Abstract
Background
Humeral bone loss is commonly encountered during revision shoulder arthroplasty and anticipating humeral bone defects can help the revision surgeon make appropriate plans to achieve adequate fixation and stability. No validated classification system exists to characterize humeral bone loss in the setting of revision shoulder arthroplasty.
Questions/purposes
The purposes of this study were (1) to create a classification system for humeral bone loss in revision shoulder arthroplasty; (2) to determine the classification system’s reliability; and (3) to determine whether humeral bone loss type is associated with intraoperative humeral-related reconstruction characteristics.
Methods
This was a comparative retrospective radiographic study. First, six surgeons from five centers collaborated to create a classification by consensus. Second, two surgeons from two other centers who had fellowship training in shoulder and elbow surgery, who were blinded to each other’s grades and all patient details other than plain radiographs, and who were not involved in creation of the system, classified true AP, AP, and lateral (axillary and/or scapular-Y) radiographs from 108 revision (413 radiographs) from one center that were performed between November 15, 2006, and January 4, 2018. Interobserver reliability was calculated by comparing those two reviews and determining Cohen’s κ. In addition, one reviewer repeated his assessments twice, 4 months apart, to determine intraobserver reliability using Cohen’s κ. Third, we performed a retrospective chart study of these same revisions to determine intraoperative humeral-related reconstruction characteristics such as the use of greater tuberosity fixation, stem length, humeral bone grafting, and the use of proximal humeral replacement or total humeral replacement; at the center where these revisions were performed during that timespan, no attempt to classify bone loss was made. During that period, the general indications for greater tuberosity fixation included the absence of a stable osseous connection between the greater tuberosity and the shaft of the humerus with a tuberosity amendable to repair; the general indications for use of longer stems were inability to obtain a minimum of two cortical widths of overlap between the implant and the humeral diaphysis and/or loss of the greater tuberosity; and the general indications for proximal and total humeral replacement were bone loss that was felt to be too severe to allow reconstruction with allograft.
Results
The classification system consists of three types of humeral bone loss: Type 1 is loss of the epiphysis with subtypes for loss of the calcar and loss of the greater tuberosity; Type 2 is loss of the metadiaphysis above the deltoid attachment with a subtype for cortical thinning; and Type 3 is bone loss extending below the deltoid attachment with a subtype for cortical thinning. We studied 108 revisions: 38 (35%) without bone loss, 34 (31%) Type 1, 27 (25%) Type 2, and nine (8%) Type 3. For reliability, interrater κ was 0.545 and in 71% (77 of 108) of revisions, the two raters agreed on a numeric type. Intrarater κ was 0.615 and in 77% (83 of 108) of revisions, the rater agreed with himself as to the numeric type. Stem length increased with class type (Type 1 median [range] 130 [70-210], Type 2 150 [70-210], Type 3 190 [70-240], p = 0.005). Most greater tuberosity fixation for intraoperative fracture was in Types 1 and 2 (13 of 18 compared with the five of 18 of greater tuberosity fixation that was within Types 0 and 3, p = 0.043). Most bone grafting was in Types 2 and 3 (eight of 13 compared with five of 13 of bone grafting was in Types 0 and 1, p = 0.044). Most proximal humeral and total humeral replacements were in Type 3 (three of four compared with one of four, p < 0.001).
Conclusions
We developed the Proximal Humeral Arthroplasty Revision Osseous inSufficiency (PHAROS) system, which has adequate, if imperfect, reliability to classify humeral bone loss in the setting of revision shoulder arthroplasty. This classification system may be useful to anticipate the complexity of humeral reconstruction. Further validation incorporating advanced imaging and further evaluators will be necessary.
Level of Evidence
Level III, diagnostic study.
Introduction
As the use of shoulder arthroplasty increases, so does the number of revision shoulder arthroplasties [5, 8, 18, 21, 22]. Humeral bone loss is commonly encountered during these revision procedures [5, 6, 8, 10, 16-18, 21, 22], and anticipating humeral bone defects can help the revision surgeon make appropriate plans to achieve adequate fixation and stability [13, 20].
However, no validated classification system exists to characterize humeral bone loss in the setting of revision shoulder arthroplasty. A classification system offers multiple advantages. Revision shoulder arthroplasties often are complex, and it can be difficult for the surgeon to determine which aspects of the pathology most influence treatment. In these types of situations, classification systems can provide a substantial benefit to the surgeon by clarifying the pathology. As a result, within orthopaedics, many classification systems have achieved widespread use. A classification system can inform diagnosis, treatment, and prognosis. In the setting of revision shoulder arthroplasty, such a system could guide surgeons with regard to a number of important clinical choices such as use of allograft, extended-length stems, proximal humeral replacement (or total humeral replacement), and the likelihood of intraoperative humeral-related complications such as greater tuberosity fractures. From a research perspective, a classification system allows future studies to compare equivalent pathologies. The ideal system would allow reliable classification based on plain radiographs alone and would provide diagnostic, therapeutic, and prognostic information to the surgeon and the patient.
The purposes of this study were to (1) create a classification system for humeral bone loss in revision shoulder arthroplasty; (2) determine the classification system’s reliability; and (3) determine whether humeral bone loss type is associated with intraoperative humeral-related reconstruction characteristics.
Materials and Methods
We conducted a multipart study. This study was approved by the institutional review board of the University of Utah, which granted a waiver of consent for chart and image review, because all data were secured, stored, and transmitted in a deidentified fashion. First, we gathered a group of experienced, high-volume shoulder arthroplasty surgeons interested in developing a classification system. A simplified classification system previously described by one member of this group (PB) was used as a starting point [2]. Within this group, we first discussed elements that each member felt should be incorporated. A draft system was then created and reviewed by each individual for commentary. This resulted in multiple rounds of revisions. These were reviewed iteratively until there were no further objections.
Second, we created a study set of radiographs and clinical histories for analysis. To create this set, we considered potentially eligible all patients who underwent revision shoulder arthroplasty between November 15, 2006, and January 4, 2018, as determined by the use of Common Procedural Terminology (CPT) codes 23331, 23332, 23333, 23335, 23473, or 23474. Five different surgeons (PNC, RZT, PG, RB, RLR) performed the operations. Only two of the surgeons (PNC, RZT) were involved with creation of the classification system. This list was reviewed and only those shoulders with prerevision AP, Grashey, and lateral-view radiographs and an operative report were included. Both axillary and scapular Y lateral-view radiographs were acceptable. We also excluded those shoulders in which the humeral component had (1) not been revised; (2) where a new permanent humeral component was never placed; (3) where there was no prior humeral component; or (4) where a platform stem was present and the fixated portion was not revised. These groups were excluded because the association between the classification system and aspects of humeral component revision could not be evaluated without humeral component revision. Our initial search based on CPT codes revealed 159 shoulders in 157 patients. Application of criteria resulted in the exclusion of 51 shoulders in 51 patients (Fig. 1). Applying these exclusions left us with the records of 108 shoulders in 106 patients for analysis.
Fig. 1.
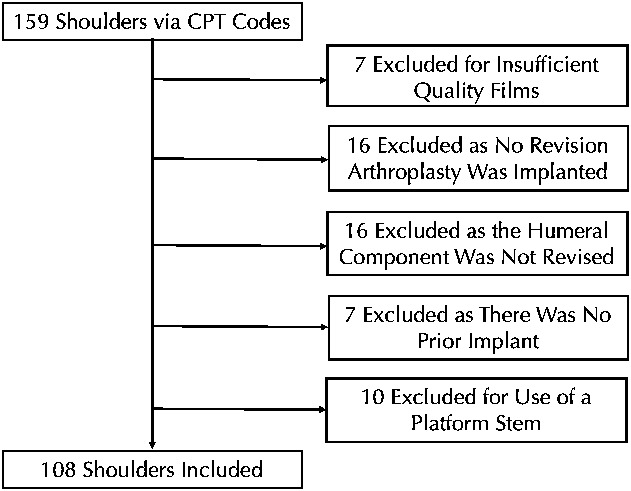
This flowchart demonstrates the results of our initial search based on CPT codes as well as study group size after the application of exclusion criteria.
Radiographic Review Protocol
To address the second purpose of this study, we took all 413 radiographs from 108 shoulders, assigned each one a study ID, and deidentified them to allow blinded review. These radiographs were then reviewed in a blinded fashion by two attending orthopaedic surgeons with fellowship training in shoulder and elbow surgery (JMG, DHS) and each radiograph was assigned a type and subtype according to the Proximal Humeral Arthroplasty Revision Osseous inSufficiency (PHAROS) Classification, which is summarized in the Results section that follows (Fig. 2). All efforts were made for these individuals to be insulated from the clinical details to provide an unbiased assessment of the clinical power of the system. Specifically, these individuals were at two centers not involved in creation of the system. Neither was at the center where the revisions were performed nor did they perform any of the revisions. These individuals were blinded to the clinical details regarding the revisions. Neither surgeon was familiar with any of the patients or radiographs. To ensure that these individuals understood how to apply the system, they graded the first 15 radiographs and these results were reviewed with the group. This process was repeated for the second 15 radiographs. The remainder of the data set was then graded and we tested interrater reliability. These reliability results were calculated both with and without these training sets. One individual (PNC) reviewed and rereviewed all radiographs twice separated by 4 months and assigned a type and subtype to determine intrarater reliability.
Fig. 2 A-B.
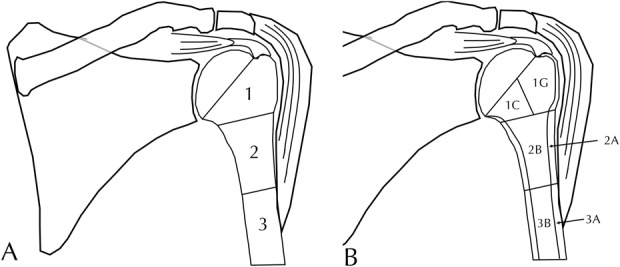
This schematic demonstrates the PHAROS Classification system for humeral bone loss in revision shoulder arthroplasty. Numeric (A) types include Type 1 with epiphyseal bone loss, Type 2 with metadiaphyseal bone loss above the deltoid attachment, and Type 3 with diaphyseal bone loss extending below the deltoid attachment. Alphanumeric (B) subtypes include Type 1C with calcar compromise, Type 1G with greater tuberosity compromise, Type 2A with cortical thinning of the metadiaphysis > 50% of the expected cortical thickness based on the noninstrumented portion of the humerus with associated epiphyseal loss or cortical thinning, Type 2B with bone loss above the deltoid of some metadiaphysis and the epiphysis, Type 3A with diaphyseal cortical thinning, and Type 3B with compromise of the majority of the diaphysis with loss of the epiphysis, metadiaphysis, and part of the diaphysis below the deltoid insertion.
Data Collection Protocol
To address the third purpose of this study, for each patient, the following data were collected by chart review: laterality, age, gender, initial implant type (hemiarthroplasty, total shoulder arthroplasty [TSA], or reverse total shoulder arthroplasty [RTSA]), surgeon, etiology of failure as described within the operative report diagnosis section, manufacturer of the revision humeral component, stem length in centimeters, method of stem fixation (cemented versus cementless), humeral liner type and thickness, method of stem extraction, whether a humeral bone graft was used and what type, how the graft was prepared, the length of the humeral bone graft used, whether the subscapularis was repaired, whether there were any concomitant tendon transfers, and whether the greater tuberosity required repair as a result of intraoperative fracture. At the center where these revisions were performed (November 15, 2006, and January 4, 2018), no attempt to classify bone loss was made. During that period, the general indications for greater tuberosity fixation included the absence of a stable osseous connection between the greater tuberosity and the shaft of the humerus with a tuberosity amendable to repair; the general indications for use of longer stems were inability to obtain a minimum of two cortical widths of overlap between the implant and the humeral diaphysis and/or loss of the greater tuberosity; and the general indications for proximal and total humeral replacement were bone loss that was felt to be too severe to allow reconstruction with allograft.
Statistical Analysis
Probability values < 0.05 were considered significant. All analyses were conducted in Excel X (Microsoft, Redmond, WA, USA) and SPSS 23 (IBM, Armonk, NY, USA). To determine reliability, we calculated Cohen’s κ. We defined a priori that a κ of > 0.5 would be considered the lower limit of acceptability because this is (1) similar to the published κ of the widely used Paprosky system [4]; (2) above 0.4, which is the lower limit of moderate agreement according to Landis and Koch [12]; and (3) above 0.4, which is the lower limit of fair to good agreement according to Fleiss [9]. Using chi-square tests, categorical variables were compared among bone loss types. Because all continuous data were nonnormally distributed (as determined by the Kolmogorov-Smirnov test), continuous variables were compared among the three numeric types using Kruskal-Wallis tests and we did not perform post hoc testing. The grades of the first of these individuals to finish grading the radiographs were used in the comparative analysis as was decided a priori.
Results
A Classification System for Humeral Bone Loss in Revision Shoulder Arthroplasty
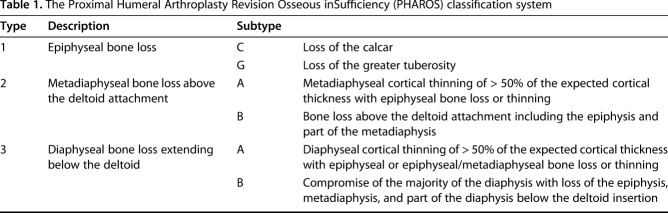
After reaching a consensus, the authors arrived at a system for classifying humeral bone loss consisting of three numeric types (Fig. 2) with these further subdivided into alphanumeric subtypes (Fig. 3). This system was named the PHAROS Classification after the ancient Greek word Pharos, a lighthouse that guides sailors in a storm. Type 1 was epiphyseal bone loss with the epiphysis including the articular surface, tuberosities, and calcar. Type 1 was subdivided into the following subtypes: Type C calcar loss (as seen on the Grashey and axillary radiographs) and Type G compromise (that is, loss or malunion) of the greater tuberosity. Type 2 was metadiaphyseal bone loss with the metadiaphysis defined as that bone above the deltoid attachment. Type 2 was subdivided into the following subtypes: Type A was cortical thinning of the metadiaphysis > 50% of the expected cortical thickness based on the noninstrumented portion of the humerus with associated epiphyseal loss or cortical thinning and Type B was bone loss above the deltoid of some metadiaphysis and the epiphysis. Type 3 was diaphyseal bone loss extending below the deltoid attachment. Type 3 was subdivided into the following subtypes: Type A was diaphyseal cortical thinning > 50% of the expected cortical thickness below the deltoid attachment based on the noninstrumented portion of the humerus with epiphyseal or epiphyseal/metaphyseal bone loss or cortical thinning and Type B was compromise of the majority of the diaphysis with loss of the epiphysis, metadiaphysis, and part of the diaphysis below the deltoid insertion (Table 1).
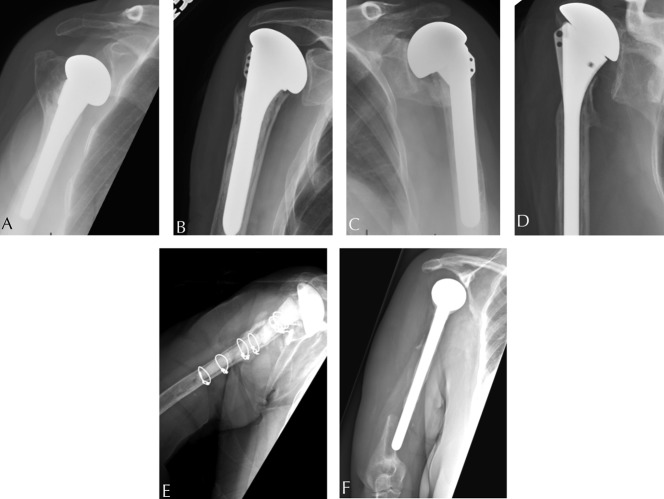
Fig. 3 A-F.
These radiographs are representative of each type and subtype of the PHAROS Classification system for humeral bone loss in revision shoulder arthroplasty. (A) This AP radiograph demonstrates Type 1C bone loss. (B) This AP radiograph demonstrates Type 1G bone loss. (C) This AP radiograph demonstrates Type 2A bone loss. (D) This AP radiograph demonstrates Type 2B bone loss. (E) This AP radiograph demonstrates Type 3A bone loss. (F) This AP radiograph demonstrates Type 3B bone loss.
Table 1.
The Proximal Humeral Arthroplasty Revision Osseous inSufficiency (PHAROS) classification system
Reliability
Among numeric types, as determined by the grades of the first of our two reviewers to finish grading the radiographs, our study included 38 of 108 (35%) without bone loss, which were used as a control group, 34 of 108 (31%) with Type 1, 27 of 108 (25%) Type 2, and nine of 108 (8%) Type 3. For intrarater reliability, using the full alphanumeric types, κ was 0.615 and using just the numeric types, κ was 0.660. In 71% (77 of 108) of shoulders, the rater agreed with himself as to the alphanumeric type. In 77% (83 of 108) of shoulders, the rater agreed with himself as to the numeric type. For interrater reliability, using the full alphanumeric type, κ was 0.545 and using just the numeric type, κ was 0.594. In 65% (70 of 108) of shoulders, the raters agreed on an alphanumeric type. In 71% (77 of 108) of shoulders, the raters agreed on a numeric type. Reliability was slightly improved if the two 15-shoulder training sets were excluded: using the full alphanumeric type, κ was 0.581 and using just the numeric type, κ was 0.625. In 69% (54 of 78) of shoulders, the raters agreed on an alphanumeric type. In 74% (58 of 78) of shoulders, the raters agreed on a numeric type.
Association Between Humeral Bone Loss Type and Intraoperative Humeral-related Reconstruction Characteristics
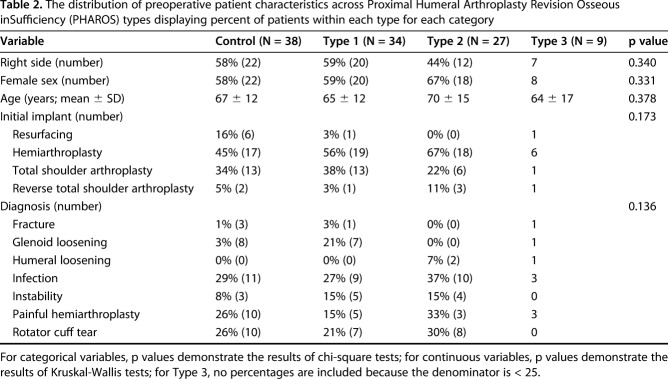
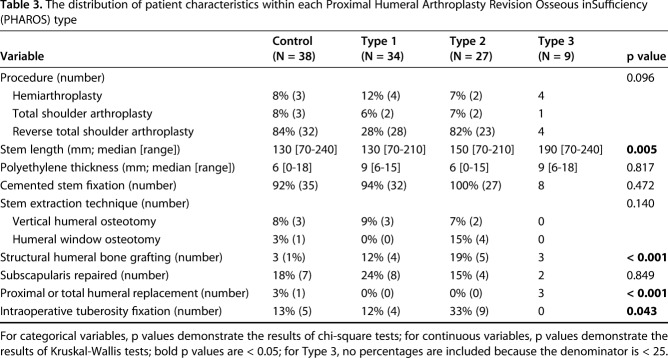
Patient characteristics did not differ between groups (Table 2). Many aspects of the humeral reconstruction differed based on the humeral bone loss type. The stem length increased sequentially with numeric type (Type 1 median [range] 130 [70-210], Type 2 150 [70-210], Type 3 190 [70-240], p = 0.005; Table 3). Most greater tuberosity fixation for intraoperative fracture was in Types 1 and 2 (13 of 18 compared with five of 18 of greater tuberosity fixation that was within Types 0 and 3, p = 0.043). Most structural bone grafting was in Types 2 and 3 (eight of 13 compared with five of 13 of bone grafting was within Types 0 and 1, p = 0.044). In particular, five of 13 Type 2B and two of four Type 3B underwent concomitant bone grafting. Of the nine structural humeral bone grafts placed, three were step-cut and six were not step-cut. These included three femoral allograft ring grafts, of which two were step-cut and one was not, which varied from 3 to 6 cm in length. These also included six proximal humeral allografts, of which only one was step-cut, which varied from 2.75 to 5 cm in length. Most proximal humeral and total humeral replacements were in Type 3 (three of four compared with one of four, p < 0.001) with two of four Type 3B shoulders undergoing proximal humeral and total humeral replacement. Most shoulders in all types had cemented stem fixation (92% [35 of 38] of those without bone loss, 94% [32 of 34] Type 1, 100% [27 of 27] Type 2, and eight of nine Type 3). Most shoulders in all types did not have a subscapularis repair (18% [seven of 38] of those without bone loss, 24% [eight of 34] Type 1, 15% [four of 27] Type 2, and two of nine Type 3, p = 0.849). There were no differences in humeral extraction method (Table 3; p = 0.140) because a vertical humeral osteotomy and humeral window osteotomy were infrequent in all types. With regard to the polyethylene liner, there were no differences in combined polyethylene/spacer thickness between types (thickness in Type 1 was median [range] 9 [6-15], Type 2 6 [0-15], and Type 3 9 [6-18], p = 0.817), suggesting that in the setting of advanced bone loss, most restoration of length was recreated within the implant and bone graft and not within the spacer or polyethylene.
Table 2.
The distribution of preoperative patient characteristics across Proximal Humeral Arthroplasty Revision Osseous inSufficiency (PHAROS) types displaying percent of patients within each type for each category
Table 3.
The distribution of patient characteristics within each Proximal Humeral Arthroplasty Revision Osseous inSufficiency (PHAROS) type
Discussion
Revision shoulder arthroplasty procedures are becoming more common [5, 8, 18, 21, 22], and humeral bone loss is commonly encountered during revisions [5, 6, 8, 10, 16-18, 21, 22]. Anticipating bone defects can help the revision surgeon achieve humeral component fixation and stability [13, 20]. The PHAROS Classification system was developed to describe humeral bone loss in revision shoulder arthroplasty situations based on the surgical experience of the authors and on the anatomic subregions of the proximal humerus. The proposed classification system has acceptable interrater and intrarater reliability and this retrospective analysis has shown the classification to be helpful in directing operative treatment strategies. In particular, Types 1 and 2 bone loss were associated with use of greater tuberosity fixation of intraoperative fractures, Types 2 and 3 were associated with use of structural humeral bone grafting, and Type 3 diaphyseal bone loss was associated with use of proximal humeral replacement or total humeral replacement. This classification system may be useful in future research regarding revision shoulder arthroplasty. This classification system may also be useful to anticipate the complexity of the humeral reconstruction. Future research will be necessary to understand the treatment implications of the PHAROS Classification.
Our study has several limitations. First, a limited number of subspecialty shoulder surgeons were involved in the creation of this system and thus the system may only apply to subspecialty shoulder surgeons. Future studies will be necessary to validate our findings with general orthopaedic surgeons and trainees. Second, this is a retrospective study. A prospective design would have allowed more consistent radiographic collection. Third, although the system was developed and reliability tested using a multicenter approach, our retrospective study was performed on a single-center basis. Thus, our results will need to be redemonstrated at other centers. At other institutions with experience or a preference for other implants or other techniques, the procedure performed for each type may differ. To mitigate this limitation, the study group includes revisions performed by five surgeons, of whom only two were involved in the creation of our classification. In addition, the surgeons assigning types by radiographic review did not perform any of the revisions. Further research will be needed to validate this classification using varying treatment strategies and with longer followup periods for predicting relevant complications. Fourth, our study is likely underpowered for some comparisons, particularly those with regard to Type 3 bone loss because bone loss of this severity is rare. Thus, the treatment implications of our system with regard to Type 3 bone loss remain uncertain. Fifth, our system only incorporates information from preoperative plain radiographs. Certainly incorporation of CT scans could provide additional information. However, these studies add cost and subject patients to additional radiation and delay before revision. In addition, many shoulders within our cohort (49% [53 of 108]) did not have preoperative CT scans and thus development of a system that requires CT scans would create substantial selection bias. In addition, bone loss may be affected by intraoperative factors such as component and cement removal or retention or the status of the soft tissues. Because this was a retrospective study, these factors were not consistently universally recorded and thus cannot be studied. Sixth, as implants change, certain elements of our system may become less relevant; for instance, with the development of platform stems, there may be fewer humeral component revisions. Seventh, our study does not include data on clinical outcomes, revision rates, or rates of humeral loosening. The purpose of this initial study was to create a system based on the clinical experience of a group of high-volume surgeons and to demonstrate the system is reliable and future studies will be necessary to determine whether the system is associated with clinical outcome or revision rates. Eighth, our study did not include those shoulders in which the humeral component was not revised and thus our classification system may not be applicable to these shoulders.
In this study, we describe a three-part system for the classification of humeral bone loss. Few prior studies have classified humeral bone loss in the setting of revision shoulder arthroplasty. One of the authors (PB) suggested three types: Type A with < 2 cm of epiphyseal bone loss (analogous to Type 1 loss in our system), Type B with < 4 cm of metaphyseal bone loss (analogous to Type 2 bone loss in our system), and Type C with > 4 cm of bone loss extending into the diaphysis (analogous to Types 3 in our system) [2]. McLendon and colleagues have suggested that bone loss be subdivided into < 5 cm (analogous to Types 1 and 2 in our system) and > 5 cm (analogous to Type 3 in our system). Within their study, this threshold was proposed as a guide for indications for a proximal humeral allograft [14]. Neither of these systems has been evaluated for reliability or an association with treatment or complications. Although the biomechanics of the proximal humerus differ drastically from those of the proximal femur, our system incorporates elements of the Paprosky classification for femoral bone loss [19]. This system is widely used [19]. For instance, Paprosky Types 1 and 2, in which the diaphysis is preserved, are analogous to Types 1 and 2 in our system. Similarly, within the Paprosky system, the division between Types 3 and 4 is whether the isthmus remains supportive, which is analogous to the Type 3A versus 3B divide in our system.
We found the PHAROS system to be reliable based on our a priori cutoff for acceptable reliability. Our numeric system, with a κ of 0.625 after training, has slightly superior reliability to the commonly used and accepted Paprosky system, which has a κ of 0.61 [4]. The authors thus feel that this system is sufficiently reliable for clinical and research use. The system is purposefully alphanumeric to allow surgeons to use either the simplified numeric system or the more complex alphanumeric system. As would be expected, reliability of the simplified numeric system was superior to the more complex alphanumeric system, likely because of difficulty with reliably determining whether cortical thinning had occurred. However, it should be noted that reliability within our system could be improved because there was disagreement between reviewers as to the alphanumeric grade in 35% (38 of 108) of shoulders and as to the numeric grade in 29% (31 of 108) of shoulders, and there was disagreement between the same reviewers on multiple occasions as to the alphanumeric grade in 29% (31 of 108) of shoulders and as to the numeric grade in 23% (25 of 108) of shoulders. Disagreement levels may be even higher with use of the system by those without fellowship training in shoulder and elbow surgery. Disagreement limits future clinical and research use of the PHAROS system. Disagreement is also a limitation of our analysis because the grades of the first reviewer to finish grading the radiographs were used in the comparative analysis. Certainly future research with inclusion of additional radiographs or advanced imaging could be helpful to reduce residual disagreement. Within our series, although training did slightly improve interobserver reliability, the difference was numerically very small and the values including the training sets were still well above our threshold of acceptability, suggesting that individuals new to the system can begin immediate use without training with reliable grades.
We found that our classification system was associated with humeral-related complications and treatment. In particular, Types 1 and 2 bone loss were associated with the use of greater tuberosity fixation of intraoperative fractures, Types 2 and 3 were associated with the use of structural humeral bone grafting, and Type 3 diaphyseal bone loss was associated with use of proximal humeral replacement or total humeral replacement. As future studies apply this classification system in other data sets, this classification system may provide a basis for treatment recommendations. For instance, in the setting of loss of the calcar and greater tuberosity (Type 2A and greater bone loss), the two most commonly utilized landmarks to determine prosthesis height are absent and thus the surgeon could consider obtaining bilateral full-length humeral radiographs to properly determine prosthesis height and avoid postoperatively undertensioning of the soft tissues and the potential for instability [2, 23]. Similarly, although revision to TSA was possible in seven of 108 shoulders, it was not possible in any shoulders with 1G bone loss, because revision to a TSA in the setting of an irreparable rotator cuff is contraindicated [3, 15] and thus the surgeon should be prepared to consider an RTSA in the setting of 1G bone loss. As the underlying pathology becomes better understood and as new implants and techniques are developed, the development of new types may be necessary, as has occurred in other classification systems [1, 7, 11, 21]. Although the methodology of the current study does not allow the authors to make specific recommendations, the authors suggest that clinic use of this classification system could help surgeons to clarify their approach when facing the full spectrum of humeral bone loss pathology encountered in revision shoulder arthroplasty.
The PHAROS system has adequate, if imperfect, reliability to classify humeral bone loss in the setting of revision shoulder arthroplasty. This classification system may be useful in future research regarding revision shoulder arthroplasty. This classification system may also be useful to anticipate the complexity of humeral reconstruction. Further validation incorporating advanced imaging and further evaluators will be necessary.
Acknowledgments
We thank Irene Stertz and Erin Granger for assistance with data collection. We also thank Dr Aaron Rosenberg for his advice regarding generalized optimal characteristics for a classification system. We thank Drs Robert Burks, Patrick Greis, and R. Lor Randall, who performed some of the included revisions.
Footnotes
One of the authors certifies that he (PNC) has received payments, during the study period, of an amount of less than USD 10,000 from DePuy (New Brunswick, NJ, USA). One of the authors certifies that he (AAR) has received payments, during the study period, of an amount greater than USD 1,000,000 from Arthrex (Naples, FL, USA) and of amounts less than USD 10,000 from the American Shoulder and Elbow Surgeons (Chicago, IL, USA), Atreon (Columbus, OH, USA, Orthopedics (Slack Inc, Thorofare, NJ, USA), Orthopedics Today (Slack Inc), SAGE Publishing (Newbury Park, CA, USA), Wolters Kluwer Health Publishing (Philadelphia, PA, USA), Aesculap/B. Braun (Bethlehem, PA, USA), Histogenics (Waltham, MA, USA), Medipost (Rockville, MD, USA), NuTech (Birmingham, AL, USA), OrthoSpace (Caesarea, Israel), Smith & Nephew (Memphis, TN, USA), Zimmer (Warsaw, IN, USA), Saunders (Philadelphia, PA, USA), the Arthroscopy Association of North American (Chicago, IL, USA), and Major League Baseball (New York, NY, USA). One of the authors certifies that he (GPN) has received payments, during the study period, of an amount less than USD 10,000 from American Shoulder and Elbow Surgeons (Chicago IL, USA), Arthrosurface (Franklin, MA, USA), Innomed (Savannah, GA, USA), and Wright Medical (Memphis, TN, USA). One of the authors certifies that he (PB) has received payments, during the study period, an amount of greater than USD 1,000,000 from Wright Medical and of amounts less than USD 10,000 from Smith & Nephew and CONMED Linvatec (Utica, NY, USA). One of the authors certifies that he (JDK) has received payments, during the study period, of an amount of USD 10,001 to 100,000 from Arthrex and of an amount less than 10,000 USD from Elite Orthopaedics (South El Monte, CA, USA) and Zimmer. One of the authors (JMG) is a board or committee member for the American Academy of Orthopaedic Surgeons and serves as a paid consultant for Wright Medical. One of the authors certifies that he (RZT) has received payments, during the study period, of an amount less than 10,000 from Zimmer, DePuy, Conextions (Salt Lake City, UT, USA), INTRAFUSE (Salt Lake City, UT, USA), KATOR (Salt Lake City, UT, USA), Wright Medical, the Journal of Bone and Joint Surgeons (Needham, MA, USA), and the Journal of Orthopaedic Trauma (Tampa, FL, USA).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the University of Utah, Salt Lake City, UT, USA.
References
- 1.Bercik MJ, Kruse K, Yalizis M, Gauci M-O, Chaoui J, Walch G. A modification to the Walch classification of the glenoid in primary glenohumeral osteoarthritis using three-dimensional imaging. J Shoulder Elbow Surg. 2016;25:1601-1606. [DOI] [PubMed] [Google Scholar]
- 2.Boileau P. Complications and revision of reverse total shoulder arthroplasty. Orthop Traumatol Surg Res . 2016;102:S33–43. [DOI] [PubMed] [Google Scholar]
- 3.Bonnevialle N, Melis B, Neyton L, Favard L, Molé D, Walch G, Boileau P. Aseptic glenoid loosening or failure in total shoulder arthroplasty: revision with glenoid reimplantation. J Shoulder Elbow Surg. 2013;22:745–751. [DOI] [PubMed] [Google Scholar]
- 4.Brown NM, Foran JRH, Valle CJD, Moric M, Sporer SM, Levine BR, Paprosky WG. The inter-observer and intra-observer reliability of the Paprosky femoral bone loss classification system. J Arthroplasty. 2014;29:1482–1484. [DOI] [PubMed] [Google Scholar]
- 5.Chin PC, Hachadorian ME, Pulido PA, Munro ML, Meric G, Hoenecke HR. Outcomes of anatomic shoulder arthroplasty in primary osteoarthritis in type B glenoids. J Shoulder Elbow Surg. 2015;24:1888–1893. [DOI] [PubMed] [Google Scholar]
- 6.Daggett M, Werner B, Collin P, Gauci M-O, Chaoui J, Walch G. Correlation between glenoid inclination and critical shoulder angle: a radiographic and computed tomography study. J Shoulder Elbow Surg. 2015;24:1948–1953. [DOI] [PubMed] [Google Scholar]
- 7.Domos P, Checchia CS, Walch G. Walch B0 glenoid: pre-osteoarthritic posterior subluxation of the humeral head. J Shoulder Elbow Surg. 2017;27:181-188. [DOI] [PubMed] [Google Scholar]
- 8.Engelhardt C, Farron A, Becce F, Place N, Pioletti DP, Terrier A. Effects of glenoid inclination and acromion index on humeral head translation and glenoid articular cartilage strain. J Shoulder Elbow Surg. 2017;26:157–164. [DOI] [PubMed] [Google Scholar]
- 9.Fleiss JL. Statistical Methods for Rates and Proportions. New York, NY, USA: John Wiley; 1981. [Google Scholar]
- 10.Hughes RE, Bryant CR, Hall JM, Wening J, Huston LJ, Kuhn JE, Carpenter JE, Blasier RB. Glenoid inclination is associated with full-thickness rotator cuff tears. Clin Orthop Relat Res. 2003;407:86–91. [DOI] [PubMed] [Google Scholar]
- 11.Iannotti JP, Jun BJ, Patterson TE, Ricchetti ET. Quantitative measurement of osseous pathology in advanced glenohumeral osteoarthritis. J Bone Joint Surg Am . 2017;99:1460–1468. [DOI] [PubMed] [Google Scholar]
- 12.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 13.Maurer A, Fucentese SF, Pfirrmann CWA, Wirth SH, Djahangiri A, Jost B, Gerber C. Assessment of glenoid inclination on routine clinical radiographs and computed tomography examinations of the shoulder. J Shoulder Elbow Surg. 2012;21:1096–1103. [DOI] [PubMed] [Google Scholar]
- 14.McLendon PB, Cox JL, Frankle MA. Humeral bone loss in revision shoulder arthroplasty. Am J Orthop (Belle Mead NJ). 2018. Feb;47(2). 10.12788/ajo.2018.0012. [DOI] [PubMed] [Google Scholar]
- 15.Melis B, Bonnevialle N, Neyton L, Levigne C, Favard L, Walch G, Boileau P. Glenoid loosening and failure in anatomical total shoulder arthroplasty: is revision with a reverse shoulder arthroplasty a reliable option? J Shoulder Elbow Surg. 2012;21:342–349. [DOI] [PubMed] [Google Scholar]
- 16.Moor BK, Bouaicha S, Rothenfluh DA, Sukthankar A, Gerber C. Is there an association between the individual anatomy of the scapula and the development of rotator cuff tears or osteoarthritis of the glenohumeral joint? A radiological study of the critical shoulder angle. Bone Joint J. 2013;95:935–941. [DOI] [PubMed] [Google Scholar]
- 17.Spiegl UJ, Horan MP, Smith SW, Ho CP, Millett PJ. The critical shoulder angle is associated with rotator cuff tears and shoulder osteoarthritis and is better assessed with radiographs over MRI. Knee Surg Sports Traumatol Arthrosc. 2016;24:2244–2251. [DOI] [PubMed] [Google Scholar]
- 18.Terrier A, Merlini F, Pioletti DP, Farron A. Total shoulder arthroplasty: downward inclination of the glenoid component to balance supraspinatus deficiency. J Shoulder Elbow Surg. 2009;18:360–365. [DOI] [PubMed] [Google Scholar]
- 19.Valle CJD, Paprosky WG. Classification and an algorithmic approach to the reconstruction of femoral deficiency in revision total hip arthroplasty. J Bone Joint Surg Am. 2003;85(Suppl 4):1–6. [DOI] [PubMed] [Google Scholar]
- 20.Van Haver A, Heylen S, Vuylsteke K, DeClercq G, Verborgt O. Reliability analysis of glenoid component inclination measurements on postoperative radiographs and computed tomography-based 3D models in total and reversed shoulder arthroplasty patients. J Shoulder Elbow Surg. 2016;25:632–640. [DOI] [PubMed] [Google Scholar]
- 21.Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14:756–760. [DOI] [PubMed] [Google Scholar]
- 22.Walch G, Young AA, Boileau P, Loew M, Gazielly D, Molé D. Patterns of loosening of polyethylene keeled glenoid components after shoulder arthroplasty for primary osteoarthritis: results of a multicenter study with more than five years of follow-up. J Bone Joint Surg Am. 2012;94:145–150. [DOI] [PubMed] [Google Scholar]
- 23.Werner BS, Ascione F, Bugelli G, Walch G. Does arm lengthening affect the functional outcome in onlay reverse shoulder arthroplasty? J Shoulder Elbow Surg. 2017;26:2152-2157. [DOI] [PubMed] [Google Scholar]