Abstract
Introduction:
Multiple mechanisms are involved in the development and persistence of neuropathic pain. Some patients with nerve damage will remain painless and develop a “loss of function” phenotype, whereas others develop painful neuropathies.
Objectives:
The aim of this study is to investigate the role of a peripheral nervous system sensitization by analyzing patients with and without pain.
Methods:
The topical application of capsaicin was investigated in peripheral nociceptors. Two groups of patients (painful vs painless) with length-dependent neuropathies and small-fiber impairment were tested. Quantitative sensory testing was assessed before and after topical application of 0.6% capsaicin in the affected skin. In addition, blood perfusion measurements and an axon reflex flare assessment were performed.
Results:
Quantitative testing revealed that heat hyperalgesia was induced in all patients and volunteers (P < 0.01) without observing any significant differences between patient groups. By contrast, the extent of the axon reflex flare reaction (P < 0.01) as well as the blood perfusion (P < 0.05) was significantly greater in patients with pain than in neuropathy patients not experiencing pain.
Conclusion:
Hyperexcitable vasoactive nociceptive C fibers might contribute to pain in peripheral neuropathies and therefore may serve as a key player in separating into a painless or painful condition.
Keywords: Peripheral neuropathy, Heat pain threshold, Vasoactive c-nociceptors, Quantitative sensory testing
1. Introduction
Peripheral neuropathic pain may follow as a direct consequence of a lesion or disease affecting the peripheral somatosensory system.57 Clinically, patients with neuropathies are characterized by negative signs and symptoms (hypoesthesia and hypoalgesia), whereas those with neuropathic pain additionally suffer from positive signs and symptoms (spontaneous and evoked pain).34 Although several hypotheses have been proposed to explain the complex processes of pain and its underlying mechanisms, the pathophysiology of neuropathic pain remains unclear.3,4,61 In this sense, the fact that some patients with nerve damage develop neuropathic pain but others do not represents a substantial issue to be addressed.
Several studies indicate that neuropathic pain is associated with damage to the nociceptive pathways.4,15,17,60 However, many patients with such lesions do not develop neuropathic pain.6 Indeed, clinical examination and other routine diagnostic tools assessing function and structure of somatosensory pathways as well as skin biopsies were unable to reveal differences between patients with and without neuropathic pain after nerve injury.28,32,58,62 The same applies to studies investigating the nociceptive pathways; it was demonstrated that quantitative sensory tests of small-fiber function did not distinguish between patients with and without peripheral neuropathic pain.28,59
By contrast, patients with central pain after spinal cord injury had preserved capsaicin-sensitive pathways as compared to individuals who had suffered spinal cord injury but did not experience pain.64 This finding is in line with evidence from basic research indicating that capsaicin-sensitive neurons play a crucial role in transmitting pain and may be involved in chronic pain.39,47,50,64
Physiologically, both heat- and capsaicin-induced pain are mediated by the majority of nociceptive C fibers through TRPV1, a heat-sensitive ion channel of the transient receptor potential family.3,11,30 Moreover, the activation of these pathways may induce an axon reflex flare, which is caused by the release of vasoactive substances from axon collaterals of afferent nociceptive C fibers, eg, calcitonin gene-related peptide (CGRP).7,22,24,26 The size of the axon reflex erythema reflects a morphological parameter that depends on the individual C-fiber topography and its degree of overlap.10 On the contrary, the blood perfusion is a more functional parameter and reflects a CGRP liberation by detecting even subtle changes in microcirculation. However, the microcirculatory blood perfusion also depends on the border of the axon reflex flare area. Thereby, single-point probe blood perfusion measurements also dependent on morphology.
A classification of different C-fiber subtypes was conducted using microneurographic studies.37,48 Thereby, the CM fibers (= mechanically sensitive C fibers) and the CMi fibers (= mechanically insensitive C fibers) were characterized.45,46,53,67 Most CM fibers are sensitive to both heat and capsaicin stimuli (ie, polymodal C fibers; CMH), whereas only a subpopulation of the CMi fibers is susceptible to both stimuli.38,46 Further on, it was demonstrated that the capsaicin-induced axon reflex erythema is only mediated through the CMi fibers,45,46 whereas the CMH fibers do not affect the axon reflex.
In this study, it was hypothesized that hyperactive C-fiber function contributes to pain. Therefore, the primary objective was to investigate the function of capsaicin-sensitive afferents in both patient groups, with and without peripheral neuropathic pain and healthy volunteers.
2. Methods
2.1. Study population
In total, 18 patients with polyneuropathies and (A) 9 healthy age- and sex-matched controls were investigated. The patient cohort included 2 subgroups ie, (B) 9 patients with spontaneous neuropathic pain and (C) 9 pain-free patients. All patients, with and without neuropathic pain, suffered from bilateral polyneuropathies with signs of warmth hypoesthesia (ie, warm detection threshold: z-profile [0] to [−2.5]), indicating loss of afferent function (verified by quantitative sensory testing [QST], see 2.3). All patients were selected according to their warm detection threshold as other studies identified WDT as a highly reliable parameter for C-fiber function.1,44 All patients suffered from polyneuropathies affecting the extremities bilaterally. Diagnosis of polyneuropathy was based on consensus criteria for a symmetric polyneuropathy18 (ordinal likelihood ++++). Subjects younger than 18 years, pregnant women, and subjects with evidence of allergy to capsaicin were excluded.
The aim of the study and the nature of the tests were explained to the subjects in accordance with the Declaration of Helsinki. The study was approved by the ethics committee of the University Hospital of Kiel, and all patients provided written informed consent.
2.2. Study protocol
According to the subgroup (A, B, and C), the testing was performed at the dorsomedial foot within the area of pain in neuropathic pain patients (B); within the area that was clinically affected by polyneuropathy in pain-free patients (C); and within the corresponding area (ie, dorsomedial foot) in control subjects (A).
The first part consisted of a baseline QST within the respective test area. Thereafter, a gauze pad was soaked with 0.3 ml capsaicin (0.6%) and applied to the test area for a duration of 15 minutes. Within this period, blood perfusion was continuously monitored by laser Doppler flowmetry. In addition, subjects were instructed to rate the change in pain perception (ie, capsaicin-induced pain) every minute on an 11-point numeric rating scale (NRS 0–10).
The second part involved the measurement of the visible capsaicin-evoked axon reflex flare area (another 15 minutes after removing the gauze pad). Thereafter, a follow-up QST was performed within the test area.
Local temperature at the test area was kept constant by a feedback-controlled heating device set at 32°C. In this way, the temperature-dependent capsaicin effects could be minimized.7,63,65 All experiments were performed in a room with a constant temperature of 22 to 23°C and a relative humidity of 50% to 60%.
2.3. Quantitative sensory testing
Quantitative sensory testing was performed according to the standards of the German Research Network on Neuropathic Pain.33,41,42 A shortened version of 4 different thermal tests was used for standardized assessment of small-fiber function. Thermal QST was performed with a thermo-test device (TSA 2001-II; Medoc, Ramat Yishai, Israel) to investigate perception thresholds for warmth (= warm detection threshold, [WDT]), cold (= cold detection threshold, [CDT]), heat pain (= heat pain threshold, [HPT]), and cold pain (= cold pain threshold, [CPT]).19,68 Thereby, the method of limits was used by applying ramp stimuli at a velocity of 1°C/second starting from 32°C applied to the skin using a Peltier type thermode (3 × 3 cm2).
The subjects were instructed to press a button when the respective thermal sensation was perceived. Cutoff temperatures were 0 and 50°C, respectively.
2.4. Capsaicin challenge
A 0.3 ml aliquot of a solution containing 0.02 M capsaicin (0.6%), dissolved in 45% ethanol (Kieler Hofapotheke, Kiel, Germany), was applied to the skin using a 3 × 3 cm2 large gauze pad for 15 minutes.3,7,11,30,64 To prevent the ethanol from evaporating, the gauze pad was covered with adhesive film.
2.5. Laser Doppler flowmetry
A perfusion measurement system (Periflux System 5000; Perimed, Stockholm, Sweden) was used to measure perfusion with the laser Doppler probe (Probe 413; Perimed) directly applied to the skin. Perfusion needed to be determined in an area (ie, primary area) surrounding the gauze pad as this prevented measurements within the stimulation area (ie, application area).
2.6. Axon reflex flare reaction
Topical capsaicin induces an axon reflex flare reaction by releasing vasodilating neuropeptides (eg, CGRP) from the axon collaterals of vasoactive capsaicin-sensitive nociceptive afferents.7,45,63–65 Fifteen minutes after removing capsaicin, the visible area of capsaicin-evoked flare was drawn on sheets of plastic film and the area was measured using an irregular area calculator software (SketchAndCalc, 2017).
2.7. Statistical analysis and z-transformation
Each QST parameter was z-transformed according to a calculation paradigm invented by Magerl et al.33,42 To generate location-, gender-, and age- matched data, this analysis was based on 180 healthy controls. Z-scores of 0 represent the exact mean value of the healthy cohort, whereas z-scores above 0 represent a gain of function and scores below 0 represent a loss of function. Demographic data were displayed as mean (±SEM). Z- transformed data, blood perfusion measurement (area under the curve [AUC]), and axon reflex flare size data were displayed as boxplot (minimum, first quartile, median, third quartile, and maximum). Further on, the time course of blood perfusion change and the change of pain ratings were displayed as mean (±SEM). The Mann–Whitney U test was applied for unpaired data analyses and the Wilcoxon signed–rank test for paired data analyses (SPSS 23.0; SPSS, Inc, Chicago, IL). All correlation analyses were conducted using Spearman correlation.
P values <0.05 were considered as statistically significant. The Bonferroni–Holm correction was applied.
3. Results
3.1. Characterization of patients
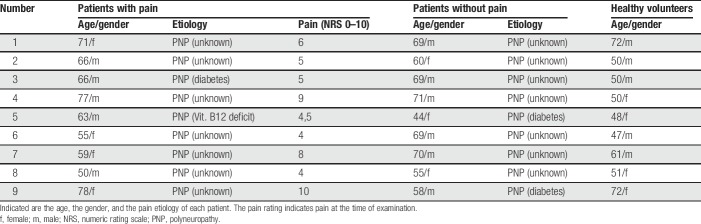
In total, 18 patients with peripheral neuropathies and 9 healthy volunteers were investigated. The patient group consisted of 2 subgroups, including (B) 9 patients with neuropathic pain (4 women, mean age 65 [±3.2] years) and (C) 9 pain-free patients (3 women, mean age 63 [±3.1] years) (Table 1). (A) The healthy volunteers group (4 women, mean age 56 [±3.4] years) consisted of 9 subjects without any history or clinical signs of neurological disorders.
Table 1.
Characterization of patient groups and healthy cohort.
3.2. Quantitative sensory testing and capsaicin challenge
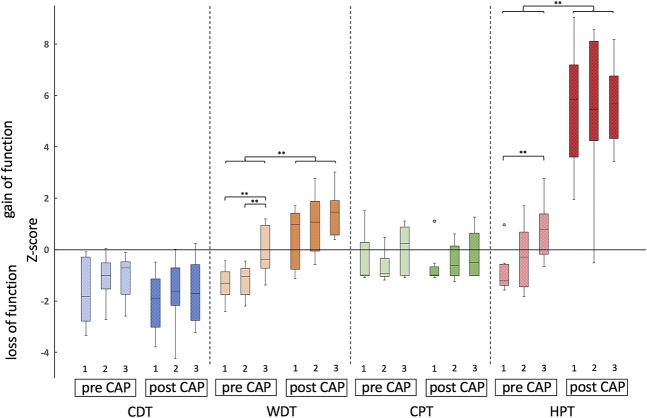
Before capsaicin challenge, baseline QST revealed a significantly increased WDT (ie, reduced z-values in WDT) in both patient groups as compared to healthy volunteers (A vs B P = 0.004; A vs C P = 0.004; the Mann–Whitney U test) but no difference between patient groups (P = 0.863; the Mann–Whitney U test). Signs of heat hypoalgesia (ie, increased HPT) were only demonstrated in the patients with pain as compared to controls (P = 0.002; the Mann–Whitney U test) (Fig. 1). After treating small-fiber afferents with topical capsaicin, a follow-up QST was conducted, without indicating any significant differences in QST parameters between groups.
Figure 1.
QST profiles. Indicated are QST profiles of the painful (1), painless (2) and healthy control group (3), before and after capsaicin (CAP) application. Each QST parameter is displayed as boxplot (minimum, maximum, median, and first and third quartiles). Intraindividual statistical testing (ie, before vs after capsaicin application) was conducted using the Wilcoxon signed–rank test. Interindividual statistical testing (ie, between patient groups and healthy cohort) was conducted using the Mann–Whitney U test. ***<0.001, **<0.01, n.s. >0.05. CPT, cold pain threshold; HPT, heat pain threshold; QST, quantitative sensory testing.
Further on, a comparison of baseline and follow-up QST parameters was conducted. Results revealed an increase of z-values in WDT and HPT in both patient groups as well as in the control group (WDT and HPT for all 3 groups prior vs after capsaicin: P < 0.01; the Wilcoxon signed-rank test) (Fig. 1).
The maximum pain ratings after capsaicin application (ie, at minute 15) (A vs B P = 0.190; A vs C P = 0.063; B vs C P = 0.489; the Mann–Whitney U test) as well as the continuous pain rating during capsaicin application (ie, AUC from minute 0–15) (A vs B P = 0.222; A vs C P = 0.063; B vs C P = 0.340; the Mann–Whitney U test) did not reach any significant differences between groups (Fig. 2).
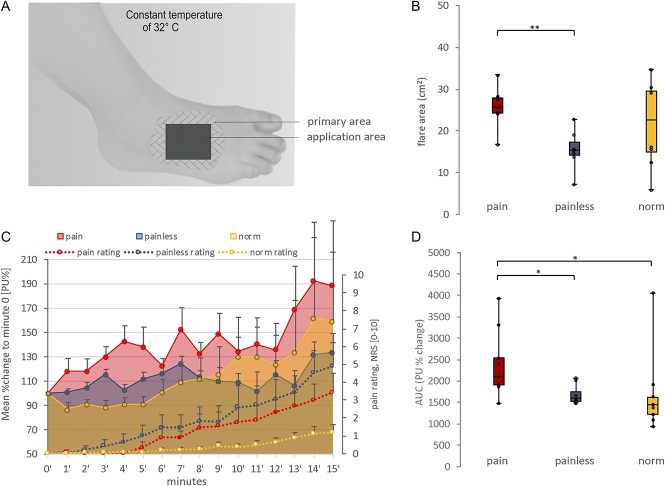
Figure 2.
Capsaicin challenge and vasoactive reaction. (A) Setup of the experimental capsaicin application procedure. In the application area, the capsaicin patch was applied and the sensory testing was conducted. In the primary area, the continuous blood perfusion measurement as well as the margin of the axon reflex erythema was determined. (B) The axon reflex flare area (cm2) after 15 minutes of topical capsaicin application is displayed as boxplot (minimum, maximum, median, and first and third quartiles). There were no significant differences between the patient groups and healthy subjects. Capsaicin induced a larger axon reflex flare in the neuropathic pain patients than in the patients without pain. The application area (9 cm2) was not included in the total axon reflex flare size. Interindividual statistical testing for axon reflex flare area was conducted using the Mann–Whitney U test. **<0.01. (C) Displayed is the time course of mean (±SEM) blood perfusion change (arbitrary perfusion units, % change [PU%]) in the primary area and the mean (±SEM) pain rating change (numeric rating scale 0–10) during capsaicin challenge. Interindividual statistical testing for maximum pain ratings (ie, at minute 15) was conducted using the Mann–Whitney U test (statistically not significant). (D) The area under the curve for blood perfusion (AUC [PU%]) is displayed as boxplot. Indicated is a significant difference between the patients with pain as compared to the painless and normal subgroups. Interindividual statistical testing for mean blood perfusion (AUC) between subgroups was conducted using the Mann–Whitney U test. *<0.05.
3.3. Capsaicin-evoked axon reflex flare and blood perfusion
Results from laser Doppler flowmetry indicate an increased response after capsaicin challenge in painful neuropathy patients as compared to healthy volunteers (P = 0.019; the Mann–Whitney U test) and painless neuropathy patients (P = 0.021; the Mann–Whitney U test) (Fig. 2).
By contrast, no differences were found in the size of capsaicin-induced flare between healthy controls and patient groups (painful polyneuropathy [PNP] vs healthy controls P = 0.17; painless PNP vs healthy controls P = 0.09; the Mann–Whitney U test). However, the axon reflex flare size seemed to change towards an increase in neuropathic pain patients as compared to patients without pain (P = 0.002; the Mann–Whitney U test) (Fig. 2).
Further on, a correlation analysis revealed a significant correlation of the HPT delta (ie, [baseline HPT] − [follow-up HPT]) and flare in neuropathic pain patients (P = 0.004, rho = 0.912; the Spearman correlation). There was no correlation between the axon reflex flare size and the maximum pain rating after capsaicin application (ie, at minute 15) (P = 0.565, rho = 0.130; Spearman correlation) or the continuous pain rating during capsaicin application (ie, AUC from minute 0–15) (P = 0.472, rho = 0.162; Spearman correlation).
4. Discussion
In this study, thermosensitive small-fiber function was assessed by quantitative thermal testing before and after a capsaicin challenge in patients with and without peripheral neuropathic pain. All patients suffered from peripheral neuropathy, with reduced warm detection indicating loss of small-fiber function due to neuropathy.
The major findings in this study were that (1) no significant differences in HPT could be detected between the 2 patient groups before applying capsaicin; (2) after topically applying capsaicin, a significant reduction in HPTs (ie, heat hyperalgesia) was found in both patient groups without any significant differences between the groups; and (3) the extent of capsaicin-induced blood perfusion was greater in patients with pain than in painless patients and in healthy volunteers.
4.1. Determinants for WDT and heat pain threshold
Our results are in line with previous studies, indicating no differences when comparing neuropathy patients with and without pain on thermal quantitative testing.28,31,59 However, these findings reflect the function of the whole thermoafferent system, implying that both centrally and peripherally located pain processing mechanisms determine QST results. To which proportion the different components of the pain pathway are modulated by chronic pain remains unclear yet. Indeed, these circumstances might contribute to the fact that no differences between patient groups could be detected on QST. Even if an isolated peripheral C-fiber dysfunction was present in 1 patient group, this sensation could be modified due to central adaption processes and thereby not detectable by psychophysical testing.
4.2. Mismatch of damage to small-fiber afferents—key to understanding neuropathic pain?
In this study, capsaicin induced an axon reflex erythema in both patient groups and in the control group, which indicates the presence and integrity of CMi fibers in neuropathy patients with and without pain. Interestingly, there was a difference in the extent of the capsaicin-induced vasoactive response. The size of capsaicin-induced flare and the blood perfusion in neuropathic pain patients were significantly increased as compared to patients not experiencing pain. However, results from the axon reflex erythema indicate that the flare size of healthy controls is within the range of both patient groups (Fig. 2). By contrast, the laser Doppler indicated a different blood perfusion change between the painful PNP patients as compared to the painless PNP group and healthy controls. This suggests that the laser Doppler might be more sensitive in detecting even subtle changes in microcirculation than estimating the flare size with the eye. However, the axon reflex flare has a sharp border; thus, the microcirculatory blood perfusion also depends on the border of the axon reflex flare area. Vice versa, the axon reflex flare size depends on the total dose of CGRP release, which increases with more C-fiber overlap or hyperexcitability. This needs to be considered when interpreting results from a single-point probe perfusion measurement.
Still, in line with these results, a microneurographic study found a different distribution between C-fiber subgroups. Interestingly, the ratio between CM fibers and CMi fibers in healthy volunteers was 2:1. In neuropathy patients with pain, the relationship was reversed between CM fibers and CMi fibers.40 In previous studies, mechanical hyperalgesia as well as central sensitization have been linked to CMi fibers.27,46,47,50 Moreover, a recent microneurography study indicated that a stimulation of CMi fibers induced secondary mechanical hyperalgesia.43 This supports the notion that CMi fibers might act as key players in neuropathic pain. Indeed, our results support either a change of C-fiber ratio, ie, (1) due to a reduction of CMH fibers but preserved number of CMi fibers or (2) due to a disease-related increase or hyperexcitability of CMi fibers.
By contrast, other studies have shown results different from those of ours. For example, Kramer et al.29 found that the size of the axon reflex flare after an electrically evoked axon reflex in patients with diabetic small-fiber neuropathy was smaller than that in healthy subjects. However, they did not differentiate whether the patients suffered from neuropathic pain or had a neuropathy without pain. Moreover, the electric stimuli applied in the latter study activated all axons directly so that the axon reflex flare reflects the activation of all vasoactive nerve fibers (eg, mechanically insensitive C-fiber-histamine positive, CMiHis) and not only the CMi fibers. Another study examined the histamine-induced flare in patients with small-fiber neuropathy and neuropathic pain in comparison to healthy subjects.8 In this study, the axon reflex flare in neuropathic pain patients was decreased. One explanation for the discrepancy of the results of this study might be that a different subgroup of vasoactive C fibers (ie, CMiHis) is histamine sensitive.47,49
Still, the question why capsaicin-induced pain ratings do not develop according to the blood perfusion increase remains (Fig. 2). Numeric rating scale pain ratings represent psychometric parameters, and alterations of such parameters may occur easily due to diverse factors (ie, socioeconomic factors, beliefs, etc.).54,55 Therefore, and also due to the small sample size, an exact pathomechanistic explanation for this observation may be too speculative at this point.
4.3. Clinical implications
Upregulation of TRPV1 channels or increased expression of voltage-gated sodium channels on the C fibers significantly contributes to the development of neuropathic pain.11–13,16,23,56,66 Therefore, the use of drugs modulating this channel was examined both in animal experiments and in controlled clinical trials.14,20,21 For example, a single application of highly concentrated capsaicin (8%) had an analgesic effect in subgroups of patients with different etiologies of neuropathic pain (ie, postherpetic neuralgia, HIV polyneuropathy, and painful radiculopathy).2,5,51,52 Highly concentrated capsaicin induces reversible degeneration of capsaicin-sensitive nociceptive endings in the epidermis. However, only in a subset of patients treated with high-dosage capsaicin (8%) an analgesic effect could be achieved. A few studies aimed at identifying responders by patient-reported outcome parameters (eg, painDETECT, pain intensity [NRS]), intraepidermal anatomical nerve fiber counts, as well as QST.9,25,36 Despite all these approaches, the most robust predictor identified so far was the duration of pain (ie, for less than 6 months), suggesting that these patients might suffer from less central sensitization.35 The fact that sensory testing, morphological nerve fiber structure, as well as questionnaires may not differentiate between functionality of CMH and CMi fibers and that central mechanisms contribute to pain might explain these observations.
However, this implicates that a more specified classification with methods such as a standardized capsaicin challenge or other techniques inducing CMi flare reaction could act as predictive parameters for potential substances targeting TRPV1.
4.4. Conclusions
Preserved or hyperexcitable vasoactive (capsaicin-sensitive) C fibers in patients with length-dependent neuropathy and signs of small-fiber dysfunction may contribute to the persistence of certain peripheral pain states. Therefore, the CMi fiber may play a crucial role in a painful condition. By targeting these nociceptors and scrutinizing their functionality, prediction of response could be determined for compounds targeting TRPV1. In the future, further studies need to assess whether a topical capsaicin (8%) treatment in patients with hyperexcitable CMi fibers may be more beneficial.
4.5. Limitations
The small sample size in both patient groups and the healthy cohort can be seen as a limitation. Therefore, further studies are needed to confirm our pathophysiological findings.
Disclosures
The authors have no conflict of interest to declare. The authors acknowledge financial support by Land Schleswig-Holstein within the funding program Open Access Publikationsfonds.
Acknowledgements
The authors thank all participating patients, colleagues, and the staff of the institutions for their contributions to data collection.
This study was financially supported by the National Health and Medical Research Council of Australia (NHMRC), the EFIC-Grünenthal Grant (EGG), the Alexander von Humboldt-Stiftung, BMBF (01EM0504), an unrestricted financial grant from Pfizer, Germany, and an unrestricted financial grant from Grünenthal GmbH.
J. Forstenpointner reports grants and personal fees from Grünenthal GmbH, during the conduct of the study; personal fees and nonfinancial support from Grünenthal GmbH and Sanofi Genzyme, personal fees from Bayer, nonfinancial support from Novartis, outside the submitted work. D. Naleschinski reports honoraria for lectures from Grünenthal, Genzyme, and Pfizer, and travel/accommodation expenses from Pfizer, Genzyme, and Grünenthal. P. Hüllemann reports speaking fees from Pfizer and Genzyme and travel reimbursement from Grünenthal. R. Baron reports grants and personal fees from Grünenthal, during the conduct of the study; grants from Pfizer, Genzyme GmbH, Grünenthal GmbH, and Mundipharma. Member of the EU Project No 633491: DOLORisk. Member of the IMI “Europain” collaboration and industry members of this are: Astra Zeneca, Pfizer, Esteve, UCB-Pharma, Sanofi Aventis, Grünenthal GmbH, Eli Lilly, and Boehringer Ingelheim Pharma GmbH & Co. KG. German Federal Ministry of Education and Research (BMBF): Member of the ERA_NET NEURON/IM-PAIN Project (01EW1503). German Research Network on Neuropathic Pain (01EM0903), NoPain system biology (0316177C). German Research Foundation (DFG), personal fees from Pfizer, Genzyme GmbH, Grünenthal GmbH, Mundipharma, Sanofi Pasteur, Medtronic Inc. Neuro-modulation, Eisai Co. Ltd., Lilly GmbH, Boehringer Ingelheim Pharma GmbH & Co. KG, Astellas, Desitin, Teva Pharma, Bayer-Schering, MSD GmbH, and Seqirus, personal fees from Pfizer, Genzyme GmbH, Grünenthal GmbH, Mundipharma, Allergan, Sanofi Pasteur, Medtronic, Eisai, Lilly GmbH, Boehringer Ingelheim Pharma GmbH & Co. KG, Astellas, Novartis, Bristol-Myers Squibb, Biogenidec, AstraZeneca, Merck, Abbvie, Daiichi Sankyo, Glenmark Pharmaceuticals, Seqirus, Teva Pharma, Genentech, Galapagos NV, Kyowa Kirin GmbH, Vertex Pharmaceuticals Inc, Biotest AG, and TAD Pharma GmbH, outside the submitted work. A. Binder reports honoraria from Astellas, Allergan, Bayer, Boehringer-Ingelheim, Grünenthal, and Pfizer, and he participated in advisory boards of Astellas, Boehringer-Ingelheim, Genzyme, and Grünenthal.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
J. Forstenpointner, D. Naleschinski contributed equally to this work.
References
- [1].Agostinho CM, Scherens A, Richter H, Schaub C, Rolke R, Treede RD, Maier C. Habituation and short-term repeatability of thermal testing in healthy human subjects and patients with chronic non-neuropathic pain. Eur J Pain 2009;13:779–85. [DOI] [PubMed] [Google Scholar]
- [2].Backonja M, Wallace MS, Blonsky ER, Cutler BJ, Malan P, Jr, Rauck R, Tobias J. NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia: a randomised, double-blind study. Lancet Neurol 2008;7:1106–12. [DOI] [PubMed] [Google Scholar]
- [3].Baron R. Capsaicin and nociception: from basic mechanisms to novel drugs. Lancet 2000;356:785–7. [DOI] [PubMed] [Google Scholar]
- [4].Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol 2010;9:807–19. [DOI] [PubMed] [Google Scholar]
- [5].Baron R, Treede RD, Birklein F, Cegla T, Freynhagen R, Heskamp ML, Kern KU, Maier C, Rolke R, Seddigh S, Sommer C, Stander S, Maihofner C. Treatment of painful radiculopathies with capsaicin 8% cutaneous patch. Curr Med Res Opin 2017;33:1401–11. [DOI] [PubMed] [Google Scholar]
- [6].Baron R, Wasner G, Binder A. Chronic pain: genes, plasticity, and phenotypes. Lancet Neurol 2012;11:19–21. [DOI] [PubMed] [Google Scholar]
- [7].Baron R, Wasner G, Borgstedt R, Hastedt E, Schulte H, Binder A, Kopper F, Rowbotham M, Levine JD, Fields HL. Effect of sympathetic activity on capsaicin-evoked pain, hyperalgesia, and vasodilatation. Neurology 1999;52:923–32. [DOI] [PubMed] [Google Scholar]
- [8].Bickel A, Kramer HH, Hilz MJ, Birklein F, Neundorfer B, Schmelz M. Assessment of the neurogenic flare reaction in small-fiber neuropathies. Neurology 2002;59:917–19. [DOI] [PubMed] [Google Scholar]
- [9].Bischoff JM, Ringsted TK, Petersen M, Sommer C, Uceyler N, Werner MU. A capsaicin (8%) patch in the treatment of severe persistent inguinal postherniorrhaphy pain: a randomized, double-blind, placebo-controlled trial. PLoS One 2014;9:e109144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Brooks J, Tracey I. From nociception to pain perception: imaging the spinal and supraspinal pathways. J Anat 2005;207:19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000;288:306–13. [DOI] [PubMed] [Google Scholar]
- [12].Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 1997;389:816–24. [DOI] [PubMed] [Google Scholar]
- [13].Catterall WA, Dib-Hajj S, Meisler MH, Pietrobon D. Inherited neuronal ion channelopathies: new windows on complex neurological diseases. J Neurosci 2008;28:11768–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cui M, Honore P, Zhong C, Gauvin D, Mikusa J, Hernandez G, Chandran P, Gomtsyan A, Brown B, Bayburt EK, Marsh K, Bianchi B, McDonald H, Niforatos W, Neelands TR, Moreland RB, Decker MW, Lee CH, Sullivan JP, Faltynek CR. TRPV1 receptors in the CNS play a key role in broad-spectrum analgesia of TRPV1 antagonists. J Neurosci 2006;26:9385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Defrin R, Ohry A, Blumen N, Urca G. Characterization of chronic pain and somatosensory function in spinal cord injury subjects. PAIN 2001;89:253–63. [DOI] [PubMed] [Google Scholar]
- [16].Drenth JP, Waxman SG. Mutations in sodium-channel gene SCN9A cause a spectrum of human genetic pain disorders. J Clin Invest 2007;117:3603–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Eide PK, Jorum E, Stenehjem AE. Somatosensory findings in patients with spinal cord injury and central dysaesthesia pain. J Neurol Neurosurg Psychiatry 1996;60:411–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].England JD, Gronseth GS, Franklin G, Miller RG, Asbury AK, Carter GT, Cohen JA, Fisher MA, Howard JF, Kinsella LJ, Latov N, Lewis RA, Low PA, Sumner AJ. Distal symmetrical polyneuropathy: definition for clinical research. Muscle Nerve 2005;31:113–23. [DOI] [PubMed] [Google Scholar]
- [19].Fruhstorfer H, Lindblom U, Schmidt WC. Method for quantitative estimation of thermal thresholds in patients. J Neurol Neurosurg Psychiatry 1976;39:1071–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gavva NR. Body-temperature maintenance as the predominant function of the vanilloid receptor TRPV1. Trends Pharmacol Sci 2008;29:550–7. [DOI] [PubMed] [Google Scholar]
- [21].Gavva NR, Treanor JJ, Garami A, Fang L, Surapaneni S, Akrami A, Alvarez F, Bak A, Darling M, Gore A, Jang GR, Kesslak JP, Ni L, Norman MH, Palluconi G, Rose MJ, Salfi M, Tan E, Romanovsky AA, Banfield C, Davar G. Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. PAIN 2008;136:202–10. [DOI] [PubMed] [Google Scholar]
- [22].Geber C, Fondel R, Kramer HH, Rolke R, Treede RD, Sommer C, Birklein F. Psychophysics, flare, and neurosecretory function in human pain models: capsaicin versus electrically evoked pain. J Pain 2007;8:503–14. [DOI] [PubMed] [Google Scholar]
- [23].Han C, Dib-Hajj SD, Lin Z, Li Y, Eastman EM, Tyrrell L, Cao X, Yang Y, Waxman SG. Early- and late-onset inherited erythromelalgia: genotype-phenotype correlation. Brain 2009;132(pt 7):1711–22. [DOI] [PubMed] [Google Scholar]
- [24].Holzer P, Jocic M. Cutaneous vasodilatation induced by nitric oxide-evoked stimulation of afferent nerves in the rat. Br J Pharmacol 1994;112:1181–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hoper J, Helfert S, Heskamp ML, Maihofner CG, Baron R. High concentration capsaicin for treatment of peripheral neuropathic pain: effect on somatosensory symptoms and identification of treatment responders. Curr Med Res Opin 2014;30:565–74. [DOI] [PubMed] [Google Scholar]
- [26].Hughes SR, Brain SD. A calcitonin gene-related peptide (CGRP) antagonist (CGRP8-37) inhibits microvascular responses induced by CGRP and capsaicin in skin. Br J Pharmacol 1991;104:738–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Klede M, Handwerker HO, Schmelz M. Central origin of secondary mechanical hyperalgesia. J Neurophysiol 2003;90:353–9. [DOI] [PubMed] [Google Scholar]
- [28].Kleggetveit IP, Jorum E. Large and small fiber dysfunction in peripheral nerve injuries with or without spontaneous pain. J Pain 2010;11:1305–10. [DOI] [PubMed] [Google Scholar]
- [29].Kramer HH, Schmelz M, Birklein F, Bickel A. Electrically stimulated axon reflexes are diminished in diabetic small fiber neuropathies. Diabetes 2004;53:769–74. [DOI] [PubMed] [Google Scholar]
- [30].LaMotte RH, Lundberg LE, Torebjork HE. Pain, hyperalgesia and activity in nociceptive C units in humans after intradermal injection of capsaicin. J Physiol 1992;448:749–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Landerholm SH, Ekblom AG, Hansson PT. Somatosensory function in patients with and without pain after traumatic peripheral nerve injury. Eur J Pain 2010;14:847–53. [DOI] [PubMed] [Google Scholar]
- [32].Lauria G, Cornblath DR, Johansson O, McArthur JC, Mellgren SI, Nolano M, Rosenberg N, Sommer C. EFNS guidelines on the use of skin biopsy in the diagnosis of peripheral neuropathy. Eur J Neurol 2005;12:747–58. [DOI] [PubMed] [Google Scholar]
- [33].Magerl W, Krumova EK, Baron R, Tolle T, Treede RD, Maier C. Reference data for quantitative sensory testing (QST): refined stratification for age and a novel method for statistical comparison of group data. PAIN 2010;151:598–605. [DOI] [PubMed] [Google Scholar]
- [34].Maier C, Baron R, Tolle TR, Binder A, Birbaumer N, Birklein F, Gierthmuhlen J, Flor H, Geber C, Huge V, Krumova EK, Landwehrmeyer GB, Magerl W, Maihofner C, Richter H, Rolke R, Scherens A, Schwarz A, Sommer C, Tronnier V, Uceyler N, Valet M, Wasner G, Treede RD. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. PAIN 2010;150:439–50. [DOI] [PubMed] [Google Scholar]
- [35].Maihofner CG, Heskamp ML. Treatment of peripheral neuropathic pain by topical capsaicin: impact of pre-existing pain in the QUEPP-study. Eur J Pain 2014;18:671–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mainka T, Malewicz NM, Baron R, Enax-Krumova EK, Treede RD, Maier C. Presence of hyperalgesia predicts analgesic efficacy of topically applied capsaicin 8% in patients with peripheral neuropathic pain. Eur J Pain 2016;20:116–29. [DOI] [PubMed] [Google Scholar]
- [37].Mano T, Iwase S, Toma S. Microneurography as a tool in clinical neurophysiology to investigate peripheral neural traffic in humans. Clin Neurophysiol 2006;117:2357–84. [DOI] [PubMed] [Google Scholar]
- [38].Namer B, Barta B, Orstavik K, Schmidt R, Carr R, Schmelz M, Handwerker HO. Microneurographic assessment of C-fibre function in aged healthy subjects. J Physiol 2009;587(pt 2):419–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ochoa JL, Campero M, Serra J, Bostock H. Hyperexcitable polymodal and insensitive nociceptors in painful human neuropathy. Muscle Nerve 2005;32:459–72. [DOI] [PubMed] [Google Scholar]
- [40].Orstavik K, Namer B, Schmidt R, Schmelz M, Hilliges M, Weidner C, Carr RW, Handwerker H, Jorum E, Torebjork HE. Abnormal function of C-fibers in patients with diabetic neuropathy. J Neurosci 2006;26:11287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Pfau DB, Geber C, Birklein F, Treede RD. Quantitative sensory testing of neuropathic pain patients: potential mechanistic and therapeutic implications. Curr Pain Headache Rep 2012;16:199–206. [DOI] [PubMed] [Google Scholar]
- [42].Rolke R, Baron R, Maier C, Tolle TR, Treede RD, Beyer A, Binder A, Birbaumer N, Birklein F, Botefur IC, Braune S, Flor H, Huge V, Klug R, Landwehrmeyer GB, Magerl W, Maihofner C, Rolko C, Schaub C, Scherens A, Sprenger T, Valet M, Wasserka B. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. PAIN 2006;123:231–43. [DOI] [PubMed] [Google Scholar]
- [43].Sauerstein K, Liebelt J, Namer B, Schmidt R, Rukwied R, Schmelz M. Low-frequency stimulation of silent nociceptors induces secondary mechanical hyperalgesia in human skin. Neuroscience 2018;387:4–12. [DOI] [PubMed] [Google Scholar]
- [44].Scherens A, Maier C, Haussleiter IS, Schwenkreis P, Vlckova-Moravcova E, Baron R, Sommer C. Painful or painless lower limb dysesthesias are highly predictive of peripheral neuropathy: comparison of different diagnostic modalities. Eur J Pain 2009;13:711–18. [DOI] [PubMed] [Google Scholar]
- [45].Schmelz M, Michael K, Weidner C, Schmidt R, Torebjork HE, Handwerker HO. Which nerve fibers mediate the axon reflex flare in human skin? Neuroreport 2000;11:645–8. [DOI] [PubMed] [Google Scholar]
- [46].Schmelz M, Schmid R, Handwerker HO, Torebjork HE. Encoding of burning pain from capsaicin-treated human skin in two categories of unmyelinated nerve fibres. Brain 2000;123(pt 3):560–71. [DOI] [PubMed] [Google Scholar]
- [47].Schmelz M, Schmidt R. Microneurographic single-unit recordings to assess receptive properties of afferent human C-fibers. Neurosci Lett 2010;470:158–61. [DOI] [PubMed] [Google Scholar]
- [48].Schmelz M, Schmidt R, Bickel A, Torebjork HE, Handwerker HO. Innervation territories of single sympathetic C fibers in human skin. J Neurophysiol 1998;79:1653–60. [DOI] [PubMed] [Google Scholar]
- [49].Schmidt R, Schmelz M, Ringkamp M, Handwerker HO, Torebjork HE. Innervation territories of mechanically activated C nociceptor units in human skin. J Neurophysiol 1997;78:2641–8. [DOI] [PubMed] [Google Scholar]
- [50].Serra J, Campero M, Bostock H, Ochoa J. Two types of C nociceptors in human skin and their behavior in areas of capsaicin-induced secondary hyperalgesia. J Neurophysiol 2004;91:2770–81. [DOI] [PubMed] [Google Scholar]
- [51].Simpson DM, Brown S, Tobias J. Controlled trial of high-concentration capsaicin patch for treatment of painful HIV neuropathy. Neurology 2008;70:2305–13. [DOI] [PubMed] [Google Scholar]
- [52].Simpson DM, Estanislao L, Brown SJ, Sampson J. An open-label pilot study of high-concentration capsaicin patch in painful HIV neuropathy. J Pain Symptom Manage 2008;35:299–306. [DOI] [PubMed] [Google Scholar]
- [53].Szolcsanyi J. Forty years in capsaicin research for sensory pharmacology and physiology. Neuropeptides 2004;38:377–84. [DOI] [PubMed] [Google Scholar]
- [54].Tarigopula R, Tyagi NK, Jackson J, Gupte C, Raju P, LaRosa J. Health care workers and ICU pain perceptions. Pain Med 2014;15:1027–35. [DOI] [PubMed] [Google Scholar]
- [55].Thumboo J, Chew LH, Lewin-Koh SC. Socioeconomic and psychosocial factors influence pain or physical function in Asian patients with knee or hip osteoarthritis. Ann Rheum Dis 2002;61:1017–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Tominaga M, Caterina MJ. Thermosensation and pain. J Neurobiol 2004;61:3–12. [DOI] [PubMed] [Google Scholar]
- [57].Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW, Hansson P, Hughes R, Nurmikko T, Serra J. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology 2008;70:1630–5. [DOI] [PubMed] [Google Scholar]
- [58].Truini A, Biasiotta A, La Cesa S, Di Stefano G, Galeotti F, Petrucci MT, Inghilleri M, Cartoni C, Pergolini M, Cruccu G. Mechanisms of pain in distal symmetric polyneuropathy: a combined clinical and neurophysiological study. PAIN 2010;150:516–21. [DOI] [PubMed] [Google Scholar]
- [59].Uceyler N, Vollert J, Broll B, Riediger N, Langjahr M, Saffer N, Schubert AL, Siedler G, Sommer C. Sensory profiles and skin innervation of patients with painful and painless neuropathies. PAIN 2018;159:1867–76. [DOI] [PubMed] [Google Scholar]
- [60].Vestergaard K, Nielsen J, Andersen G, Ingeman-Nielsen M, Arendt-Nielsen L, Jensen TS. Sensory abnormalities in consecutive, unselected patients with central post-stroke pain. PAIN 1995;61:177–86. [DOI] [PubMed] [Google Scholar]
- [61].von Hehn CA, Baron R, Woolf CJ. Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron 2012;73:638–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Vrethem M, Boivie J, Arnqvist H, Holmgren H, Lindstrom T. Painful polyneuropathy in patients with and without diabetes: clinical, neurophysiologic, and quantitative sensory characteristics. Clin J Pain 2002;18:122–7. [DOI] [PubMed] [Google Scholar]
- [63].Wasner G, Binder A, Kopper F, Baron R. No effect of sympathetic sudomotor activity on capsaicin-evoked ongoing pain and hyperalgesia. PAIN 2000;84:331–8. [DOI] [PubMed] [Google Scholar]
- [64].Wasner G, Lee BB, Engel S, McLachlan E. Residual spinothalamic tract pathways predict development of central pain after spinal cord injury. Brain 2008;131(pt 9):2387–400. [DOI] [PubMed] [Google Scholar]
- [65].Wasner G, Naleschinski D, Baron R. A role for peripheral afferents in the pathophysiology and treatment of at-level neuropathic pain in spinal cord injury? A case report. PAIN 2007;131:219–25. [DOI] [PubMed] [Google Scholar]
- [66].Waxman SG, Dib-Hajj SD. Erythromelalgia: a hereditary pain syndrome enters the molecular era. Ann Neurol 2005;57:785–8. [DOI] [PubMed] [Google Scholar]
- [67].Weidner C, Schmelz M, Schmidt R, Hansson B, Handwerker HO, Torebjork HE. Functional attributes discriminating mechano-insensitive and mechano-responsive C nociceptors in human skin. J Neurosci 1999;19:10184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Yarnitsky D, Ochoa JL. Warm and cold specific somatosensory systems. Psychophysical thresholds, reaction times and peripheral conduction velocities. Brain 1991;114(pt 4):1819–26. [DOI] [PubMed] [Google Scholar]