Abstract
Background
Many patients undergo both THA and spinal arthrodesis, and those patients may not fare as well as those who undergo one procedure but not the other. The mechanisms of how spinal arthrodesis affects patient function after THA remain unclear.
Questions/purposes
The aims of our study were to (1) determine how patient-reported outcome measures (PROMs), including the Oxford hip score as well as dislocations and complications compare after THA between patients with and without spinal arthrodesis; (2) characterize sagittal pelvic changes in these patients that occur when moving between different functional positions and test for differences between patients with and without spinal arthrodesis; and (3) assess whether differences in sagittal pelvic dynamics are associated with PROMs, complications, and dislocations after THA.
Methods
In this case-control study, we identified 42 patients (60 hips) who had undergone both THA and spinal arthrodesis between 2002 and 2016 and who were available for followup at a minimum of 12 months (mean, 6 ± 5 years) after the later of the two procedures. These cases were case-control-matched for age, gender, and body mass index with 42 patients (60 hips) who underwent only THA and had no known spinal pathology. All patients completed PROMs, including the Oxford hip score, and underwent four radiographs of the pelvis and spinopelvic complex in three positions (supine, standing, and deep-seated). Cup orientation and various spinopelvic parameters, including pelvic tilt and pelvic-femoral angle, were measured. The difference in pelvic tilt between standing and seated allowed for patient classification based on spinopelvic mobility into normal (± 10°-30°), stiff (< ± 10°) or hypermobile (> ± 30°) groups.
Results
Compared with the THA-only group, the THA-spinal arthrodesis group had inferior PROMs (Oxford hip score, 33 ± 10 versus 43 ± 6; p < 0.001) and more surgery-related complications (such as dislocation, loosening, periprosthetic fracture or infection, psoas irritation) (12 versus 3; p = 0.013), especially dislocation (5 versus 0; p = 0.023). We detected no difference in change of pelvic tilt between supine and standing positions between the groups. When standing, patients undergoing THA-spinal arthrodesis had greater pelvic tilt (25° ± 11° versus 17° ± 8°; p < 0.001) and the hip was more extended (193° ± 22° versus 185° ± 30°; p = 0.012). We found that patients undergoing THA-spinal arthrodesis were more likely to have spinopelvic hypermobility (12 of 42 versus three of 42; odds ratio, 5.2; p = 0.02) with anterior tilting of the pelvis. Of all biomechanical parameters, only spinopelvic hypermobility was associated with inferior PROMs (Oxford hip score, 35 ± 9 versus 40 ± 7 in normal mobility; p = 0.049) and was also present in dislocating hips that underwent revision despite acceptable cup orientation.
Conclusions
In patients with spinal arthrodesis who have undergone THA, spinopelvic hypermobility is associated with inferior outcomes, including hip instability. Spinopelvic hypermobility should be routinely assessed because these patients may have a narrow zone of optimum cup orientation that would require new technology to define and assist the surgeon in obtaining it.
Level of Evidence Level III, therapeutic study.
Introduction
The demand both for THA and spinal arthrodesis is projected to increase in the future because the population is aging [20, 21, 43, 45]. As such, the number of patients undergoing both THA and spinal arthrodesis is likely to increase; it has been reported that 2% to 5% of patients with a spinal arthrodesis also undergo THA [3, 4, 37]. Recent studies noted that increased complications, in particular dislocation, may be common in these patients [3, 4, 37]. Consequently, the interaction between the hip and spine has received increased attention [7, 18, 24-28, 38, 49].
Lumbar spine position affects pelvic position, which in turn influences acetabular orientation [7, 18, 24-28, 38, 44, 49]. Acetabular component (cup) orientation is an important determinant of THA outcome [15]. Under physiological conditions, when a person transitions from a supine to a standing position, the pelvis tilts posteriorly as the anterior iliac spine moves posteriorly relative to pubic tubercles [23, 44]. This spinopelvic movement is associated with an increase in cup anteversion and inclination, providing increased clearance for the femur to flex. However, we do not know what happens dynamically in the presence of a spinal arthrodesis, especially when moving to other positions (such as sitting flexed, a position associated with instability). Such knowledge would help us understand whether and how sagittal dynamics contribute to THA outcomes, and it might provide evidence-based recommendations to minimize instability risk.
The aims of this study were to (1) determine how patient-reported outcome measures (PROMs), including the Oxford hip score as well as dislocations and complications compare after THA between patients with and without spinal arthrodesis; (2) characterize sagittal pelvic changes in these patients that occur when moving between different functional positions and test for differences between patients with and without spinal arthrodesis; and (3) assess whether differences in sagittal pelvic dynamics are associated with PROMs, complications, and dislocations after THA.
Patients and Methods
This is an institutional review board-approved, retrospective, case-control-matched study registered at clinicaltrials.gov (NCT03240484). It stems from a tertiary referral center with 13 fellowship-trained hip arthroplasty surgeons and 12 fellowship-trained spine surgeons.
We considered for inclusion patients who underwent both THA and a spinal arthrodesis (cases) and patients who underwent THA only; the latter group had no history of spinal problems (controls). We excluded patients who were not able to answer questionnaires, had severe dementia, were unable to undergo radiographs as a result of medical reasons, were > 85 years, had evidence of spinal arthrodesis nonunion, or had THA or a spinal procedure performed within 12 months of the study’s initiation. Cases and controls were matched for age, gender, and body mass index (BMI), factors that have been reported to influence sagittal plane dynamics [12].
Power analysis based on one prior study [3] on the proportion of patients who experienced dislocation after THA either with or without spinal arthrodesis determined that 43 hips in each group would be needed for sufficient power (β = 0.8, α = 0.05).
Cases (THA-Spinal Arthrodesis Group)
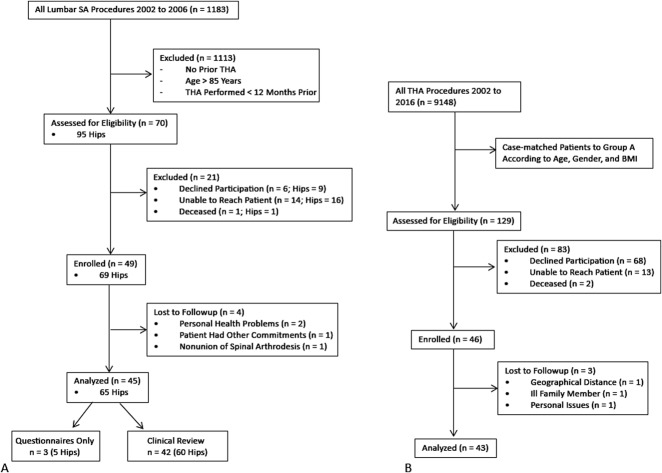
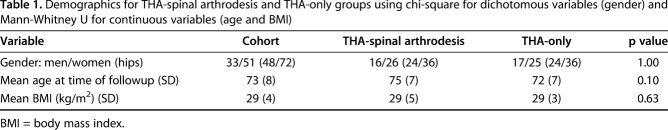
We queried the hospital’s database to identify 1183 patients who underwent lumbar spine arthrodesis between 2002 and 2016. Of these, 70 patients (95 hips) also had THA. A followup at a minimum of 12 months (6 ± 5 years) after the later of the two procedures was essential for participation because outcome plateaus at the 1-year mark and all early complications (especially dislocation) would have been captured. Of the 70 patients, one had died. All remaining 69 patients (94 hips) were invited to participate in the study. Six patients (nine hips) refused participation, 14 patients (16 hips) were unreachable (contact details no longer valid; total lost to followup: 16 of 94 [17%]), and three patients (five hips) elected to answer questionnaires only and not present for clinical review because they currently lived out of the region and because they could not present for radiographic assessment, they were excluded from the study. The remaining 42 patients (60 hips) presented for clinical review and formed the study’s cases (Fig. 1 A-B). Most patients were women (n = 36 [60%]) and the mean age at review was 75 years (± 7 years). The mean BMI was 29 kg/m2 (± 5 kg/m2). Detailed demographics of the cases are provided (Table 1).
Fig. 1 A-B.
Flow diagram that shows the method for recruiting patients to the (A) spinal arthrodesis (SA) and THA group and (B) the THA-only group. Case-matched cohorts were recruited from separate patient populations.
Table 1.
Demographics for THA-spinal arthrodesis and THA-only groups using chi-square for dichotomous variables (gender) and Mann-Whitney U for continuous variables (age and BMI)
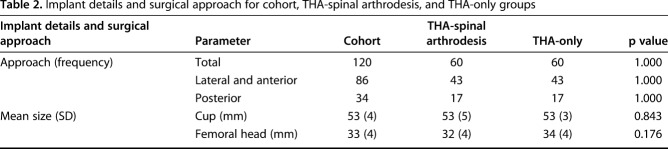
The most common diagnosis for THA was osteoarthritis (110 of 120 [92%]). Twenty-one of 60 THAs (35%) were performed before the spinal arthrodesis, whereas 39 of 60 (65%) were performed after spinal arthrodesis. The mean interval between THA and spinal arthrodesis was 6 years (± 5 years). The most common approach used was a lateral (n = 34) approach followed by posterior (n = 17) and direct anterior (n = 9; Table 2). Most hips received uncemented implants (95%). The mean cup size was 53 mm (± 5 mm) and the median femoral head size was 32 mm (28-48 mm). The most common spinal arthrodesis level was L4-L5 (n = 12) followed by L3-L5 (n = 7). Most spinal arthrodeses were one- (27) or two-level fusions (42); the remaining 15 were fusions of three or more levels. Twenty spinal arthrodeses extended into the sacrum.
Table 2.
Implant details and surgical approach for cohort, THA-spinal arthrodesis, and THA-only groups
Controls (THA-only Group)
Controls were volunteers after THA who were recruited from followup clinics, who were matched for age, gender, and BMI and had a minimum of 1 year of followup after THA. The same surgeons operated on the patients in both groups (Table 2). We aimed for a one-to-one ratio of cases and controls. Cases had slightly longer followup since index THA (6 ± 4 years) than controls (5 ± 6 years; p = 0.018).
Assessments
Patient Review–Outcome Measures
All patients presenting for review were asked to complete five validated PROM questionnaires assessing hip and spine function as well as overall well-being, including the Oxford hip score [33], the WOMAC score [5], the Oswestry Disability Index [13], and the SF-12 physical and mental scores [51]. Patients with bilateral THAs were asked to complete WOMAC and Oxford hip questionnaires for each hip.
Thereafter, an arthroplasty fellow (GG) performed a clinical review, during which surgical complications (such as dislocation, loosening, periprosthetic fracture or infection, psoas irritation) and reoperations were recorded.
Radiographic Assessments
All patients underwent four radiographs for the study purposes including (1) supine AP pelvis; (2) standing AP pelvis; (3) lateral, standing spine, pelvis, hip, and proximal femur; and (3) lateral, flexed (deep) seated spine, pelvis, hip, and proximal femur, which we chose because this is considered a position of increased dislocation risk and edge loading [32, 42].
The same radiology technicians, using the same protocol, obtained all radiographs (see Appendix, Supplemental Digital Content 1). In brief, the AP pelvic radiographs were performed using a previously described technique [9]. For the lateral standing spine, pelvis, hip, and proximal femur radiographs, the patient’s operated hip was placed adjacent to the cassette with arms resting on a support. Thereafter, the beam was centered at the greater trochanter perpendicular to the axial skeleton. The cassette-source distance was set at 150 cm. In the flexed seated position, the patient sat in a chair of comfort (range of height, 35-60 cm) and was asked to lean as far forward as possible without any discomfort. Thereafter a radiograph was taken with the same specifications as described in the standing position.
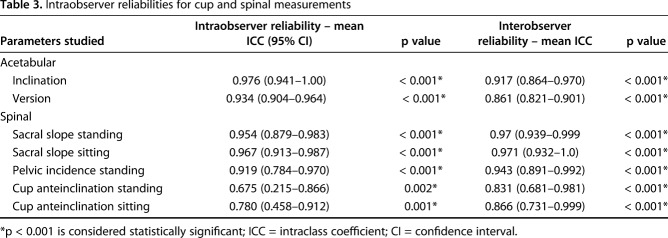
Radiographic cup orientation (inclination/anteversion) was measured from the AP pelvic radiographs using validated software (EBRA-cup; University of Innsbruck, Innsbruck, Austria) [19, 22]. We defined ideal orientation as an inclination/anteversion of 40°/20° ± 10° in the supine position. Two observers (an arthroplasty fellow [GG] and a resident [MC]) blinded to patient outcome independently performed cup measurements of all patients; furthermore, 10 patients underwent a repeat measurement by the fellow. Intra- and interobserver reliabilities of the measurements were evaluated using single-measure intraclass coefficients with a two-way random-effects model for absolute agreement. We identified excellent intra- and interobserver reliabilities for the acetabular cup and spinal measurements (Table 3).
Table 3.
Intraobserver reliabilities for cup and spinal measurements
The differences in inclination/anteversion between the standing and sitting positions, defined as Δ, were calculated as: Δinclination = standing_inclination – supine_inclination; Δanteversion = standing_anteversion – supine_anteversion.
The difference in cup orientation is a reflection of the change in pelvic sagittal plane orientation (ie, pelvic tilt) that occurs when transitioning from the two positions; the anteversion changes by an average of 0.75° for every degree of change in pelvic tilt [2, 31, 44]. A positive value in the pelvic tilt reflects a posterior tilt of the pelvis (the anterosuperior iliac spine [ASIS] moves posteriorly relative to pubic tubercles), whereas a negative value represents an anterior tilt of the pelvis (the ASIS moves anteriorly relative to the pubic tubercles). Therefore, we calculated the change in pelvic tilt (ΔPelvicTiltsupine standing) between supine and standing as: ΔPelvicTiltsupine standing = Δanteversion * (1/0.75).
We measured several spinopelvic parameters from the spine, pelvis, and hip radiographs (Fig. 2). These included both static (pelvic incidence) and dynamic measurements. The latter included sacral slope, pelvic tilt, pelvic-femoral angle, anterior-pelvic plane angle, and anteinclination (AI) of the cup [49]. Pelvic incidence is patient-specific and the same whether sitting or standing [24]. It reflects the pelvic width; the higher the value, the more anterior the hip relative to the spine [29, 30]. Pelvic incidence is the algebraic sum of sacral slope and pelvic tilt. Pelvic-femoral angle is a measure of femoral extension when standing and femoral flexion when sitting [40]. A normal standing pelvic-femoral angle is 180° ± 10° [40]. Anteinclination of the cup is a combination of both inclination and anteversion and is a dynamic measurement of the opening of the cup in the sagittal plane [18].
Fig. 2 A-B.
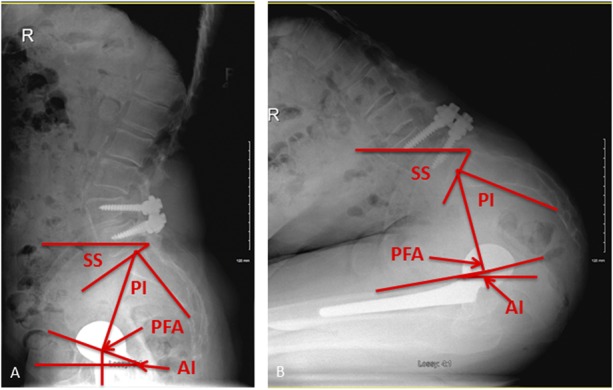
(A) Lateral standing and (B) flexed-seated spinopelvic radiographs of a patient with an L4-L5 spinal arthrodesis and an uncemented THA. Pelvic incidence, 53°; sacral slope (SS), standing, 33°; SS seated, 61°; AI of cup standing, 17°; AI seated, -16°; pelvic femoral angle (PFA) standing, 196°; PFA seated, 80°.
The difference in pelvic tilt between standing and sitting (ΔPelvicTiltstanding2seated) was calculated as: ΔPelvicTiltstanding seated = PelvicTiltseated - PelvicTiltstanding.
ΔPelvicTiltstanding seated allowed us to classify each patient based on spinopelvic mobility into normal (± 10°–30°), stiff (< ± 10°), or hypermobile (> ± 30°) [18, 49].
It is important to note that spinopelvic mobility reflects the movement of the spinopelvic complex (ie, pelvic ring) relative to the horizontal (measured by the change in sacral slope values in different positions, which use the horizontal as their reference); it does not reflect movement within the spinopelvic complex (ie, movement of the sacrum relative to the lumbar spine).
The difference in pelvic-femoral angle between standing and sitting (ΔPelvicFemoralAnglestanding seated) reflects the arc of hip movement in this transition and was calculated as:
ΔPelvicFemoralAnglestanding seated = PelvicFemoralAngleseated - PelvicFemoralAnglestanding.
A fellowship-trained, staff musculoskeletal radiologist (ZJ) who was blinded to patient outcome made all measurements. Repeat measurements for 10 patients at a minimum interval of 4 weeks measured intraobserver reliability. A second assessor (MC) also read the radiographs of these 10 patients, thereby testing interobserver reliability.
Statistical Analysis
Statistical analysis was carried out with nonparametric tests. The Mann-Whitney U and Kruskal-Wallis tests were used for scale data. The Fisher’s exact and chi-square tests were used for categorical data. Statistical significance was set at p < 0.05. All statistical analyses were carried out using SPSS software, version 22.0 (IBM, Chicago, IL, USA).
Results
Clinical Outcomes of THA With or Without Spinal Arthrodesis
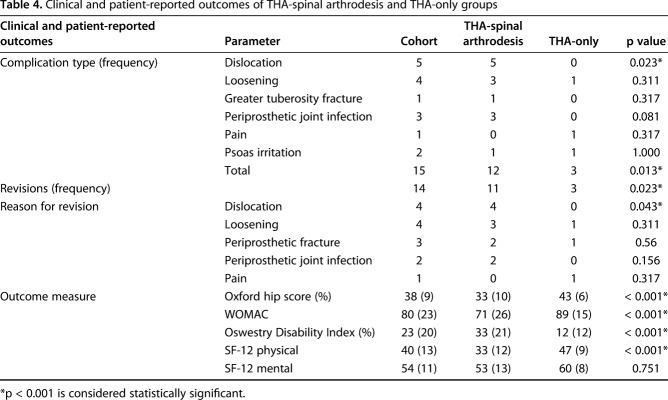
Compared with the THA-only group, the THA-spinal arthrodesis group had inferior hip PROMs (Oxford hip score: 33 ± 10 versus 43 ± 6, p < 0.001; WOMAC: 71 ± 26 versus 89 ± 15, p < 0.001; SF-12 physical: 33 ± 12 versus 47 ± 9, p < 0.001). Spine PROM scores were lower for the THA-spinal arthrodesis group as well: Oswestry, 33% ± 21% versus 12% ± 12% (p < 0.001) (Table 4). The THA-spinal arthrodesis group had more complications (12 of 60 hips) compared with the THA-only group (three of 60 hips; p = 0.013). The most common complication was dislocation (all within 3 months post-THA), which occurred in five patients in the THA-spinal arthrodesis group and was seen in none of the THA-only group (p = 0.023); four patients had recurrent dislocations (two anterior; two posterior) and underwent revision surgery; one patient dislocated early after discharge and has not undergone further surgery. To date, 14 hips have been revised; the THA-spinal arthrodesis group has had more revisions (11 of 60 hips) compared with the THA-only group (three of 60; p = 0.023; Table 4).
Table 4.
Clinical and patient-reported outcomes of THA-spinal arthrodesis and THA-only groups
Dynamic Sagittal Pelvic Position and Cup Orientation
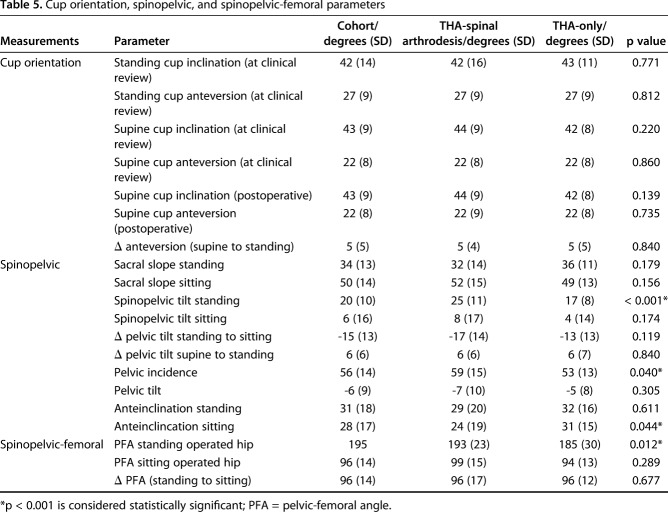
The Δinclination and Δanteversion were not different between THA-spinal arthrodesis and THA-only groups (Table 5). The mean ΔPelvicTiltsupine standing was 6° ± 6° and was not different for the two groups (Fig. 3). Pelvic incidence was higher in the THA-spinal arthrodesis group (59 ± 15) compared with the THA-only group (53 ± 13; p = 0.040). For the whole cohort, the standing pelvic tilt was +20° ± 10°, whereas the pelvic tilt when deep-seated was +6° ± 16°; ΔPelvicTiltstanding seated was -15° ± 13° (Fig. 4). Of the 84 patients in the entire cohort, 44 (51%) showed normal spinopelvic mobility, 25 (31%) showed rigidity, and 15 (18%) showed hypermobility. When standing, the pelvic tilt was greater in the THA-spinal arthrodesis group (25° ± 11°) compared with the THA-only group (17° ± 8°; p < 0.001). Pelvic-femoral anglestanding was greater (ie, more extended femur) in the THA-spinal arthrodesis group (193° ± 23°) compared with the THA-only group (185° ± 30°; p = 0.01). In all, 12 patients undergoing THA-spinal arthrodesis (28%) showed evidence of spinopelvic hypermobility compared with three patients undergoing THA only (5%; odds ratio, 5.1; p = 0.010).
Table 5.
Cup orientation, spinopelvic, and spinopelvic-femoral parameters
Fig. 3.
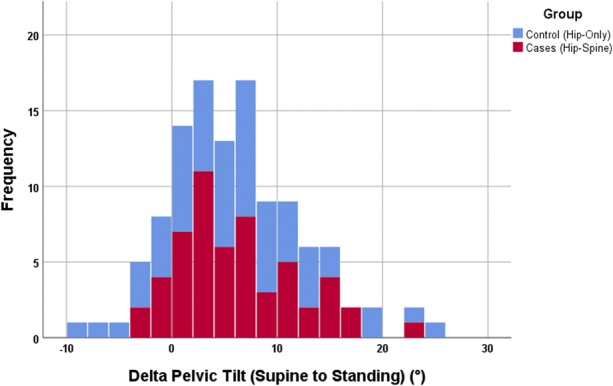
Bar chart of ΔPelvic tiltsupine standing color-coded as per group. The mean ∆Pelvic tiltsupine standing was 6° ± 6° and was not different for the two groups (p = 0.7).
Fig. 4.
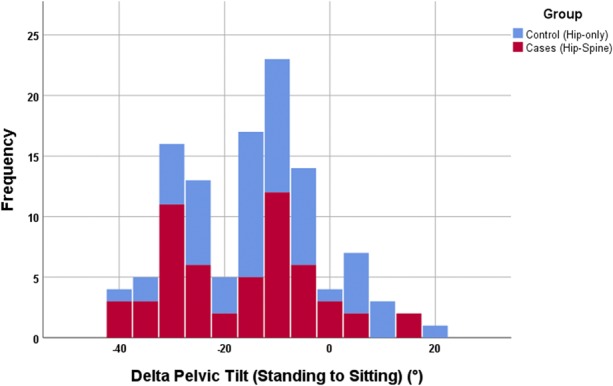
Bar chart of ΔPelvic tiltstanding seated color-coded as per group. For the whole cohort, the ΔPelvicTiltstanding seated was -15° ± 13°.
Among the THA-spinal arthrodesis group, there was no difference in complications between patients who had spinal arthrodesis extending into the sacrum (four of 29) and those who had spinal arthrodesis ending proximal to the sacrum (eight of 31; p = 0.201). Similarly, there was no difference in PROMs measured between the two groups (arthrodesis into the sacrum—Oxford hip score: 32 ± 10, WOMAC: 72 ± 19, Oswestry: 32 ± 18 versus lumbar arthrodesis only—Oxford hip score: 34 ± 10 [p = 0.463], WOMAC: 70 ± 30 [p = 0.589], Oswestry: 34 ± 10 [p = 0.662]).
Clinical Outcome and Sagittal Pelvic Position
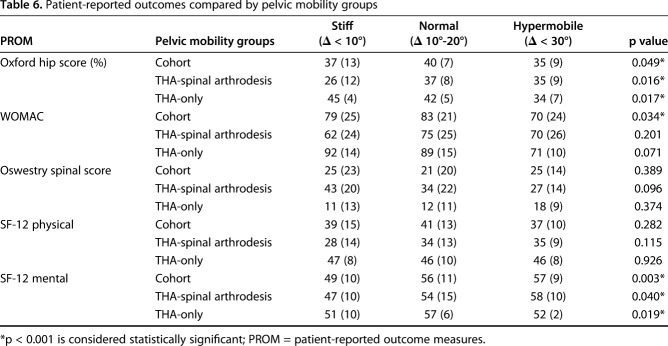
For the entire cohort, we saw differences in PROMs between the pelvic mobility groups. The normal mobility group had superior WOMAC scores (83 ± 21; p = 0.034) and Oxford hip score (40 ± 7; p = 0.049) compared with the stiff group (WOMAC, 79 ± 25; Oxford hip score, 37 ± 13) and the hypermobile group (WOMAC, 70 ± 24; Oxford hip score, 35 ± 9) (Table 6). Of the five patients who had instability, three had cup orientation outside the ideal zone. The two patients who dislocated despite appropriate cup orientation had spinopelvic hypermobility (ΔPTstanding seated ≥ -30°) with the pelvis tilting anteriorly on deep flexion. In the latter two patients, instability resolved after revision surgery, which altered the supine cup orientation (inclination/anteversion) from 50°/27° (anterior instability) to 48°/19° and from 32°/20° (posterior instability) to 35°/33°.
Table 6.
Patient-reported outcomes compared by pelvic mobility groups
Discussion
Gaining a better understanding of what factors influence outcome after THA and their link with adverse events and PROMs is of growing interest in a bundled payment environment [47, 52]. This is especially relevant where early readmissions and adverse events resulting from complications such as hip instability will negatively affect institutional performance and reimbursement. Like in other studies, we found that outcomes (including complications, revisions, and PROMs) after THA in patients with spinal arthrodesis are inferior. By performing assessments in different functional positions, it was evident that patients with spinal arthrodesis exhibit different spinopelvic mechanics and have a greater prevalence of spinopelvic hypermobility. Spinopelvic hypermobility was associated with inferior PROMs and was present in the two patients with recurrent instability despite ideal component orientation.
This study has several limitations. First, the length of followup was longer by a mean of 1 year (4.9 versus 6.2 years) in the cases compared with the controls. We do not believe this to be a serious limitation, because most complications (especially dislocations) would take place early postoperatively (as seen in this study) and because PROMs such as the Oxford hip score plateau at 1 year postoperatively [14]. Second, we did not perform any subanalysis as per approach or different numbers of fusion levels to determine whether different approaches have an effect on spinopelvic dynamics. This study would not have been powered to answer this question and further study with larger cohorts would be needed. Third, the lack of preoperative scores prevented us from determining the effect of the THA in improving outcome. This would have been a better measure of assessing effectiveness of the hip procedure because patients with spinal arthrodesis in situ may have lower baseline pre-THA and at followup because of the overlapping influences of the two kinds of pathology that were present in those patients. Fourth, several different spinal fusion levels were present in our THA-spinal arthrodesis cohort. A recent report has shown that complication risk increases with the number of levels fused [6]. It is therefore possible that this study may suffer from selection biases. However, this is a pragmatic study; the recruitment of cases involved all patients who underwent THA plus spinal arthrodesis at our center by 13 surgeons over 15 years. In addition, we only measured sagittal plane movements. Coronal and axial rotation of the spinopelvic complex would also affect cup orientation and THA biomechanics. It is likely, however, that such movements are small in comparison to the large variability of movement seen in the sagittal plane. Furthermore, any substantial obliquity would normally be detected in the standing AP pelvic radiograph. Lastly, 16 of 94 hips (17%) were lost to followup and could not be included. Nevertheless, the study had adequate power to address the research questions.
The patients who underwent both THA and spinal arthrodesis had a higher incidence of complications and revisions compared with the THA-only cohort. The most common complication was dislocation followed by periprosthetic joint infection, which is in line with previous reports [3, 4, 6]. In addition, the THA-spinal arthrodesis cohort demonstrated inferior PROMs and this study provides insight as to why this may be. Spinopelvic hypermobility was more common in the THA-spinal arthrodesis group and was associated with inferior PROMs. The association between inferior PROMs and spinopelvic hypermobility requires further study. It may be the result of subclinical impingement of the hip, the lack of sagittal balance of the spine [36], or problems of soft tissues such as abnormal pelvic muscle recruitment secondary to the increased mobility/lack of balance [16]. However, it was the hip-related PROMs rather than the Oswestry Disability Index that showed the association with hypermobility, pointing toward the hip and/or pelvic soft tissue as a strong contributor to the inferior outcomes observed.
Overall for the whole cohort, the change in pelvic tilt with different positions was considerable with variability across the entire cohort. Moving from supine to standing, the pelvis on average tilted posteriorly by 6° with a wide range (33°), in line with previous reports [2, 41, 44]. The mean Pelvic Tiltseated for the whole cohort was +5°. This differs considerably from other studies that reported the pelvic tilt in the seated-upright position to be between -25° and -36° [10, 18, 35, 40]. This probably reflects the body’s position at the time of the assessment (in this case, deep-flexed rather than upright-seated). The deep-seated position arguably represents a better assessment because it replicates what occurs when rising from a chair or when bending forward or tying up laces, the body leaning forward allowing for a biomechanically efficient sit to stand [34]. The value in this study is similar to Pierrepont et al. [41] (+1°) where the assessments were also performed when deep-seated. Importantly, like in all studies, a wide variability in Pelvic tiltseated was seen (66°). When moving from a standing to a flexed-seated position, the pelvis tilted forward (ΔPelvicTiltstanding2seated = -15°), and this movement showed great (59°) variability, too. It is noteworthy that the variability was seen in both groups, illustrating that spinopelvic movements do occur in THA-spinal arthrodesis, even if the spinal arthrodesis extends to the sacrum.
There is little doubt that instability can be of multifactorial origin such as offset, leg-length discrepancy, restoration of center of rotation, laxity, surgical approach, or femoral head size in addition to cup orientation [11, 15, 46, 50], which has challenged the safe zone concept [1, 11]. Of the five hips in patients who experienced dislocations, three had malpositioned cups with normal spinopelvic mobility and two had well-positioned cups and spinopelvic hypermobility. In the latter two, instability resolved after revision although one of the cups was placed with excess anteversion (33°; posterior dislocation) and was thereby outside the ideal zone; and the other cup had only a small reduction in anteversion (8°; anterior dislocation) and was still in the ideal zone. The findings of this study may explain the increased dislocation risk seen in patients with THA and spinal arthrodesis. When standing, the patients undergoing THA-spinal arthrodesis have a greater Pelvic tiltstanding (that is, a posteriorly tilted pelvis, which increases cup anteversion) and a greater pelvic-femoral angle (that is, the femur more extended). Such a combination would place patients undergoing THA-spinal arthrodesis at increased risk of posterior impingement and anterior instability in extension. Furthermore, when seated, the patients undergoing THA-spinal arthrodesis had an odds ratio of 5 in being more likely to have a ΔPelvic tiltstanding2seated > -30°, effectively reducing cup anteversion by > 22°. Therefore, the THA-spinal arthrodesis group is at increased risk of being functionally retroverted in flexion with an associated risk of anterior impingement and posterior dislocation.
To improve outcomes in this challenging cohort, a preoperative assessment of spinopelvic mobility should be performed. Whether this is performed with radiographs [41, 42] (Appendix 1) or modern imaging such as EOS (EOS Imaging SA, Paris, France) [12] would depend on local resources. However, such assessment would identify at-risk patients. In such patients, identifying the target cup orientation may benefit from preoperative modeling. Furthermore, surgeons managing this at-risk cohort should be cognizant of the narrower zone of ideal cup orientation and perhaps consider options to reliably achieve the ideal patient-specific orientation [48]. Patients undergoing THA-spinal arthrodesis may have a much narrower zone of safe cup orientation, which may be patient-specific; too much anteversion would render them unstable when standing, whereas too little anteversion would lead to instability when seated. The use of a larger femoral head and dual-mobility cups would increase jump distance, thereby reducing impingement risk and should be considered [17, 39]. However, the use of dual-mobility cups has been associated with abnormal sagittal dynamics [8]; whether and how dual-mobility cups interact with spinal arthrodesis remain unknown.
In conclusion, THA with a spinal arthrodesis is associated with inferior outcomes and higher complication rates. Patients undergoing spinal arthrodesis exhibit different sagittal spinopelvic movement, especially when standing (greater posterior tilt) and seated (greater anterior tilt), which place the THA at increased dislocation risk. The presence of spinopelvic hypermobility is associated with an inferior outcome and should be routinely assessed in patients with spinal arthrodesis when THA is considered. Spinopelvic hypermobility should be routinely assessed because these patients may have a narrow zone of optimum cup orientation that would require new technology to define and assist the surgeon in obtaining it.
Acknowledgments
We thank Nicole Harris for assistance with the collection of questionnaire data related to this study. Furthermore, we thank Diane Bonin, Kimberley Huywan, Stephanie Medeiros, and the team of radiographic technicians at The Ottawa Hospital for the collection of radiographic imaging.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
The work was performed at The Ottawa Hospital, Ottawa, Canada.
References
- 1.Abdel MP, von Roth P, Jennings MT, Hanssen AD, Pagnano MW. What safe zone? The vast majority of dislocated THAs are within the Lewinnek safe zone for acetabular component position. Clin Orthop Relat Res. 2016;474:386–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babisch JW, Layher F, Amiot LP. The rationale for tilt-adjusted acetabular cup navigation. J Bone Joint Surg Am. 2008;90:357–365. [DOI] [PubMed] [Google Scholar]
- 3.Barry JJ, Sing DC, Vail TP, Hansen EN. Early outcomes of primary total hip arthroplasty after prior lumbar spinal fusion. J Arthroplasty. 2017;32:470–474. [DOI] [PubMed] [Google Scholar]
- 4.Bedard NA, Martin CT, Slaven SE, Pugely AJ, Mendoza-Lattes SA, Callaghan JJ. Abnormally high dislocation rates of total hip arthroplasty after spinal deformity surgery. J Arthroplasty. 2016;31:2884–2885. [DOI] [PubMed] [Google Scholar]
- 5.Bellamy N. Validation study of WOMAC: a health status instrument for measuring clinically-important patient-relevant outcomes following total hip or knee arthroplasty in osteoarthritis. J Orthop Rheumatol. 1988;1:95–108. [PubMed] [Google Scholar]
- 6.Buckland AJ, Puvanesarajah V, Vigdorchik J, Schwarzkopf R, Jain A, Klineberg EO, Hart RA, Callaghan JJ, Hassanzadeh H. Dislocation of a primary total hip arthroplasty is more common in patients with a lumbar spinal fusion. Bone Joint J. 2017;99:585–591. [DOI] [PubMed] [Google Scholar]
- 7.Buckland AJ, Vigdorchik J, Schwab FJ, Errico TJ, Lafage R, Ames C, Bess S, Smith J, Mundis GM, Lafage V. Acetabular anteversion changes due to spinal deformity correction: bridging the gap between hip and spine surgeons. J Bone Joint Surg Am. 2015;97:1913–1920. [DOI] [PubMed] [Google Scholar]
- 8.Catelli DS, Kowalski E, Beaule PE, Lamontagne M. Does the dual-mobility hip prosthesis produce better joint kinematics during extreme hip flexion task? J Arthroplasty. 2017;32:3206–3212. [DOI] [PubMed] [Google Scholar]
- 9.Clohisy JC, Carlisle JC, Beaule PE, Kim YJ, Trousdale RT, Sierra RJ, Leunig M, Schoenecker PL, Millis MB. A systematic approach to the plain radiographic evaluation of the young adult hip. J Bone Joint Surg Am. 2008;90(Suppl 4):47–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DiGioia AM, Hafez MA, Jaramaz B, Levison TJ, Moody JE. Functional pelvic orientation measured from lateral standing and sitting radiographs. Clin Orthop Relat Res. 2006;453:272–276. [DOI] [PubMed] [Google Scholar]
- 11.Esposito CI, Gladnick BP, Lee YY, Lyman S, Wright TM, Mayman DJ, Padgett DE. Cup position alone does not predict risk of dislocation after hip arthroplasty. J Arthroplasty. 2015;30:109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esposito CI, Miller TT, Kim HJ, Barlow BT, Wright TM, Padgett DE, Jerabek SA, Mayman DJ. Does degenerative lumbar spine disease influence femoroacetabular flexion in patients undergoing total hip arthroplasty? Clin Orthop Relat Res. 2016;474:1788–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine (Phila Pa 1976). 2000;25:2940–2952; discussion 2952. [DOI] [PubMed] [Google Scholar]
- 14.Field RE, Cronin MD, Singh PJ. The Oxford hip scores for primary and revision hip replacement. J Bone Joint Surg Br. 2005;87:618–622. [DOI] [PubMed] [Google Scholar]
- 15.Grammatopoulos G, Thomas GE, Pandit H, Beard DJ, Gill HS, Murray DW. The effect of orientation of the acetabular component on outcome following total hip arthroplasty with small diameter hard-on-soft bearings. Bone Joint J. 2015;97:164–172. [DOI] [PubMed] [Google Scholar]
- 16.Greenwood NL, Duffell LD, Alexander CM, McGregor AH. Electromyographic activity of pelvic and lower limb muscles during postural tasks in people with benign joint hypermobility syndrome and non hypermobile people. A pilot study. Man Ther. 2011;16:623–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guyen O, Chen QS, Bejui-Hugues J, Berry DJ, An KN. Unconstrained tripolar hip implants: effect on hip stability. Clin Orthop Relat Res. 2007;455:202–208. [DOI] [PubMed] [Google Scholar]
- 18.Kanawade V, Dorr LD, Wan Z. Predictability of acetabular component angular change with postural shift from standing to sitting position. J Bone Joint Surg Am. 2014;96:978–986. [DOI] [PubMed] [Google Scholar]
- 19.Krismer M, Bauer R, Tschupik J, Mayrhofer P. EBRA: a method to measure migration of acetabular components. J Biomech. 1995;28:1225–1236. [DOI] [PubMed] [Google Scholar]
- 20.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–785. [DOI] [PubMed] [Google Scholar]
- 21.Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96:624–630. [DOI] [PubMed] [Google Scholar]
- 22.Langton DJ, Sprowson AP, Mahadeva D, Bhatnagar S, Holland JP, Nargol AV. Cup anteversion in hip resurfacing: validation of EBRA and the presentation of a simple clinical grading system. J Arthroplasty. 2010;25:607–613. [DOI] [PubMed] [Google Scholar]
- 23.Lazennec JY, Boyer P, Gorin M, Catonne Y, Rousseau MA. Acetabular anteversion with CT in supine, simulated standing, and sitting positions in a THA patient population. Clin Orthop Relat Res. 2011;469:1103–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lazennec JY, Brusson A, Rousseau MA. Hip-spine relations and sagittal balance clinical consequences. Eur Spine J. 2011;20(Suppl 5):686–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lazennec JY, Brusson A, Rousseau MA. Lumbar-pelvic-femoral balance on sitting and standing lateral radiographs. Orthop Traumatol Surg Res. 2013;99:S87–103. [DOI] [PubMed] [Google Scholar]
- 26.Lazennec JY, Riwan A, Gravez F, Rousseau MA, Mora N, Gorin M, Lasne A, Catonne Y, Saillant G. Hip spine relationships: application to total hip arthroplasty. Hip Int. 2007;17(Suppl 5):S91–104. [PubMed] [Google Scholar]
- 27.Lazennec JY, Rousseau MA, Brusson A, Folinais D, Amel M, Clarke I, Pour AE. Total hip prostheses in standing, sitting and squatting positions: an overview of our 8 years practice using the EOS imaging technology. Open Orthop J. 2015;9:26–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lazennec JY, Rousseau MA, Rangel A, Gorin M, Belicourt C, Brusson A, Catonne Y. Pelvis and total hip arthroplasty acetabular component orientations in sitting and standing positions: measurements reproductibility with EOS imaging system versus conventional radiographies. Orthop Traumatol Surg Res. 2011;97:373–380. [DOI] [PubMed] [Google Scholar]
- 29.Le Huec JC, Aunoble S, Philippe L, Nicolas P. Pelvic parameters: origin and significance. Eur Spine J. 2011;20(Suppl 5):564–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le Huec JC, Saddiki R, Franke J, Rigal J, Aunoble S. Equilibrium of the human body and the gravity line: the basics. Eur Spine J. 2011;20(Suppl 5):558–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lembeck B, Mueller O, Reize P, Wuelker N. Pelvic tilt makes acetabular cup navigation inaccurate. Acta Orthop. 2005;76:517–523. [DOI] [PubMed] [Google Scholar]
- 32.McDonnell SM, Boyce G, Bare J, Young D, Shimmin AJ. The incidence of noise generation arising from the large-diameter Delta Motion ceramic total hip bearing. Bone Joint J. 2013;95:160–165. [DOI] [PubMed] [Google Scholar]
- 33.Murray DW, Fitzpatrick R, Rogers K, Pandit H, Beard DJ, Carr AJ, Dawson J. The use of the Oxford hip and knee scores. J Bone Joint Surg Br. 2007;89:1010–1014. [DOI] [PubMed] [Google Scholar]
- 34.Nadzadi ME, Pedersen DR, Yack HJ, Callaghan JJ, Brown TD. Kinematics, kinetics, and finite element analysis of commonplace maneuvers at risk for total hip dislocation. J Biomech. 2003;36:577–591. [DOI] [PubMed] [Google Scholar]
- 35.Nishihara S, Sugano N, Nishii T, Ohzono K, Yoshikawa H. Measurements of pelvic flexion angle using three-dimensional computed tomography. Clin Orthop Relat Res. 2003;411:140–151. [DOI] [PubMed] [Google Scholar]
- 36.Ochi H, Homma Y, Baba T, Nojiri H, Matsumoto M, Kaneko K. Sagittal spinopelvic alignment predicts hip function after total hip arthroplasty. Gait Posture. 2017;52:293–300. [DOI] [PubMed] [Google Scholar]
- 37.Perfetti DC, Schwarzkopf R, Buckland AJ, Paulino CB, Vigdorchik JM. Prosthetic dislocation and revision after primary total hip arthroplasty in lumbar fusion patients: a propensity score matched-pair analysis. J Arthroplasty. 2017;32:1625–1640.e1. [DOI] [PubMed] [Google Scholar]
- 38.Phan D, Bederman SS, Schwarzkopf R. The influence of sagittal spinal deformity on anteversion of the acetabular component in total hip arthroplasty. Bone Joint J. 2015;97:1017–1023. [DOI] [PubMed] [Google Scholar]
- 39.Philippot R, Adam P, Reckhaus M, Delangle F, Verdot F, Curvale G, Farizon F. Prevention of dislocation in total hip revision surgery using a dual mobility design. Orthop Traumatol Surg Res. 2009;95:407–413. [DOI] [PubMed] [Google Scholar]
- 40.Philippot R, Wegrzyn J, Farizon F, Fessy MH. Pelvic balance in sagittal and Lewinnek reference planes in the standing, supine and sitting positions. Orthop Traumatol Surg Res. 2009;95:70–76. [DOI] [PubMed] [Google Scholar]
- 41.Pierrepont J, Hawdon G, Miles BP, Connor BO, Bare J, Walter LR, Marel E, Solomon M, McMahon S, Shimmin AJ. Variation in functional pelvic tilt in patients undergoing total hip arthroplasty. Bone Joint J. 2017;99:184–191. [DOI] [PubMed] [Google Scholar]
- 42.Pierrepont JW, Feyen H, Miles BP, Young DA, Bare JV, Shimmin AJ. Functional orientation of the acetabular component in ceramic-on-ceramic total hip arthroplasty and its relevance to squeaking. Bone Joint J. 2016;98:910–916. [DOI] [PubMed] [Google Scholar]
- 43.Rajaee SS, Bae HW, Kanim LE, Delamarter RB. Spinal fusion in the United States: analysis of trends from 1998 to 2008. Spine (Phila Pa 1976). 2012;37:67–76. [DOI] [PubMed] [Google Scholar]
- 44.Ranawat CS, Ranawat AS, Lipman JD, White PB, Meftah M. Effect of spinal deformity on pelvic orientation from standing to sitting position. J Arthroplasty. 2016;31:1222–1227. [DOI] [PubMed] [Google Scholar]
- 45.Rushton A, White L, Heap A, Heneghan N. Evaluation of current surgeon practice for patients undergoing lumbar spinal fusion surgery in the United Kingdom. World J Orthop. 2015;6:483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheth NP, Melnic CM, Paprosky WG. Evaluation and management of chronic total hip instability. Bone Joint J. 2016;98:44–49. [DOI] [PubMed] [Google Scholar]
- 47.Siddiqi A, White PB, Mistry JB, Gwam CU, Nace J, Mont MA, Delanois RE. Effect of bundled payments and health care reform as alternative payment models in total joint arthroplasty: a clinical review. J Arthroplasty. 2017;32:2590–2597. [DOI] [PubMed] [Google Scholar]
- 48.Spencer-Gardner L, Pierrepont J, Topham M, Bare J, McMahon S, Shimmin AJ. Patient-specific instrumentation improves the accuracy of acetabular component placement in total hip arthroplasty. Bone Joint J. 2016;98:1342–1346. [DOI] [PubMed] [Google Scholar]
- 49.Stefl M, Lundergan W, Heckmann N, McKnight B, Ike H, Murgai R, Dorr LD. Spinopelvic mobility and acetabular component position for total hip arthroplasty. Bone Joint J. 2017;99:37–45. [DOI] [PubMed] [Google Scholar]
- 50.Timperley AJ, Biau D, Chew D, Whitehouse SL. Dislocation after total hip replacement–there is no such thing as a safe zone for socket placement with the posterior approach. Hip Int. 2016;26:121–127. [DOI] [PubMed] [Google Scholar]
- 51.Ware JE, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. [DOI] [PubMed] [Google Scholar]
- 52.Williams J, Kester BS, Bosco JA, Slover JD, Iorio R, Schwarzkopf R. The association between hospital length of stay and 90-day readmission risk within a total joint arthroplasty bundled payment initiative. J Arthroplasty. 2017;32:714–718. [DOI] [PubMed] [Google Scholar]