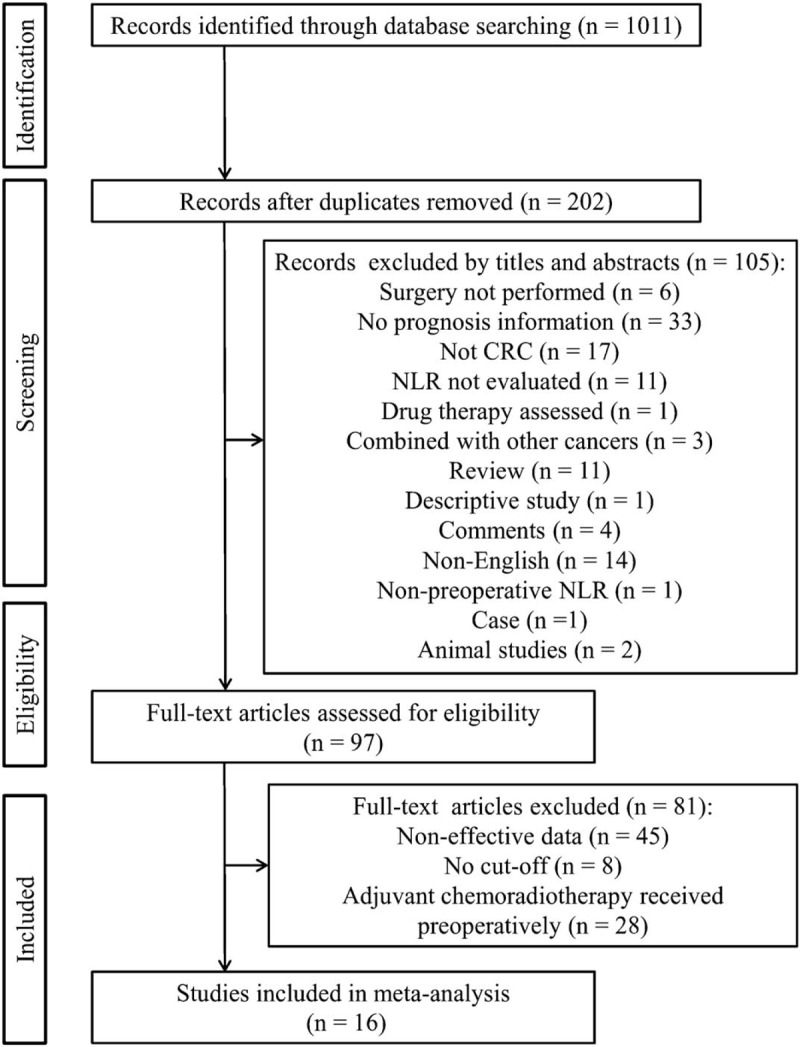
Flowchart of searching relevant studies used in this meta-analysis.
Table 1.
Characteristics of all eligible studies.

Table 2.
Quality of the included studies based on the Newcastle–Ottawa scale.
3.2. Meta-analysis results
There were 11 studies to investigate the prognostic significance of preoperative NLR for OS in CRC patients. A significant heterogeneity was present between the studies (I2 = 56.3%, P = .011) and thus a random-effects model was chosen to pool the study results. A pooled HR of 1.66 (95%CI: 1.36–2.02, P < .001; Fig. 2) showed that patients with an elevated NLR were expected to have lower OS after treatment.
Figure 2.
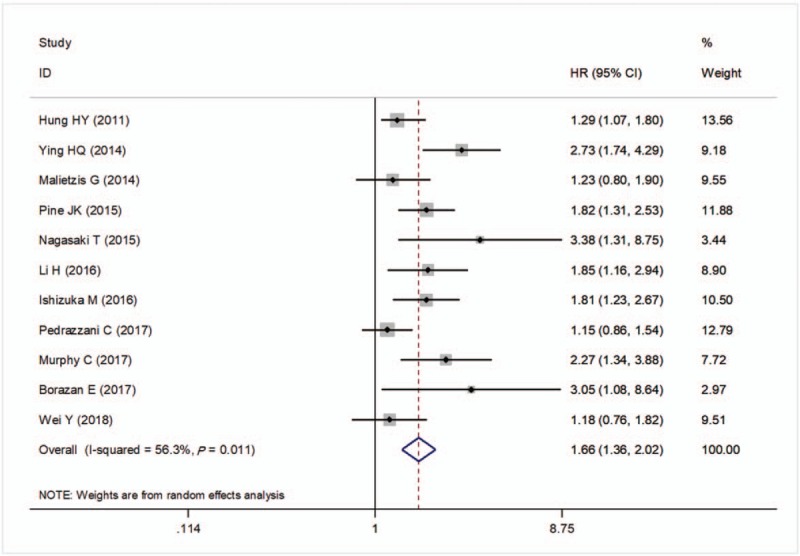
Forest plots of the correlation of NLR with overall survival. Squares indicate HR; horizontal lines indicate 95% CI; diamond indicates the summary HR estimate with its 95% CI. CI = confidence intervals, HR = hazard ratio, NLR = neutrophil to lymphocyte ratio.
There were 9 studies to investigate the prognostic significance of preoperative NLR for DFS in CRC patients. A significant heterogeneity was detected between these studies (I2 = 52.3%, P = .032) and thus a random-effects model was applied to pool the study results. A pooled HR of 1.54 (95%CI: 1.18–2.02, P = .002; Fig. 3) showed that patients with an elevated NLR were associated with lower DFS after treatment.
Figure 3.
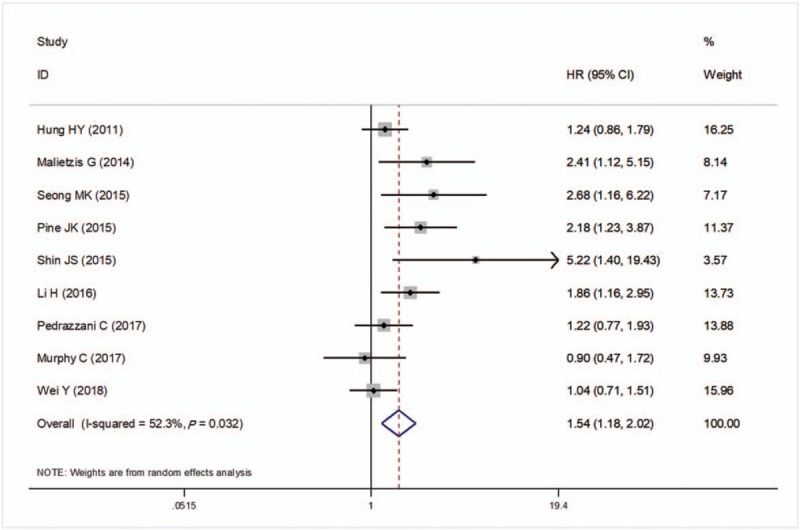
Forest plots of the correlation of neutrophil to lymphocyte ratio (NLR) with disease-free survival. Squares indicate hazard ratio (HR); horizontal lines indicate 95% confidence intervals (CI) (if it was larger, it was generated as an arrow automatically by the software); diamond indicates the summary HR estimate with its 95% CI. CI = confidence intervals, HR = hazard ratio, NLR = neutrophil to lymphocyte ratio.
There were 3 studies to investigate the prognostic significance of preoperative NLR for RFS in CRC patients. No significant heterogeneity was present between these studies (I2 = 0%, P = .424) and thus a fixed-effects model was adopted to pool the study results. A pooled HR of 2.31 (95%CI: 1.68–3.17, P < .001; Fig. 4) showed that patients with an elevated NLR were correlated with lower RFS after treatment.
Figure 4.
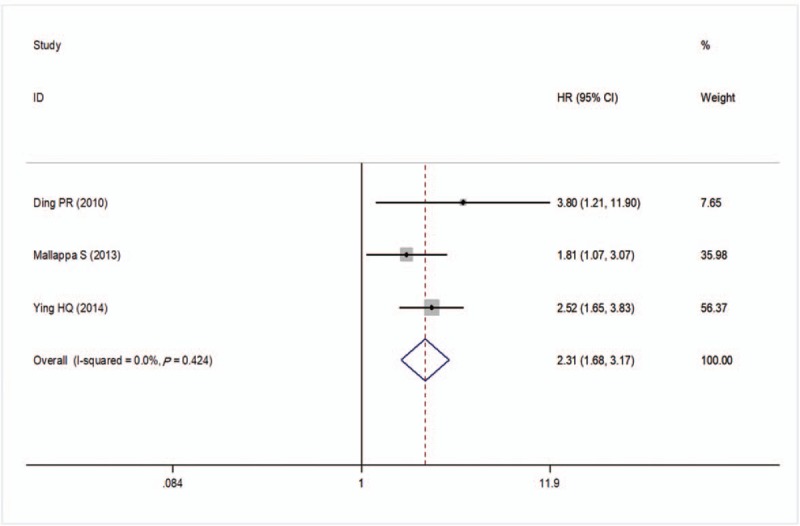
Forest plots of the correlation of NLR with recurrent-free survival Squares indicate HR; horizontal lines indicate 95% CI; diamond indicates the summary HR estimate with its 95% CI. CI = confidence intervals, HR = hazard ratio, NLR = neutrophil to lymphocyte ratio.
There were 4 studies to investigate the relationship between preoperative NLR and DSS in CRC patients. No evidence heterogeneity was observed between these studies (I2 = 0%, P = .451) and thus a fixed-effects model was adopted to pool the study results. The pooled estimates demonstrated DSS was significantly worse in the high NLR group compared with the lower NLR group after treatment (HR = 2.27; 95% CI: 1.75–2.96, P < .001; Fig. 5).
Figure 5.
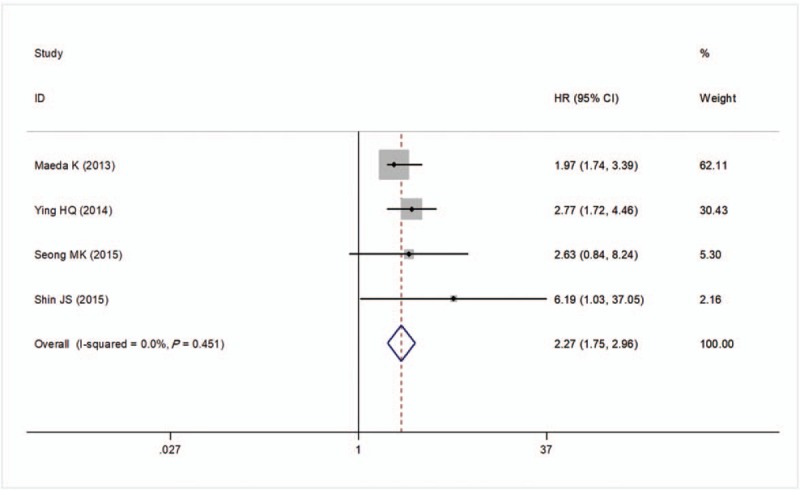
Forest plots of the correlation of NLR with disease-specific survival. Squares indicate HR; horizontal lines indicate 95% CI (if it was larger, it was generated as an arrow automatically by the software); diamond indicates the summary HR estimate with its 95% CI. CI = confidence intervals, HR = hazard ratio, NLR = neutrophil to lymphocyte ratio.
3.3. Publication bias
Because heterogeneity was present in studies to evaluate the prognostic significance of preoperative NLR for OS and DFS, thus, a publication bias estimate was used to evaluate the reliability of the meta-analysis results for these 2 indicators. Funnel plots were constructed (Fig. 6A and B), and the Egger's test showed that P = .028 and P = .030, respectively, indicating the publication bias was indeed present. Subsequently, a trim-and-fill method was performed and the HR was recalculated (Fig. 6C and D). The filled meta-analysis still indicated a positive outcome for OS (HR = 1.43; 95%CI: 1.15–1.76, P = .001). However, the level of NLR could not predict the DFS after the filled meta-analysis (HR = 1.32; 95%CI: 1.00–1.76, P = .053).
Figure 6.
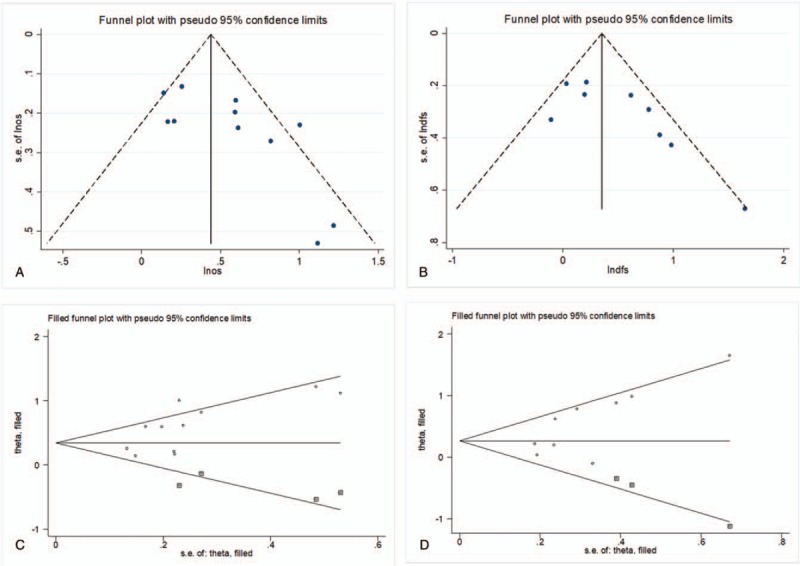
Funnel plot for the assessment of potential publication bias. A, Egger's funnel plot for OS; B, Egger's funnel plot for DFS; C, Trim-and-fill funnel plot for OS; D, Trim-and-fill funnel plot for DFS. The solid and dashed lines represent the estimated hazard ratio (HR) and its 95% CI. The horizontal axis was ln (HR); SE: standard error. CI = confidence intervals, DFS = disease-free survival, HR = hazard ratio, OS = overall survival.
3.4. Sensitivity analyses
A single study involved in the meta-analysis was deleted each time to unveil the influence of the individual data set to the pooled HR. The results showed that no single study could materially affect the pooled HRs in the present meta-analysis (Fig. 7).
Figure 7.

Sensitivity analysis. The middle vertical axis indicates the overall HR and the 2 vertical axes indicate its 95% CI. Every hollow round indicates the pooled OR when the left study was omitted in this meta-analysis. The 2 ends of every broken line represent the 95% CI. The horizontal axis was ln (HR). CI = confidence intervals, HR = hazard ratio.
3.5. Subgroup analysis
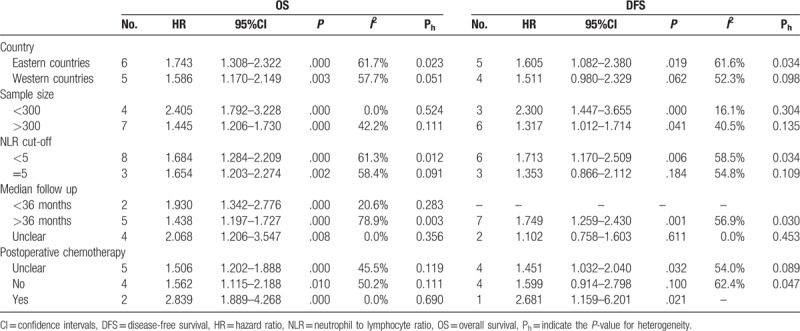
Only the studies investigating the relationship between NLR and OS/DFS could be stratified by country, sample size, NLR cut-off, follow up time and postoperative chemotherapy. The results indicated that the elevated NLR predicted poor OS in all stratified categories, while high NLR only predicted poor DFS in eastern countries (HR = 1.61, 95%CI = 1.082–2.38, P = .019) and studies with NLR cut-off <5 (HR = 1.71, 95%CI = 1.17–2.51, P = .006), follow up time <36 months (HR = 1.75, 95%CI = 1.26–2.43, P = .001), postoperative chemotherapy (HR = 2.68, 95%CI = 1.16–6.20, P = .021) and sample size <300 (HR = 2.30, 95%CI = 1.45–3.66, P < .001) or >300 (HR = 1.32, 95%CI = 1.01–1.71, P = .041) (Table 3).
Table 3.
Subgroup analysis for the association between elevated preoperative NLR and prognosis of patients with colorectal cancer.
4. Discussion
The present study, to our knowledge, was the first meta-analysis assessing the prognostic value of preoperative NLR and prognosis in patients with CRC undergoing curative surgery. The results indicated that, compared with low NLR, elevated NLR was associated with worse OS, DFS, RFS, and DSS in patients with CRC. Heterogeneity was present in studies investigating the relationship between NLR and OS/DFS, and thus a filled meta-analysis was performed. Consequently, the elevated NLR was still significantly related with OS, but not DFS. Subsequent subgroup analysis further demonstrated the high NLR was an OS-related factor for patients with CRC undergoing only surgery or combined with postoperative chemotherapy no matter the studies were performed in eastern or western country, with sample size > or < 300, follow up > 36 or < months, NLR cut-off < or = 5. Our study seemed to be in line with the previous meta-analyses that investigated the prognostic value of preoperative NLR for patients with other cancers, such as epithelial ovarian cancer,[34] upper tract urothelial carcinoma,[35] hepatocellular carcinoma[36] and all solid tumors.[37] But compared with these studies, our inclusion criteria may be more strict, including that HR and 95%CI only could be obtained by multivariate Cox regression analysis and adjuvant chemoradiotherapy could not be received preoperatively, both guaranteeing the reliability to confirm the prognostic value of preoperative NLR. Our findings suggested that the patients with an elevated NLR may need other adjuvant treatments (such as inflammation inhibitors pre- and post-operatively) to combine with surgery to improve their prognosis.[38]
Although the exact mechanisms underlying the association of an elevated NLR with the prediction of poor survival in CRC patients remains unclear, increasing evidence suggests inflammatory and anti-inflammatory imbalance mediated by neutrophils and lymphocytes may play important roles. It had been reported that neutrophils were able to activate stromal cells in a NF-κB-dependent manner and then induced them transformation towards an inflammatory lymphoid stroma phenotype to trigger the survival of malignant B-cell lymphomas cells.[39] Similarly, Donati et al found neutrophil counts and its derived IL-16 were both elevated in pre-metastatic lungs in a mouse model using 4T1 tumor cells. IL-16 promoted cell adhesiveness, invasiveness, and migration, which could be reversed by using an IL-16 neutralizing antibody.[40] Furthermore, inflammatory neutrophils were speculated to enhance cancer cell growth and invasion by producing and releasing matrix metalloproteinases and angiogenesis-related gene vascular endothelial growth factor.[41–44] Conversely, infiltrating lymphocytes might exert cytotoxic roles on cancer stem cells and induce their apoptosis, ultimately preventing the progression of cancer.[45] Thus, the level of infiltrating lymphocytes may be decreased in cancer, which had been observed in the studies of Youssef et al[46] and Gulubova et al[47]. Apoptosis of T lymphocytes was also reported to be significantly correlated with Dukes’ stage (P = .02), lymphatic metastasis (P = .03), vascular metastasis (P = .01), lymph node metastasis (P = .02) and age (P = .01) of patients with CRC.[46] Accordingly, the increased neutrophils and decreased lymphocytes lead to the higher NLR, which is beneficial to the development and progression of CRC and induce poor prognosis.
The data identified in our current meta-analysis provide only limited information about the precise clinical utility of NLR for prognosis of CRC patients. The first limitation of this study was the retrospective nature present in the majority of enrolled studies that may be at high risk of patient selection bias. Secondly, detailed treatment (surgery, chemotherapy) procedures and demographic data of patients (such as age, sex, TNM staging, differentiation, tumor grade, location, etc.) could not be linked to the level of NLR. Thirdly, the cut-off value for defining high NLR was heterogeneous among studies and the prognosis outcomes were determined at different follow-up time. Fourthly, the sample size was not large, which may result in result bias. For example, only 3 or 4 studies were included to evaluate the prognostic significance of NLR for RFS and DSS, which may lead to the overestimation or underestimation of its value. Furthermore, only articles published in English language were included which also may cause a potential bias because some negative results may be published in native language. Hereby, our results should be further confirmed by more prospective and large-scale studies.
In conclusion, this meta-analysis demonstrates preoperative high blood-based NLR is associated with worse prognosis in patients who underwent surgery for CRC, especially OS. Detection of NLR may represent an inexpensive and widely available method for prognosis prediction and may be beneficial to identify the -high-risk patients who need other antitumor therapies in addition to surgery.
Author contributions
Conceptualization: Hongcai Li.
Data curation: Hongcai Li and Yan Zhao.
Formal analysis: Hongcai Li.
Investigation: Hongcai Li, Yan Zhao, and Fengying Zheng.
Methodology: Hongcai Li and Yan Zhao.
Validation: Fengying Zheng.
Writing – original draft: Hongcai Li.
Writing – review & editing: Hongcai Li.
Footnotes
Abbreviations: CI = confidence intervals, CRC = colorectal cancer, DFS = disease-free survival, DSS = disease specific survival, HR = hazard ratio, IL = interleukin, NLR = neutrophil-lymphocyte ratio, NOS = Newcastle–Ottawa Scale, OS = overall survival, PRISMA = Preferred Reporting Items for Systematic Review and Meta-analysis, RFS = recurrence free survival, TNM = tumor-node-metastasis.
The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Stillwell AP, Buettner PG, Ho YH. Meta-analysis of survival of patients with stage IV colorectal cancer managed with surgical resection versus chemotherapy alone. World J Surg 2010;34:797–807. [DOI] [PubMed] [Google Scholar]
- [3].Gennari L, Doci R, Rossetti C. Prognostic factors in colorectal cancer. Hepatogastroenterology 2000;47:310–4. [PubMed] [Google Scholar]
- [4].Kanas GP, Taylor A, Primrose JN, et al. Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clin Epidemiol 2012;4:283–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Peng J, Li C, Wang F, et al. Right- and left-sided stage III colon cancers present different prognostic outcomes of oxaliplatin-based adjuvant chemotherapy after curative resection. Cancer Manag Res 2018;10:2095–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Davoodi H, Hashemi SR, Seow HF. Increased NFk-B activity in HCT116 colorectal cancer cell line harboring TLR4 Asp299Gly variant. Iran J Allergy Asthma Immunol 2012;11:121–32. [PubMed] [Google Scholar]
- [7].Jiang H, Dong L, Gong F, et al. Inflammatory genes are novel prognostic biomarkers for colorectal cancer. Int J Mol Med 2018;42:368–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Abdalkareem E, Huat LB, Mohamed AHS, et al. Silencing of IL-21 on HT29 and HCT116 cells and determining its possible role in the proliferation of colorectal cancer associated with Schistosoma mansoni infection. Aust J Basic Appl Sci 2015;9:39–44. [Google Scholar]
- [9].Chang CJ, Chien Y, Lu KH, et al. Oct4-related cytokine effects regulate tumorigenic properties of colorectal cancer cells. Biochem Biophys Res Commun 2011;415:245–51. [DOI] [PubMed] [Google Scholar]
- [10].Li BH, Xu SB, Li F, et al. Stat6 activity-related Th2 cytokine profile and tumor growth advantage of human colorectal cancer cells in vitro and in vivo. Cell Signal 2012;24:718–25. [DOI] [PubMed] [Google Scholar]
- [11].Li H, Song J, Cao M, et al. Preoperative neutrophil-to-lymphocyte ratio is a more valuable prognostic factor than platelet-to-lymphocyte ratio for nonmetastatic rectal cancer. Int Immunopharmacol 2016;40:327–31. [DOI] [PubMed] [Google Scholar]
- [12].Mitsuru Ishizuka HN, Kazutoshi Takagi, Yoshimi Iwasaki, et al. Clinical significance of the C-reactive protein to albumin ratio for survival after surgery for colorectal cancer. Ann Surg Oncol 2016;doi 10.1245/s10434-015-4948-7. [DOI] [PubMed] [Google Scholar]
- [13].Zheng DC, Zheng C, Wu J, et al. Neutrophil-lymphocyte ratio predicts the prognosis of patients with colorectal cancer: a meta-analysis. Int J Clin Exp Med 2016;9:78–90. [Google Scholar]
- [14].Li MX, Liu XM, Zhang XF, et al. Prognostic role of neutrophil-to-lymphocyte ratio in colorectal cancer: a systematic review and meta-analysis. I Int J Cancer 2014;134:2403–13. [DOI] [PubMed] [Google Scholar]
- [15].Malietzis G, Giacometti M, Kennedy RH, et al. The emerging role of neutrophil to lymphocyte ratio in determining colorectal cancer treatment outcomes: a systematic review and meta-analysis. Ann Surg Oncol 2014;21:3938–46. [DOI] [PubMed] [Google Scholar]
- [16].Shamseer L, Moher D, Clarke M., et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;349:g7647. [DOI] [PubMed] [Google Scholar]
- [17].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [18].Egger MDSG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Duval S, Tweedie R, Trim Fill. A simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–63. [DOI] [PubMed] [Google Scholar]
- [20].Wei Y, Zhang X, Wang G, et al. The impacts of pretreatment circulating eosinophils and basophils on prognosis of stage I–III colorectal cancer. Asia Pac J Clin Oncol 2018;doi: 10.1111/ajco.12871. [DOI] [PubMed] [Google Scholar]
- [21].Pedrazzani C, Mantovani G, Fernandes E, et al. Assessment of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and platelet count as predictors of long-term outcome after R0 resection for colorectal cancer. Sci Rep 2017;7:1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Moo-Kyung S. Prognostic inflammation score in surgical patients with colorectal cancer. J Korean Med Sci 2015;30:1793–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pine JK, Morris E, Hutchins GG, et al. Systemic neutrophil-to-lymphocyte ratio in colorectal cancer: the relationship to patient survival, tumour biology and local lymphocytic response to tumour. Br J Cancer 2015;113:204–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ying HQ, Deng QW, He BS, et al. The prognostic value of preoperative NLR, d-NLR, PLR and LMR for predicting clinical outcome in surgical colorectal cancer patients. Med Oncol 2014;31:305. [DOI] [PubMed] [Google Scholar]
- [25].Mallappa S, Sinha A, Gupta S, et al. Preoperative neutrophil to lymphocyte ratio >5 is a prognostic factor for recurrent colorectal cancer. Colorectal Dis 2013;15:323–8. [DOI] [PubMed] [Google Scholar]
- [26].Murphy C, Turner N, Wong HL, et al. Examining the impact of regular aspirin use and PIK3CA mutations on survival in stage 2 colon cancer. Intern Med J 2017;47:88–98. [DOI] [PubMed] [Google Scholar]
- [27].Ding PR, An X, Zhang RX, et al. Elevated preoperative neutrophil to lymphocyte ratio predicts risk of recurrence following curative resection for stage IIA colon cancer. Int J Colorectal Dis 2010;25:1427–33. [DOI] [PubMed] [Google Scholar]
- [28].Nagasaki T, Akiyoshi T, Fujimoto Y, et al. Prognostic impact of neutrophil-to-lymphocyte ratio in patients with advanced low rectal cancer treated with preoperative chemoradiotherapy. Dig Surg 2015;32:496–503. [DOI] [PubMed] [Google Scholar]
- [29].Shin JS, Suh KW, Oh SY. Preoperative neutrophil to lymphocyte ratio predicts survival in patients with T1-2N0 colorectal cancer. J Surg Oncol 2015;112:654–7. [DOI] [PubMed] [Google Scholar]
- [30].Malietzis G, Giacometti M, Askari A, et al. A preoperative neutrophil to lymphocyte ratio of 3 predicts disease-free survival after curative elective colorectal cancer surgery. Ann Surg 2014;260:287–92. [DOI] [PubMed] [Google Scholar]
- [31].Borazan E, Balik AA, Bozdağ Z, et al. Assessment of the relationship between neutrophil lymphocyte ratio and prognostic factors in non-metastatic colorectal cancer. Turk J Surg 2017;33:185–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Maeda K, Shibutani M, Otani H, et al. Prognostic value of preoperative inflammation-based prognostic scores in patients with stage IV colorectal cancer who undergo palliative resection of asymptomatic primary tumors. Anticancer Res 2013;33:5567–73. [PubMed] [Google Scholar]
- [33].Hung HY, Chen JS, Yeh CY, et al. Effect of preoperative neutrophil–lymphocyte ratio on the surgical outcomes of stage II colon cancer patients who do not receive adjuvant chemotherapy. Int J Colorectal Dis 2011;26:1059–65. [DOI] [PubMed] [Google Scholar]
- [34].Yang Z, Gu JH, Guo CS, et al. Preoperative neutrophil-to-lymphocyte ratio is a predictor of survival of epithelial ovarian cancer: a systematic review and meta-analysis of observational studies. Oncotarget 2017;8:46414–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Vartolomei MD, Kimura S, Ferro M, et al. Is neutrophil-to-lymphocytes ratio a clinical relevant preoperative biomarker in upper tract urothelial carcinoma? A meta-analysis of 4385 patients. World J Urol 2018;36:1019–29. [DOI] [PubMed] [Google Scholar]
- [36].Sun XD, Shi XJ, Chen YG, et al. Elevated preoperative neutrophil-lymphocyte ratio is associated with poor prognosis in hepatocellular carcinoma patients treated with liver transplantation: a meta-analysis. Gastroenterol Res Pract 2016;2016:4743808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Paramanathan A, Saxena A, Morris DL. A systematic review and meta-analysis on the impact of pre-operative neutrophil lymphocyte ratio on long term outcomes after curative intent resection of solid tumours. Surg Oncol 2014;23:31–9. [DOI] [PubMed] [Google Scholar]
- [38].Halazun KJ, Aldoori A, Malik HZ, et al. Elevated preoperative neutrophil to lymphocyte ratio predicts survival following hepatic resection for colorectal liver metastases. Eur J Surg Oncol 2008;34:55–60. [DOI] [PubMed] [Google Scholar]
- [39].Grégoire M, Guilloton F, Pangault C, et al. Neutrophils trigger a NF-κB dependent polarization of tumor-supportive stromal cells in germinal center B-cell lymphomas. Oncotarget 2015;6:16471–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Donati K, Sépult C, Rocks N, et al. Neutrophil-derived interleukin 16 in premetastatic lungs promotes breast tumor cell seeding. Cancer Growth Metastasis 2017;10:179064417738513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lin C, Lin W, Yeh S, et al. Infiltrating neutrophils increase bladder cancer cell invasion via modulation of androgen receptor (AR)/MMP13 signals. Oncotarget 2015;6:43081–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Gong Y, Koh DR. Neutrophils promote inflammatory angiogenesis via release of preformed VEGF in an in vivo corneal model. Cell Tissue Res 2010;339:437–48. [DOI] [PubMed] [Google Scholar]
- [43].Tan KW, Chong SZ, Wong FH, et al. Neutrophils contribute to inflammatory lymphangiogenesis by increasing VEGF-A bioavailability and secreting VEGF-D. Blood 2013;122:3666–77. [DOI] [PubMed] [Google Scholar]
- [44].Song W, Yeh CR, He D, et al. Infiltrating neutrophils promote renal cell carcinoma progression via VEGFa/HIF2α and estrogen receptor β signals. Oncotarget 2015;6:19290–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Inoda S, Hirohashi Y, Torigoe T, et al. Cytotoxic T lymphocytes efficiently recognize human colon cancer stem-like cells. Am J Pathol 2011;178:1805–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Youssef MM, Paish EC, Murray JC, et al. Tumor infiltrating T lymphocytes and apoptosis in colorectal cancer. Egypt J Immunol 2015;22:19–28. [PubMed] [Google Scholar]
- [47].Gulubova M, Manolova I, Kyurkchiev D, et al. Decrease in intrahepatic CD56 + lymphocytes in gastric and colorectal cancer patients with liver metastases. APMIS 2009;117:870–9. [DOI] [PubMed] [Google Scholar]


