Abstract
Background
One of the most commonly identified pathogens responsible for orthopaedic implant infection is Staphylococcus epidermidis, which can form biofilms on surfaces. Currently, orthopaedic implants made of various surface materials are available, each with features influencing osseointegration, biocompatibility, and adherence of bacteria to the surface, which is the first step in biofilm formation. The aim of this experimental study was to investigate the effect of a high tribologic-resistant 2.5-µm zirconium nitride top coat on an antiallergic multilayer ceramic-covered cobalt-chromium-molybdenum surface on the formation of S. epidermidis biofilm compared with other commonly used smooth and rough orthopaedic implant surface materials.
Questions/purposes
(1) When evaluating the surfaces of a cobalt-chromium-molybdenum (CoCrMo) alloy with a zirconium (Zr) nitride coating, a CoCrMo alloy without a coating, titanium alloy, a titanium alloy with a corundum-blasted rough surface, and stainless steel with a corundum-blasted rough surface, does a Zr coating reduce the number of colony-forming units of S. epidermidis in an in vitro setting? (2) Is there quantitatively less biofilm surface area on Zr-coated surfaces than on the other surfaces tested in this in vitro model?
Methods
To determine bacterial adhesion, five different experimental implant surface discs were incubated separately with one of 31 different S. epidermidis strains each and subsequently sonicated. Twenty test strains were obtained from orthopaedic patients undergoing emergency hip prosthesis surgeries or revision of implant infection and 10 further strains were obtained from the skin of healthy individuals. Additionally, one reference strain, S. epidermidis DSM 3269, was tested. After serial dilutions, the number of bacteria was counted and expressed as colony-forming units (CFUs)/mL. For biofilm detection, discs were stained with 0.1% Safranin-O for 15 minutes, photographed, and analyzed with computer imaging software.
Results
The lowest bacterial count was found in the CoCrMo + Zr surface disc (6.6 x 104 CFU/mL ± 4.6 x 104 SD) followed by the CoCrMo surface (1.1 x 105 CFU/mL ± 1.9 x 105 SD), the titanium surface (1.36 x 105 CFU/mL ± 1.8 x 105 SD), the rough stainless steel surface (2.65 x 105 CFU/mL ± 3.8 x 105 SD), and the rough titanium surface (2.1 x 105 CFU/mL ± 3.0 x 105 SD). The mean CFU count was lower for CoCrMo + Zr discs compared with the rough stainless steel surface (mean difference: 2.0 x 105, p = 0.021), the rough titanium alloy surface (mean difference: 1.4 x 105, p = 0.002), and the smooth titanium surface (mean difference: 7.0 x 104, p = 0.016). The results of biofilm formation quantification show that the mean covered area of the surface of the CoCrMo + Zr discs was 19% (± 16 SD), which was lower than CoCrMo surfaces (35% ± 23 SD), titanium alloy surface (46% ± 20 SD), rough titanium alloy surface (66% ± 23 SD), and rough stainless steel surface (58% ± 18 SD).
Conclusions
These results demonstrate that a multilayer, ceramic-covered, CoCrMo surface with a 2.5-µm zirconium nitride top coat showed less S. epidermidis biofilm formation compared with other surface materials used for orthopaedic implants.
Clinical Relevance
CoCrMo with a 2.5-µm zirconium nitride top coat seems to be a promising surface modification technology able to reduce bacterial attachment on the surface of an implant and, hence, may further prevent implant infection with S. epidermidis biofilm formation.
Introduction
Staphylococcus epidermidis is the most common causative agent of prosthetic implant infection [8, 10]. This bacterium can produce biofilms with high persistence on implanted medical devices [1, 5, 19, 20] and has an adverse impact on the patient’s quality of life [6, 12]. If infection occurs, removal of an infected implant and wide débridement of the infected tissue are required in most patients [15, 23, 24].
To prevent bacterial attachment to an implant, several surface modifications have been made to prosthetic implants. Ideally, such surface modifications have a beneficial impact on implant osseointegration yet at the same time have a detrimental impact on bacteria adhesion. Currently, several orthopaedic implants made of different materials with distinct surface properties are available. Titanium alloys (Ti6Al4V, ISO 5832-3) show good osseointegration and biocompatibility; however, the material has a comparatively low resistance to tribocorrosion. Implant steel (ISO 5832-9) demonstrates favorable mechanical properties, yet because of its nickel content, its biocompatibility is lower than other materials. Cobalt-chromium-molybdenum alloy (CoCr29Mo6, ISO 5832-12) has the highest stiffness and its corrosion resistance is high, but its biocompatibility may be limited as a result of induction of allergy [13]. Rough surfaces such as corundum-blasted structures not only promote implant engraftment into the bone, but also are considered to support bacterial adhesion. Other surfaces and coatings have demonstrated better ability to prevent bacteria adherence and biofilm formation [7, 24].
The aim of this experimental study therefore was to investigate the effect of a high tribologic-resistant 2.5-µm zirconium nitride (Zr) top coat on an antiallergic multilayer ceramic-covered cobalt-chromium-molybdenum surface on the formation of S. epidermidis biofilm compared with other commonly used smooth and rough orthopaedic implant surface materials.
Specifically, we asked: (1) When evaluating the surfaces of a cobalt-chromium-molybdenum (CoCrMo) alloy with a Zr nitride coating, a CoCrMo alloy without a coating, titanium alloy, a titanium alloy with a corundum-blasted rough surface, and stainless steel with a corundum-blasted rough surface, does a Zr coating reduce the number of colony-forming units of S. epidermidis in an in vitro setting? (2) Is there quantitatively less biofilm surface area on Zr-coated surfaces than on the other surfaces tested in this in vitro model?
Materials and Methods
Bacterial Isolates and Implant Infection
Twenty S. epidermidis strains previously obtained from orthopaedic patients undergoing débridement and resection arthroplasties performed for acute periprosthetic joint infection [11] were tested together with one S. epidermidis (DSM 3269) reference strain. Additionally, 10 S. epidermidis strains isolated from the skin of healthy volunteers were tested. In total, 31 S. epidermidis strains were tested against five different surface discs each, resulting in 155 separate test panels. Because all test panels were repeated in triplicate, a total of 465 data sets were available for final analysis. All test strains were identified using routine laboratory identification methods and stored at -70° C before conducting the experiments.
Surface discs with a diameter of 10 mm were prepared for this in vitro study. The following surface discs were used: (1) a novel multilayer antiallergic surface (Braun Aesculap, Melsungen, Germany) with a multilayer ceramic-covered CoCrMo surface and a high tribologic-resistant 2.5-µm Zr nitride top coat (CoCrMo + Zr; Fig. 1A); (2) a cobalt-chromium-molybdenum alloy (CoCr29Mo6, ISO 5832-12; Fig. 1B); (3) a titanium alloy (Ti6Al4V, ISO 5832-3; Fig. 1C); (4) a titanium alloy (Ti6Al4V, ISO 5832-3; Fig. 1D) with a corundum-blasted rough surface; and (5) a stainless steel implant (ISO 5832-9; Fig. 1E) with a corundum-blasted rough surface.
Fig. 1 A-E.
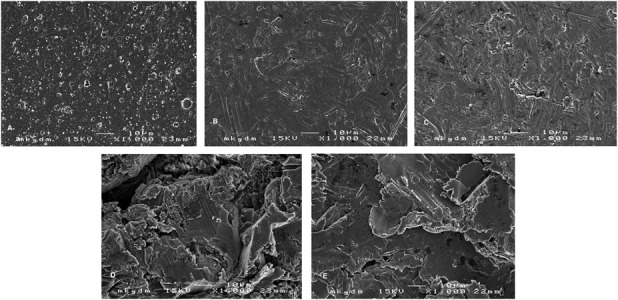
Presented are the images of the tested orthopaedic implant material surfaces: (A) CoCrMo + Zr; (B) CoCrMo; (C) titanium alloy; (D) titanium alloy, corundum-blasted; and (E) implant steel, corundum-blasted.
Biofilm Formation on Orthopaedic Surface Discs
Experiments with each surface material were carried out in the following manner. Overnight cultures of the bacterial isolates grown on Columbia agar plates (Biomerieux, Marcy-l’Étoile, France) were adjusted at a McFarland 0.5 to an average density of 1 x 106 cells/µL in 3 mL 0.9% NaCl solution. The bacteria suspension was diluted 1:100 in Mueller-Hinton broth (Sigma-Aldrich, Darmstadt, Germany) and seeded in a 24-well cell culture plate (Greiner Bio-One International, Kremsmuenster, Austria). A test surface disc was added into each well and incubated for 24 hours.
Biofilm formation was tested by measuring the number of colony-forming units (CFUs) and staining the biofilms. After 24-hour incubation, the discs were put into 3 mL phosphate-buffered saline (PBS; Gibco, Invitrogen, Auckland, New Zealand), gently shaken, and washed. Then they were transferred into 3 mL new PBS, vortexed for 10 seconds, and subsequently sonicated for 10 minutes at an intensity of 44 kHz with a routine laboratory water bath sonicator. The bacteria-PBS solution was diluted 1:100 and 20 µL of this dilution were streaked on Columbia agar plates. After incubation at 35° C for 24 hours at ambient air, we counted colonies and calculated the number of CFUs/mL. The method of counting bacteria followed standard bacteriologic practice. Serial dilutions of -1 to -3 (1:10 to 1:1000) were made; the number of CFUs was counted and multiplied by the dilution factor, which yielded CFU counts in the range between 30 and 300 CFUs. All tests were carried out in triplicate; however, blinding of the surface coating was technically not possible.
Additionally, discs were gently washed in PBS and fixed with 2% glutaraldehyde (Calbiochem; Merck, Darmstadt, Germany) for 15 minutes. Thereafter, discs were washed gently again in distilled water and stained with 0.1% Safranin-O (Sigma-Aldrich, Darmstadt, Germany) solution for 15 minutes. After the staining procedure, discs were rinsed in distilled water, air-dried, and photographed for quantification of biofilm formation. One disc was incubated in medium only and served as a positive control. To quantify the percentage of the biofilm overgrowth area on discs, we took and analyzed photographs using the open-source JAVA image processing software ImageJ 1.45r (National Institutes of Health, Bethesda, MD, USA).
Statistical Analysis
Results are presented as mean ± SD. Data from the quantification of biofilm formation, which showed normal distribution, were analyzed using one-way analysis of variance with a Bonferroni post hoc test. Nonnormally distributed values from the evaluation of CFUs were analyzed using the Kruskal-Wallis test followed by Dunn’s multiple comparison test. A value of p ≤ 0.05 was considered significant. Statistical analysis was performed with PRISM (Graph Pad, San Diego, CA, USA) version 7.0 for Macintosh.
Results
Evaluation of Colony-forming Units
The lowest bacterial count was found in the CoCrMo + Zr surface disc (6.6 x 104 CFU/mL ± 4.6 x 104 SD; Fig. 2) followed by the CoCrMo surface (1.1 x 105 CFU/mL ± 1.9 x 105 SD), the titanium surface (1.4 x 105 CFU/mL ± 1.8 x 105 SD), the rough titanium surface (2.1 x 105 CFU/mL ± 3.0 x 105 SD), and the rough stainless steel surface (2.7 x 105 CFU/mL ± 3.8 x 105 SD). However, the mean CFU counts were fewer for the CoCrMo + Zr surface disc compared with the rough stainless steel surface (mean difference 2.0 x 105, p = 0.021), the rough titanium surface (mean difference 1.4 x 105, p = 0.002), and the smooth titanium surface (mean difference 7.0 x 104, p = 0.016). Compared with the CoCrMo surface, the Zr nitride-coated discs showed no differences in bacterial growth.
Fig. 2.
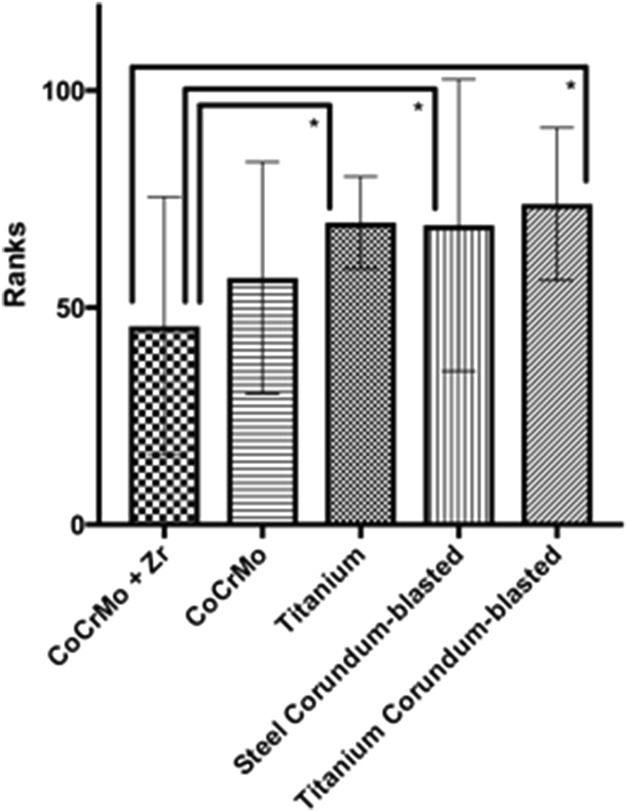
The figure shows the number of CFUs growing on Columbia agar plates after 24 hours of incubation (*significant difference).
Quantification of Biofilm Formation
The results of biofilm formation quantification show that the mean covered area of the surface of the CoCrMo + Zr discs was 19% (± 16 SD; confidence interval [CI], 12-25) (Fig. 3), which is lower than the other surfaces: CoCrMo (35% ± 23 SD, CI, 26-44; mean difference 16, p = 0.001, CI, 6.48-38.59), titanium alloy (46% ± 20 SD, CI, 38-54; mean difference 27, p < 0.0001, CI, 11.44-43.55), rough titanium alloy (66% ± 23 SD, CI, 57-75; mean difference 47, p < 0.0001, CI, 31.21-63.32), and rough stainless steel (58% ± 18 SD, CI, 50-65; mean difference 39, p < 0.0001, CI, 22.63-54.74). The CoCrMo surface additionally showed lower biofilm formation compared with the steel corundum-blasted (mean difference 23; p = 0.001; CI, 6.48-38.59) and titanium alloy corundum-blasted (mean difference 31; p < 0.0001; CI, 15.06-47.17) surfaces. Furthermore, the titanium alloy surface was less overgrown with biofilm compared with the titanium alloy corundum-blasted (mean difference 20; p = 0.006; CI, -35.83 to -3.72) surfaces.
Fig. 3.
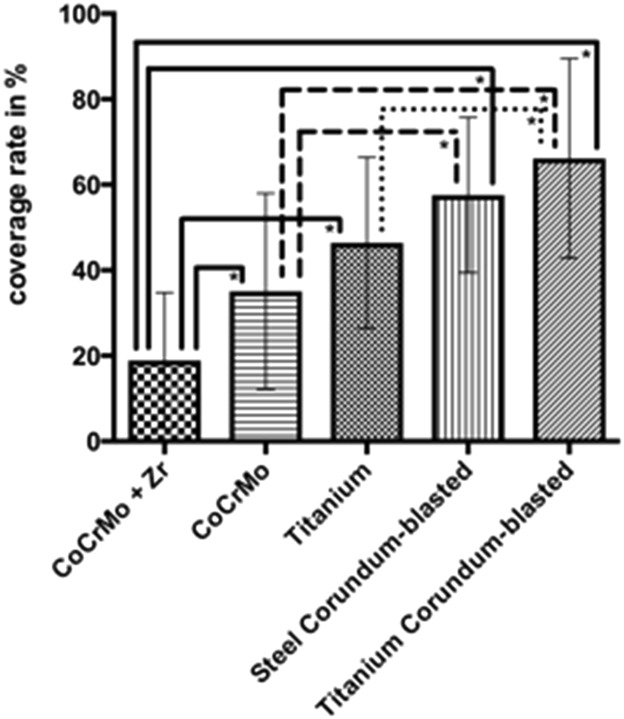
The figure depicts the biofilm coverage rate in percent on all tested surfaces.
Discussion
Staphylococcus epidermidis is the most common causative agent of prosthetic implant infection, able to produce biofilms with high persistence on implanted medical devices [1, 5, 19, 20] if bacterial adhesion occurs to the implant surface. To prevent bacterial attachment, several surface modifications have been made, which allow improved implant osseointegration yet at the same time decrease the potential for bacteria adhesion. In this study, we demonstrated that a multilayer, ceramic-covered CoCrMo surface with a Zr nitride top coat showed lower biofilm formation compared with other surfaces commonly used for orthopaedic implants. Additionally, we found fewer CFUs growing on agar plates after sonication of the various surfaces between the Zr nitride-coated surface and both rough surface samples.
However, the chief aspect of the present work was to measure the amount of biofilm formation on different types of surfaces. The number of bacteria obtained from surfaces after sonication represents only an indirect measurement, which must be interpreted in conjunction with biofilm mass. When test bacteria are exposed on test discs, only a fraction will attach to the surface, colonize it, and start formation of biofilm. Because all experiments were conducted under identical conditions, it can be concluded that only the type of surface influenced the number of bacteria able to attach. The fewer bacteria attaching to the surface, the fewer biofilm will be generated, and the fewer bacteria can be leached out from the biofilm during sonication. Hence, our results do not demonstrate an antibacterial effect on the surface or an antibacterial compound leaching from the surface material, but rather lower initial bacterial attachment on smooth surfaces compared with rough surfaces. Furthermore, adding Zr to smooth surfaces reduces further the probability of bacteria attachment and colonization.
A rapid and complete integration of an implant into tissue is relevant to prevent bacterial attachment and, hence, biofilm formation [17, 18]. Usually, the surfaces of titanium devices are moderately roughened to support osseointegration. Osteoblast-like cells prefer microstructured surfaces, but unfortunately, it has also been shown that roughened surfaces improve bacteria adherence [22]. These results correspond with our findings and demonstrate that more biofilm was measured on both tested rough corundum-blasted surfaces than on the smooth surfaces. Previous reports published by Größner-Schreiber et al. [3, 4] combined with our results show that Zr nitride coatings reduce biofilm formation compared with rough and smooth surfaces. Like the results shown by Koseki et al. [9], we could furthermore demonstrate that less biofilm was detectable on the CoCrMo surface compared with titanium alloy and stainless steel. In addition to the favorable characteristic of decreased biofilm formation, also the biocompatibility is favorable and the potential for allergies and inflammation of such CoCrMo alloys with zirconium is very low, as was demonstrated in an in vivo animal study [16].
Currently, different materials are used for implants in orthopaedic surgery, including stainless steel, titanium and its alloys, and CoCrMo alloy; each material has specific advantages and limitations [9]. Aside from important characteristics such as biocompatibility, stiffness, and osseointegration, another factor may play a key role for the successful outcome of prosthetic implantation: bacteria adherence to implants, a process necessary for biofilm formation [7]. In contrast to planktonic bacteria, bacteria within a biofilm are less susceptible to systemic antibiotics because of the protective glycocalyx of biofilms [2]. A drug-free decrease in bacterial adhesion to the medical device presents an attractive method in the prevention of biofilm formation, particularly in view of the increasing resistance of bacteria against multiple antibiotics globally [14].
In conclusion, we demonstrated that a multilayer, ceramic-covered CoCrMo surface with a Zr nitride 2.5-µm top coat had less biofilm formation compared with other commonly used surface materials for orthopaedic implants. In conjunction with favorable corrosion resistance, demonstrated low toxicity, and excellent biocompatibility [21] of Zr nitride, this surface seems to be a promising candidate to reduce bacterial attachment on the surface of an implant and, hence, may further prevent implant infections with S. epidermidis biofilm formation.
Footnotes
The institution of one or more of the authors (EP) has received, during the study period, funding from CuraSolutions (Wiener Neustadt, Austria), outside this submitted work. One of the authors certifies that he (RW), or a member of his immediate family, has received or may receive payments or benefits, during the study period, an amount of less than USD 10,000 from DePuy Synthes/Johnson & Johnson Medical Products GmbH (Vienna, Austria), less than USD 10,000 from Pfizer Corporation Austria GmbH (Vienna, Austria), less than USD 10,000 from Stryker Austria GmbH (Vienna, Austria), less than USD 10,000 from Takeda Pharmaceutical Company Ltd (Tokyo, Japan), and less than USD 10,000 from Zimmer Biomet Austria GmbH (Vienna, Austria) for consulting. This work was performed using the routine research fund of the Department for Orthopedic and Traumatology, Medical University of Vienna, Austria (RW).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution waived approval for the reporting of this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Donlan RM. Biofilm formation: a clinically relevant microbiological process. Clin Infect Dis. 2001;33:1387-1392. [DOI] [PubMed] [Google Scholar]
- 2.Dunne WM., Jr. Bacterial adhesion: Seen any good biofilms lately? Clin Microbiol Rev. 2002;15:155-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Größner-Schreiber B, Griepentrog M, Haustein I, Müller WD, Lange KP, Briedigkeit H, Göbel UB. Plaque formation on surface modified dental implants. Clin Oral Implants Res. 2001;12:543-551. [DOI] [PubMed] [Google Scholar]
- 4.Größner-Schreiber B, Teichmann J, Hannig M, Dörfer C, Wenderoth DF, Ott SJ. Modified implant surfaces show different biofilm compositions under in vivo conditions. Clin Oral Implants Res. 2009;20:817-826. [DOI] [PubMed] [Google Scholar]
- 5.Harimawan A, Ting YP. Investigation of extracellular polymeric substances (EPS) properties of P. aeruginosa and B. subtilis and their role in bacterial adhesion. Colloids Surf B Biointerfaces. 2016;146(Suppl C):459–467. [DOI] [PubMed] [Google Scholar]
- 6.Hebert CK, Williams RE, Levy RS, Barrack RL. Cost of treating an infected total knee replacement. Clin Orthop Relat Res. 1996;331:140-145. [DOI] [PubMed] [Google Scholar]
- 7.Huang HL, Chang YY, Weng JC, Chen YC, Lai CH, Shieh TM. Anti-bacterial performance of zirconia coatings on titanium implants. Thin Solid Films. 2013;528:151–156. [Google Scholar]
- 8.Kiedrowski MR, Horswill AR. New approaches for treating staphylococcal biofilm infections. Ann N Y Acad Sci. 2011;1241:104-121. [DOI] [PubMed] [Google Scholar]
- 9.Koseki H, Yonekura A, Shida T, Yoda I, Horiuchi H, Morinaga Y, Yanagihara K, Sakoda H, Osaki M, Tomita M. Early Staphylococcal biofilm formation on solid orthopaedic implant materials: in vitro study. PLoS One. 2014;9:e107588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matthews PC, Berendt AR, McNally MA, Byren I. Diagnosis and management of prosthetic joint infection. BMJ. 2009;338:b1773. [DOI] [PubMed] [Google Scholar]
- 11.Parvizi J, Gehrke T; International Consensus Group on Periprosthetic Joint Infection. Definition of periprosthetic joint infection. J Arthroplasty. 2014;29:1331. [DOI] [PubMed] [Google Scholar]
- 12.Peel TN, Dowsey MM, Buising KL, Liew D, Choong PF. Cost analysis of debridement and retention for management of prosthetic joint infection. Clin Microbiol Infect. 2013;19:181-186. [DOI] [PubMed] [Google Scholar]
- 13.Plecko M, Sievert C, Andermatt D, Frigg R, Kronen P, Klein K, Stübinger S, Nuss K, Bürki A, Ferguson S, Stoeckle U, von Rechenberg B. Osseo-integration and biocompatibility of different metal implants--a comparative experimental investigation in sheep. BMC Musculoskelet Disord. 2012;13:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puckett SD1, Taylor E, Raimondo T, Webster TJ. The relationship between the nanostructure of titanium surfaces and bacterial attachment. Biomaterials. 2010;31:706-713. [DOI] [PubMed] [Google Scholar]
- 15.Senthi S, Munro JT, Pitto RP. Infection in total hip replacement: meta-analysis. Int Orthop. 2011;35:253-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sonofuchi K, Hagiwara Y, Koizumi Y, Chiba A, Kawano M, Nakayama M, Ogasawara K, Yabe Y, Itoi E. Quantitative in vivo biocompatibility of new ultralow-nickel cobalt-chromium-molybdenum alloys. J Orthop Res. 2016;34:1505-1513. [DOI] [PubMed] [Google Scholar]
- 17.Subbiahdoss G, Kuijer R, Busscher H, van der Mei H. Mammalian cell growth versus biofilm formation on biomaterial surfaces in an in vitro post-operative contamination model. Microbiology. 2010;156:3073-3078. [DOI] [PubMed] [Google Scholar]
- 18.Subbiahdoss G, Pidhatika B, Coullerez G, Charnley M, Kuijer R, van der Mei H, Textor M, Busscher HJ. Bacterial biofilm formation versus mammalian cell growth on titanium-based mono- and bi-functional coatings. Eur Cell Mater. 2010;19:205-213. [DOI] [PubMed] [Google Scholar]
- 19.Trampuz A, Zimmerli W. Prosthetic joint infections: update in diagnosis and treatment. Swiss Med Wkly. 2005;135:243-251. [DOI] [PubMed] [Google Scholar]
- 20.Tran PL, Hammond AA, Mosley T, Cortez J, Gray T, Colmer-Hamood JA, Shashtri M, Spallholz JE, Hamood AN, Reid TW. Organoselenium coating on cellulose inhibits the formation of biofilms by Pseudomonas aeruginosa and Staphylococcus aureus. Appl Environ Microbiol. 2009;75:3586-3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wickens DJ, West G, Kelly PJ, Verran J, Lynch S, Whitehead KA. Antimicrobial activity of nanocomposite zirconium nitride/silver coatings to combat external bone fixation pin infections. Int J Artif Organs. 2012;35:817-825. [DOI] [PubMed] [Google Scholar]
- 22.Wu Y, Zitelli JP, TenHuisen KS, Yu X, Libera MR. Differential response of Staphylococci and osteoblasts to varying titanium surface roughness. Biomaterials. 2011;32:951-960. [DOI] [PubMed] [Google Scholar]
- 23.Zimmerli W, Ochsner PE. Management of infection associated with prosthetic joints. Infection. 2003;31:99-108. [DOI] [PubMed] [Google Scholar]
- 24.Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351:1645-1654. [DOI] [PubMed] [Google Scholar]


