Abstract
Background:
Programmed cell death ligand 1 (PD-L1) expression was reported to be associated with poor prognosis in various solid tumors. However, the prognosis value of PD-L1 in pancreatic cancer remained inconclusive. We performed a meta-analysis to assess the clinical value of PD-L1 as a novel prognostic biomarker of pancreatic cancer.
Methods:
PubMed, Embase, and Web of Science were searched up to October 2018. The HRs and 95% CIs for overall survival (OS) and cancer-specific survival (CSS) according to the expressional status of PD-L1 were pooled. The combined odd ratios (ORs) and 95% CIs were utilized to assess the association between PD-L1 and clinicopathological characteristics.
Results:
A total of 9 studies with 993 patients were included. Elevated PD-L1 expression was related with poor OS (HR = 1.63, 95% CI = 1.34–1.98, P < .001) and CSS (HR = 1.86, 95% CI = 1.34–2.57, P < .001). Furthermore, high PD-L1 expression was also demonstrated to be associated with positive N stage (OR = 1.81, 95% CI = 1.21–2.71, P = .004), advanced T stage (OR = 1.86, 95% CI = 1.08–3.19, P = .025), and low differentiation (OR = 2.24, 95% CI = 1.16–4.33, P = .017). However, PD-L1 has nonsignificant correlation with M stage, gender, or age.
Conclusion:
This study suggests that PD-L1 is a potential prognostic biomarker and may be helpful to clinicians aiming to select the appropriate immunotherapy for pancreatic cancer.
Keywords: clinical features, meta-analysis, pancreatic cancer, PD-L1, survival
1. Introduction
Pancreatic cancer is a highly lethal malignancy with 5-year survival rate as low as 6%.[1] Pancreatic cancer is the seventh leading cause of cancer-related death both in men and women worldwide.[2] Therapeutic strategies of pancreatic cancer include surgery, chemotherapy, radiotherapy, and palliative care. Although treatment techniques have been developed in recent years, the prognosis of pancreatic cancer is not significantly improved. To date, a group of prognostic factors are identified for clinical management of pancreatic cancer.[3–5] However, these markers are lack of accuracy to predict and are not widely adopted. Therefore, it is still important to find out novel and available prognostic biomarkers for patients with pancreatic cancer.
Programmed cell death 1 (PD-1)/programmed cell death ligand 1 (PD-L1) pathway plays an important role in cancer immune editing.[6,7] In the tumor microenvironment, cancer cells and infiltrating immune cells express PD-L1,[8,9] which can combine with PD-1 on T cells and then suppress the proliferative and effector responses of T cells. Blockade of PD-L1 is a prevalent strategy of cancer immunotherapy,[10,11] which is called immune checkpoint inhibitors.[9] Previous studies reported the prognostic significance of PD-L1 expression in malignant solid tumors including breast cancer,[12] gastric cancer,[13] hepatocellular carcinoma,[14] non-small cell lung cancer,[15] and renal cell carcinoma.[16] A number of studies also investigated the association of PD-L1 and prognosis of pancreatic cancer,[17–21] with controversial results presented. For example, Nomi et al reported PD-L1 overexpression as a prognostic factor of poor overall survival (OS) (P = .016) in patients with pancreatic cancer receiving surgery.[17] However, other studies showed nonsignificant prognostic value of PD-L1 in pancreatic cancer.[20,22,23] In the present study, a meta-analysis was carried out to assess the correlation between PD-L1 expression and survival outcomes and clinicopathological characteristics in pancreatic cancer patients.
2. Materials and methods
This meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement.[24] An ethical approval was not necessary since meta-analysis was based on secondary data.
2.1. Search strategy
Electronic databases of PubMed, Embase, and Web of Science were searched. Different combinations of the following keywords were used: “PD-L1”, “programmed cell death ligand 1”, “B7-H1”, “CD274”, “pancreatic neoplasm”, “pancreatic cancer”, “prognosis”, “prognostic,” and “outcome”. The last search was up to October 2018. References of the retrieved studies were also manually searched for possible inclusions.
2.2. Selection criteria
Inclusion criteria were as follows:
-
(1)
immunohistochemistry (IHC) was used to measure PD-L1 expression in pancreatic tissues;
-
(2)
studies focus on pancreatic cancer;
-
(3)
studies evaluating the relationship between PD-L1 and survival outcomes and/or clinical features. If survival outcomes were not directly provided, enough information was given to compute the HR and 95% CI by using Tierney's method[25];
-
(4)
English articles.
Studies were excluded by the following exclusion criteria:
-
(1)
reviews, conference abstracts, or letters;
-
(2)
using other method than IHC to detect PD-L1;
-
(3)
overlapping studies.
Cancer-specific survival (CSS) was defined as the period from the time of surgery to patient death of pancreatic cancer. OS was defined as the period from the time of surgery to patient death of any cause.
2.3. Data extraction and quality assessment
Two investigators independently reviewed eligible articles and extracted information as follows: name of first author, publication year, country, number of cases, age, tumor stage, treatment, detection method, pathologic data, and survival outcomes. Disagreements between the two authors were resolved through discussion. Quality assessment of included studies was performed using the Newcastle–Ottawa Quality Assessment Scale (NOS) checklist.[26] The maximum score of NOS is 9 points and studies with a score ≥6 are considered high-quality studies.
2.4. Statistical analysis
The HRs and 95% CIs for OS and CSS according to the expressional status of PD-L1 were pooled. Moreover, the combined odd ratios (ORs) and 95% CIs were utilized to assess the association between PD-L1 and clinicopathological characteristics. Statistical heterogeneity among studies was assessed using Cochran's Q test and Higgins I2 statistic. A P value <.1 or an I2 > 50% indicated significant heterogeneity among studies; in this case, a random-effects model was used. Otherwise, a fixed-effects model was selected. Publication bias was measured using Begg's funnel plots. Statistical analyses were performed with Stata 12.0 software (Stata Corporation, College Station, TX). A P-value <.05 was considered statistically significant.
3. Results
3.1. Study characteristics
A total of 230 studies were identified through database searching. After duplicates were removed, 154 studies were screened by title and abstract and 139 records were further excluded. The remaining 15 studies were examined by reading full-texts. Eight studies were removed with reasons and 7 studies were eligible (Fig. 1). Moreover, the updated search found another 2 eligible studies. At last, 9 studies[17–23,27,28] were included in meta-analysis. The detailed information of the included studies was shown in Table 1. Five studies were from China,[18,19,23,27,28] three were from Japan,[17,21,22] and one was from United States.[20] The total sample size was 993, ranging from 36 to 373. Eight studies[17,19–23,27,28] investigated the prognostic role of PD-L1 for OS and 3 studies[18,22,27] explored the association between PD-L1 and CSS. They were published between 2007 and 2018. All studies were with a NOS score ≥6.
Figure 1.
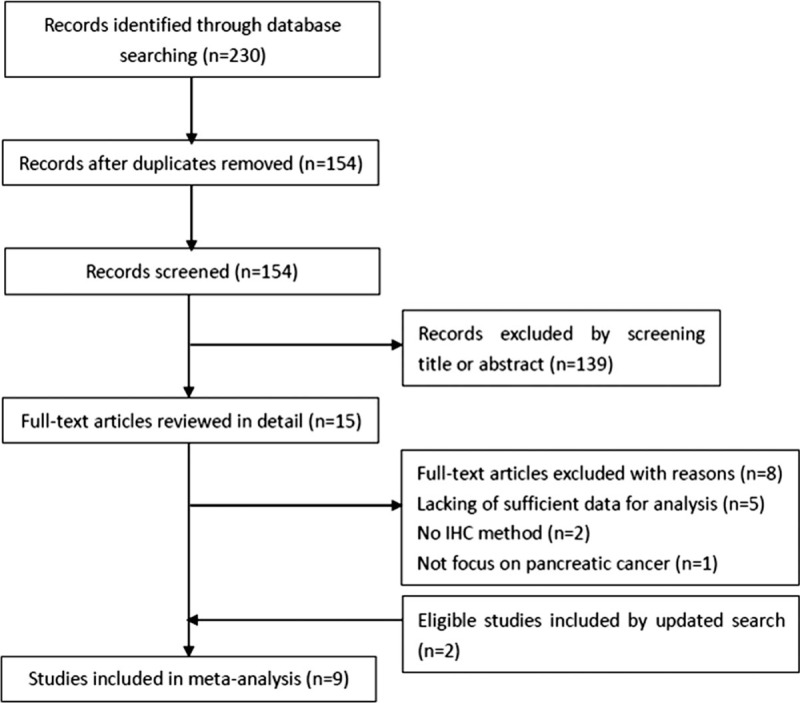
Flow diagram of literature search and study selection.
Table 1.
Characteristics of included studies.
3.2. Prognostic value of PD-L1 for OS and CSS
Eight studies with a total of 912 patients were included to explore the association between PD-L1 expression and OS. A fixed-effects model was used due to nonsignificant heterogeneity (I2 = 0, P = .678; Fig. 2). The pooled HR was 1.63, with 95% CI = 1.34–1.98, P < .001. The impact of PD-L1 on prognosis of CSS was shown in 3 studies.[18,22,27] The combined results were: HR = 1.86, 95% CI = 1.34–2.57, P < .001, with nonsignificant heterogeneity (I2 = 0, P = .878; Fig. 3).
Figure 2.
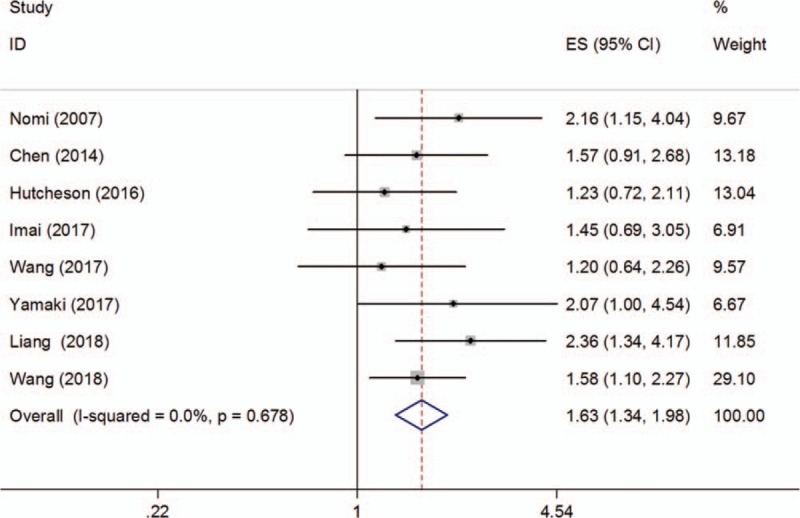
Forest plot describing the association between PD-L1 expression and OS of patients with pancreatic cancer. OS = overall survival, PD-L1 = programmed cell death ligand 1.
Figure 3.

Forest plot describing the association between PD-L1 expression and CSS of patients with pancreatic cancer. CSS = cancer-specific survival, PD-L1 = programmed cell death ligand 1.
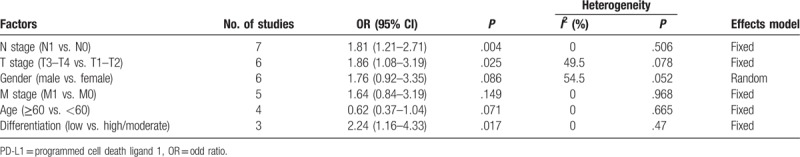
3.3. Correlation of PD-L1 expression with clinicopathological characteristics
As shown in Figure 4 and Table 2, the association of PD-L1 and 6 clinicopathological characteristics was investigated through meta-analysis. The pooled data demonstrated that high PD-L1 expression was associated with positive N stage (OR = 1.81, 95% CI = 1.21–2.71, P = .004; fixed effect), advanced T stage (OR = 1.86, 95% CI = 1.08–3.19, P = .025; fixed effect), and low differentiation (OR = 2.24, 95% CI = 1.16–4.33, P = .017; fixed effect). However, PD-L1 has nonsignificant correlation with gender (OR = 1.76, 95% CI = 0.92–3.35, P = .086; random effect), M stage (OR = 1.64, 95% CI = 0.84–3.19, P = .149; fixed effect), or age (OR = 0.62, 95% CI = 0.37–1.04, P = .071; fixed effect).
Figure 4.
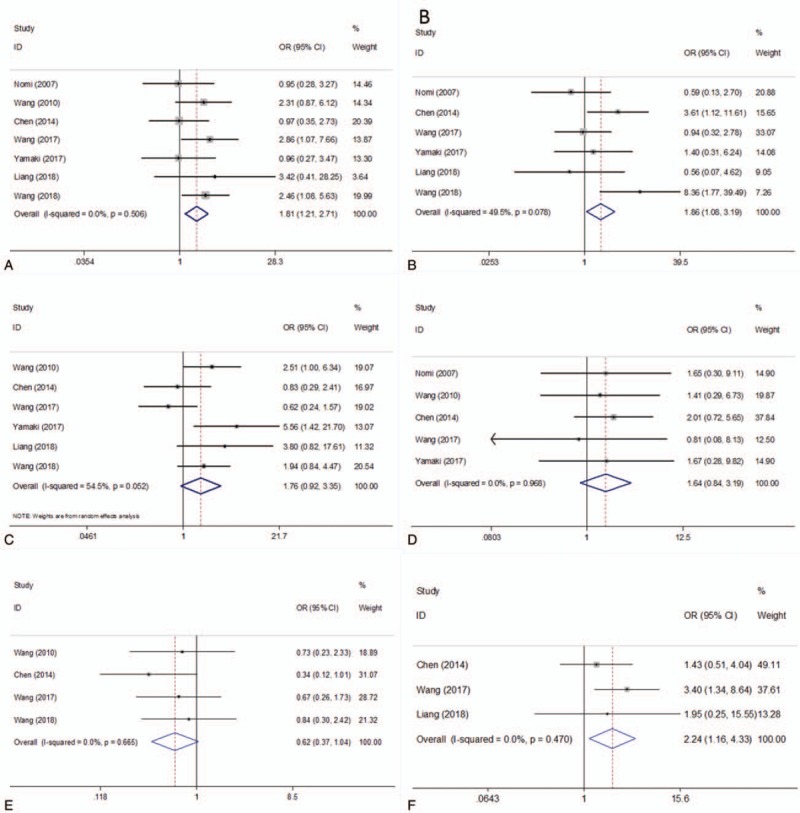
Forest plots for the association between PD-L1 expression and clinicopathological features. (A) N stage, (B) T stage, (C) gender, (D) M stage, (E) age, and (F) differentiation. PD-L1 = programmed cell death ligand 1.
Table 2.
Relation between PD-L1 expression and clinicopathological features of pancreatic cancer.
3.4. Publication bias
Begg's funnel plots were used for publication bias evaluation. The results were Begg's P = .902 for OS and Begg's P = 1 for CSS (Fig. 5). The data suggested that there was nonsignificant publication bias in this meta-analysis.
Figure 5.
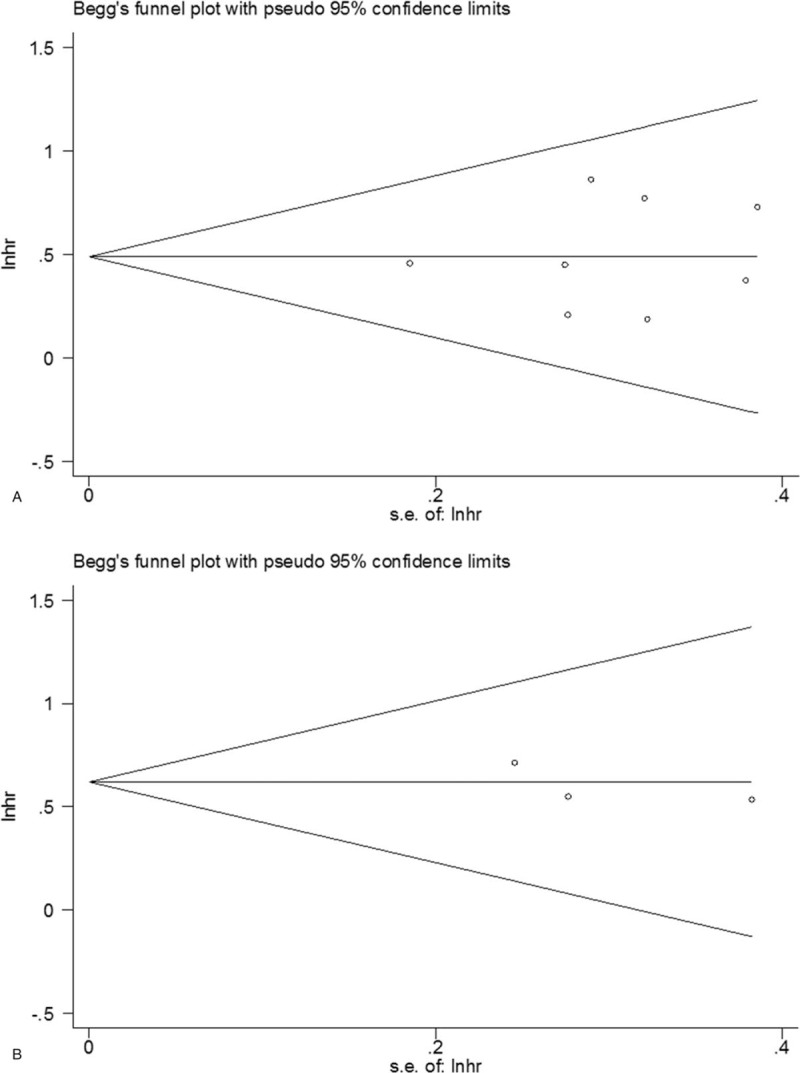
Publication bias test for (A) OS and (B) CSS. CSS = cancer-specific survival, OS = overall survival.
4. Discussion
The prognostic value of PD-L1 in pancreatic cancer remained inconsistent according to previous studies. By pooling data of 9 relevant studies including 993 patients, this meta-analysis showed that elevated PD-L1 expression was related with poor OS (HR = 1.63, 95% CI = 1.34–1.98, P < .001) and CSS (HR = 1.86, 95% CI = 1.34–2.57, P < .001). Furthermore, high PD-L1 expression was also demonstrated to be associated with positive N stage (OR = 1.81, 95% CI = 1.21–2.71, P = .004), advanced T stage (OR = 1.86, 95% CI = 1.08–3.19, P = .025), and low differentiation (OR = 2.24, 95% CI = 1.16–4.33, P = .017). This meta-analysis suggested that PD-L1 overexpression was a potential biomarker for survival prediction of patients with pancreatic cancer. Those patients with high PD-L1 expression might be suffering from more aggressive disease because of poor differentiation and advanced stage.
PD-L1, as known as B7-H1, which was first cloned in 1999,[29] is the ligand of PD-1. PD-L1 is expressed on various cell types, including cancer cells, muscle, mesenchymal stem cells, B cells, T cells, dendritic cells, and placenta.[30,31] PD-1 is a T-cell immune checkpoint involved in dampening autoimmunity during T-cell activation. In a variety of cancer types, the combination of PD-1 and PD-L1 generates an immunosuppressive tumor microenvironment and protect cancer cells from T cell cytolysis.[32,33] Binding of PD-L1 to PD-1 facilitates immune escape of tumor cells and results in poor prognosis.[34] PD-L1 overexpression was observed in multiple solid tumors and hematologic malignancies and was associated with clinical outcomes.[35–38]
A large number of meta-analyses also investigated the prognostic value of PD-L1 in various types of cancer.[13,39–44] A recent meta-analysis including 61 studies showed that PD-L1 overexpression was correlated with worse OS in patients with various solid tumors, although the correlations differed according to tumor types.[43] Wang's work[12] suggested PD-L1 overexpression in breast cancer associated with multiple clinicopathological parameters that indicated poor outcomes.[12] In addition, Dai et al showed that the expression of PD-L1 is associated with worse OS in digestive system cancers, especially in gastric cancer and pancreatic cancer.[45] However, we noticed that this study on digestive system cancers only included 3 studies of pancreatic cancer and the correlation between PD-L1 and clinicopathological features was not investigated. In our meta-analysis, we included 9 most recent studies and investigated the association between PD-L1 expression and survival outcomes as well as clinical factors in pancreatic cancer. A recent meta-analysis conducted by Zhuan-Sun et al[46] showed that elevated PD-L1 expression was associated with poor OS in pancreatic cancer. Zhuan-Sun's work was performed according to PRISMA guideline. Zhuan-Sun's study included eligible studies up to March 21, 2017 without language restriction. Compared with Zhuan-Sun's study, the present meta-analysis included eligible studies up to October 2018 published in English using IHC method. Our study used more strict inclusion criteria and updated data; therefore, the results were more recent.
Several limitations should be noted when interpreting our results. First, the sample size was relatively small. Although 9 studies were included, only 993 patients were recruited. Second, most studies were performed in Asia, especially in China and Japan. Therefore, the results should be treated with caution in non-Asian patients. Third, to guarantee the homogeneity of the meta-analysis, only studies using IHC method were included. Therefore, the results may not be applicable for other types of specimens such as serum. Fourth, only 3 studies were included for CSS analysis, which may undermine the persuasiveness of the results.
In summary, this study demonstrated that high PD-L1 expression was associated with poor OS in patients with pancreatic cancer. Moreover, PD-L1 overexpression was correlated with positive N stage, advanced T stage, and poor tumor differentiation. The results suggest that PD-L1 may be helpful to clinicians aiming to select the appropriate immunotherapy for pancreatic cancer.
Author contributions
Conceptualization: Ying Hu, Wanzhen Chen, Fangshi Zhu.
Data curation: Ying Hu, Zhanpeng Yan, Jingxia Ma, Fangshi Zhu.
Formal analysis: Ying Hu, Wanzhen Chen, Zhanpeng Yan, Jingxia Ma.
Funding acquisition: Fangshi Zhu, Jiege Huo.
Investigation: Zhanpeng Yan, Fangshi Zhu.
Methodology: Zhanpeng Yan, Jingxia Ma.
Project administration: Ying Hu, Jingxia Ma, Jiege Huo.
Resources: Wanzhen Chen.
Software: Wanzhen Chen, Jingxia Ma, Fangshi Zhu, Jiege Huo.
Supervision: Jingxia Ma, Fangshi Zhu, Jiege Huo.
Validation: Zhanpeng Yan, Jingxia Ma.
Visualization: Jingxia Ma, Jiege Huo.
Writing – original draft: Wanzhen Chen, Zhanpeng Yan.
Writing – review & editing: Jiege Huo.
Footnotes
Abbreviations: CSS = cancer-specific survival, IHC = immunohistochemistry, OR = odd ratio, OS = overall survival, PD-1 = programmed cell death 1, PD-L1 = programmed cell death ligand 1, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
No potential conflicts of interest were disclosed.
This study was supported by National Chinese Medicine Clinical Research Foundation (JDZX2012089).
References
- [1].Kamisawa T, Wood LD, Itoi T, et al. Pancreatic cancer. Lancet 2016;388:73–85. [DOI] [PubMed] [Google Scholar]
- [2].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [3].Cheng H, Long F, Jaiswar M, et al. Prognostic role of the neutrophil-to-lymphocyte ratio in pancreatic cancer: a meta-analysis. Sci Rep 2015;5:11026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Li XP, Zhao HJ, Gu JC, et al. Prognostic value of cancer stem cell marker CD133 expression in pancreatic ductal adenocarcinoma (PDAC): a systematic review and meta-analysis. Int J Clin Exp Pathol 2015;8:12084–92. [PMC free article] [PubMed] [Google Scholar]
- [5].Huang SS, Zheng JW, Huang YF, et al. Impact of S100A4 expression on clinicopathological characteristics and prognosis in pancreatic cancer: a meta-analysis. Dis Markers 2016;2016:8137378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science 2015;348:74–80. [DOI] [PubMed] [Google Scholar]
- [7].Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011;331:1565–70. [DOI] [PubMed] [Google Scholar]
- [8].Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sharma P, Allison JP. The future of immune checkpoint therapy. Science 2015;348:56–61. [DOI] [PubMed] [Google Scholar]
- [10].Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014;515:558–62. [DOI] [PubMed] [Google Scholar]
- [11].Brahmer JR, Tykodi SS, Chow LQM, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wang CJ, Zhu HJ, Zhou YD, et al. Prognostic value of PD-L1 in breast cancer: a meta-analysis. Breast J 2017;23:436–43. [DOI] [PubMed] [Google Scholar]
- [13].Gu L, Chen M, Guo D, et al. PD-L1 and gastric cancer prognosis: a systematic review and meta-analysis. PLoS One 2017;12:e0182692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gu XB, Gao XS, Xiong W, et al. Increased programmed death ligand-1 expression predicts poor prognosis in hepatocellular carcinoma patients. Onco Targets Ther 2016;9:4805–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fan YW, Ma K, Hu Y, et al. Prognostic value of PD-L1 expression in non-small cell lung cancer: a meta-analysis. Int J Clin Exp Med 2017;10:8735–44. [Google Scholar]
- [16].Iacovelli R, Nolè F, Verri E, et al. Prognostic role of PD-L1 expression in renal cell carcinoma. A systematic review and meta-analysis. Target Oncol 2016;11:143–8. [DOI] [PubMed] [Google Scholar]
- [17].Nomi T, Sho M, Akahori T, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res 2007;13:2151–7. [DOI] [PubMed] [Google Scholar]
- [18].Wang LC, Ma QY, Chen XL, et al. Clinical significance of B7-H1 and B7-1 expressions in pancreatic carcinoma. World J Surg 2010;34:1059–65. [DOI] [PubMed] [Google Scholar]
- [19].Chen Y, Sun J, Zhao H, et al. The coexpression and clinical significance of costimulatory molecules B7-H1, B7-H3, and B7-H4 in human pancreatic cancer. Onco Targets Ther 2014;7:1465–72. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [20].Hutcheson J, Balaji U, Porembka MR, et al. Immunologic and metabolic features of pancreatic ductal adenocarcinoma define prognostic subtypes of disease. Clin Cancer Res 2016;22:3606–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yamaki S, Yanagimoto H, Tsuta K, et al. PD-L1 expression in pancreatic ductal adenocarcinoma is a poor prognostic factor in patients with high CD8+ tumor-infiltrating lymphocytes: highly sensitive detection using phosphor-integrated dot staining. Int J Clin Oncol 2017;22:726–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Imai D, Yoshizumi T, Okano S, et al. The prognostic impact of programmed cell death ligand 1 and human leukocyte antigen class I in pancreatic cancer. Cancer Med 2017;6:1614–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wang Y, Lin JC, Cui JJ, et al. Prognostic value and clinicopathological features of PD-1/PD-L1 expression with mismatch repair status and desmoplastic stroma in Chinese patients with pancreatic cancer. Oncotarget 2017;8:9354–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006–12. [DOI] [PubMed] [Google Scholar]
- [25].Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [27].Liang X, Sun J, Wu H, et al. PD-L1 in pancreatic ductal adenocarcinoma: a retrospective analysis of 373 Chinese patients using an in vitro diagnostic assay. Diagn Pathol 2018;13:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wang L, Ma Q, Li D, et al. Indoleamine 2,3-dioxygenase and B7-H1 expressions as prognostic and follow-up markers in human pancreatic carcinoma. Pathol Res Pract 2018;214:1309–14. [DOI] [PubMed] [Google Scholar]
- [29].Dong HD, Zhu GF, Tamada K, et al. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med 1999;5:1365–9. [DOI] [PubMed] [Google Scholar]
- [30].Sharpe AH, Wherry EJ, Ahmed R, et al. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol 2007;8:239–45. [DOI] [PubMed] [Google Scholar]
- [31].Blank C, Mackensen A. Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion: an update on implications for chronic infections and tumor evasion. Cancer Immunol Immunother 2007;56:739–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Iwai Y, Ishida M, Tanaka Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A 2002;99:12293–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Blank C, Gajewski TF, Mackensen A. Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol Immunother 2005;54:307–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wang X, Teng FF, Kong L, et al. PD-L1 expression in human cancers and its association with clinical outcomes. Onco Targets Ther 2016;9:5023–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Karim R, Jordanova ES, Piersma SJ, et al. Tumor-expressed B7-H1 and B7-DC in relation to PD-1 + T-cell infiltration and survival of patients with cervical carcinoma. Clin Cancer Res 2009;15:6341–7. [DOI] [PubMed] [Google Scholar]
- [36].Mu CY, Huang JA, Chen Y, et al. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol 2011;28:682–8. [DOI] [PubMed] [Google Scholar]
- [37].Rossille D, Gressier M, Damotte D, et al. High level of soluble programmed cell death ligand 1 in blood impacts overall survival in aggressive diffuse large B-Cell lymphoma: results from a French multicenter clinical trial. Leukemia 2014;28:2367–75. [DOI] [PubMed] [Google Scholar]
- [38].Armand P, Nagler A, Weller EA, et al. Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: results of an international phase II trial. J Clin Oncol 2013;31:4199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zhu Z, Jin Z, Zhang M, et al. Prognostic value of programmed death-ligand 1 in sarcoma: a meta-analysis. Oncotarget 2017;8:59570–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zhang MH, Sun HB, Zhao S, et al. Expression of PD-L1 and prognosis in breast cancer: a meta-analysis. Oncotarget 2017;8:31347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Xue S, Song G, Yu J. The prognostic significance of PD-L1 expression in patients with glioma: a meta-analysis. Sci Rep 2017;7:4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Xia H, Shen J, Hu F, et al. PD-L1 over-expression is associated with a poor prognosis in Asian non-small cell lung cancer patients. Clin Chim Acta 2017;469:191–4. [DOI] [PubMed] [Google Scholar]
- [43].Wang Q, Liu F, Liu L. Prognostic significance of PD-L1 in solid tumor: an updated meta-analysis. Medicine 2017;96:e6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Li J, Wang P, Xu YL. Prognostic value of programmed cell death ligand 1 expression in patients with head and neck cancer: a systematic review and meta-analysis. PLoS One 2017;12:e0179536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Dai C, Wang M, Lu J, et al. Prognostic and predictive values of PD-L1 expression in patients with digestive system cancer: a meta-analysis. Onco Targets Ther 2017;10:3625–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zhuan-Sun Y, Huang F, Feng M, et al. Prognostic value of PD-L1 overexpression for pancreatic cancer: evidence from a meta-analysis. Onco Targets Ther 2017;10:5005–12. [DOI] [PMC free article] [PubMed] [Google Scholar]


