Supplemental Digital Content is Available in the Text.
Keywords: tDCS, rTMS, Neuropathic pain, Nociceptive pain, Mixed pain
Abstract
Introduction:
Chronic pain (CP) is highly prevalent and generally undertreated health condition. Noninvasive brain stimulation may contribute to decrease pain intensity and influence other aspects related to CP.
Objective:
To provide consensus-based recommendations for the use of noninvasive brain stimulation in clinical practice.
Methods:
Systematic review of the literature searching for randomized clinical trials followed by consensus panel. Recommendations also involved a cost-estimation study.
Results:
The systematic review wielded 24 transcranial direct current stimulation (tDCS) and 22 repetitive transcranial magnetic stimulation (rTMS) studies. The following recommendations were provided: (1) Level A for anodal tDCS over the primary motor cortex (M1) in fibromyalgia, and level B for peripheral neuropathic pain, abdominal pain, and migraine; bifrontal (F3/F4) tDCS and M1 high-definition (HD)-tDCS for fibromyalgia; Oz/Cz tDCS for migraine and for secondary benefits such as improvement in quality of life, decrease in anxiety, and increase in pressure pain threshold; (2) level A recommendation for high-frequency (HF) rTMS over M1 for fibromyalgia and neuropathic pain, and level B for myofascial or musculoskeletal pain, complex regional pain syndrome, and migraine; (3) level A recommendation against the use of anodal M1 tDCS for low back pain; and (4) level B recommendation against the use of HF rTMS over the left dorsolateral prefrontal cortex in the control of pain.
Conclusion:
Transcranial DCS and rTMS are recommended techniques to be used in the control of CP conditions, with low to moderate analgesic effects, and no severe adverse events. These recommendations are based on a systematic review of the literature and a consensus made by experts in the field. Readers should use it as part of the resources available to decision-making.
1. Introduction
Chronic pain (CP) is highly prevalent worldwide and has been acknowledged as a major public health problem in many countries.40 Chronic pain has been recently suggested to be more prevalent in countries with low human development indices.48,56 Indeed, pain affects 20% to 40% of the general population in Latin America (LA) and constitutes a major public health challenge.39,88,92,93,112 The most frequent pain syndromes are osteoarthritis-related pain, low back pain (LBP), headaches, and neuropathic pain syndromes.2,5,39,40,92,93,95 For instance, the lifetime prevalence of acute LBP is close to 70%, and it has been suggested that more than half will eventually experience chronification,12,47 ranking chronic LBP as the first cause of years lived with disability80,114 worldwide. Chronic pain has known associations with depressed mood, fatigue, and catastrophizing thoughts. It is also widely recognized that even for CP directly triggered by peripheral structures such as joint and muscle, there exist a wide range of central nervous system (CNS) modifications occurring in CP, leading to a series of central changes that will allow for the perpetuation and maintenance of the CP status.9,71,72 Pain is linked to maladaptive plasticity in the CNS,10,11,31,32,98–100 which is related to the severity of symptoms.46,49,97
Although the different pain syndromes have different treatments and response rates, CP is generally undertreated. For instance, LBP is the main reason why people seek medical attention, and still, up to 40% of patients persist with uncontrolled symptoms.14 Neuropathic Pain, which affects up to 7% of the general population, may be pharmacoresistant in up to 40% of cases.25,111 This suggests that the current pharmacological agents available and the way they are used have provided relatively low efficacy as monotherapy strategies, with relatively high potential side effects, adding a supplementary layer of burden on patients, family members, and society already fighting against CP.30 As an example, one can cite the relatively high number necessary to treat seen with first- and second-line treatments for neuropathic pain,30 as well as the continuously alarming issue related to opioid misuse and abuse in the setting of noncancer CP treatment.61
The above limitations have stimulated the blossoming of several lines of research focused at innovative treatments for CP. These nonpharmacological approaches include a broad range of interventions, which are either potentially less expensive than conventional drug treatments (eg, mindfulness-based approaches) or supposed to act directly on CNS structures implicated in the occurrence of pain and positively affect a broader range of pain-associated symptoms such as fatigue, catastrophizing, and mood. Definitely, it has been reported that in some CP conditions such as fibromyalgia, nonpharmacological approaches can decrease not only pain intensity but may also have more efficacious effects in other domains such as sleep, cognitive complaints, and fatigue than pharmacological treatment.86
Among the currently available neuromodulation techniques, noninvasive brain stimulation (NIBS) has been extensively studied over the past 30 years to control CP. These techniques are known to influence neuronal cell membrane potential23 or to induce its depolarization/hyperpolarization with different degrees of focality, cost, and complexity,55,106 and can influence pain-processing regions in the CNS.89,109,117,118 As a consequence, they are believed to drive plastic changes120 that lead to better pain control and gain in function.
Several neuromodulation techniques such as transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS) are cleared by numerous national and regional control agencies worldwide. However, there is a paucity of local or regional guidelines to guide clinicians on the best way to use these techniques. The analgesic effects of the most frequently used noninvasive neuromodulation techniques have been comprehensively scrutinized in recent reviews and meta-analyses, and most of these publications provided a broad view of the available evidence supporting the use of these techniques in some CP settings.37,57,60,82 Although meta-analyses are the backbone of some policy and guideline recommendations,21 they may be of limited use to guide the clinical recommendations of therapeutic interventions having numerous parametric variables or when the object of study has several subcategories. This is the case of neuromodulation approaches, with its different techniques and parametric variables (ie, frequency of stimulation, CNS target, and number of sessions) and CP, with its different pain syndromes, different etiologies, prevalence, and prognosis. More important, the different CP syndromes have very heterogeneous degree of evidence-based treatments available for their control. In such instances, a more individualized approach is preferred. As an illustration, one recent publication considered the adequate sample size of a NIBS trial to be of at least 400 patients,81 and trials with lower numbers of patients were penalized (downgraded) and considered as inconsistent and imprecise, unless more participants were randomized. However, for some pain syndromes, these relatively high sample size values are unrealistic and virtually no treatment to date included this number of patients in any trial. For instance, a medium-sized double-blinded controlled trial to treat complex regional pain syndrome (CRPS) may add more to the already existing (scarce) literature on CRPS evidence-based treatment compared with a study of same sample size on the use of the same technique to treat neuropathic pain due to diabetes, a situation where several other therapeutic interventions have already shown to have significant analgesic effects.28,115 Frequently, these clinically relevant nuances are missed out or diluted in recommendations based exclusively on meta-analyses.
Another approach to synthesize clinical evidence and translate it into clinical practice is the guideline approach based on systematic reviews and standardized classification of trials and recommendations, which have been used in the NIBS context,57,58,60 and provided similar findings in their literature review compared with previous meta-analyses, but lead to higher-level recommendations of some of the NIBS techniques by their respective consensus panel. Evidence-based consensus aims to guide professionals on the best way to treat certain clinical conditions, representing a community-based expression to guide decision-making, contextualized to current available resources already available for the medical condition under scrutiny.
Based on the paucity of regional clinically oriented recommendations for the potential use of NIBS in the treatment of patients with CP, the aim of this study was to perform a comprehensive and updated systematic review of all the NIBS used to relieve CP, classify studies according to the class of evidence they provide according to established categorizations,16 and provide a consensus recommendations for the use of NIBS in clinical practice in LA and Caribbean region, with emphasis on the clinical significance of the interventions in context of the currently available treatments for each pain syndrome26,27 regionally.
Recommendations were based on a modified Delphi design that included a systematic review of the literature, and formulation of recommendations by a consensus panel composed of pain and/or neuromodulation specialists, and a patient's representative, followed by a cost-estimation study based on the regional costs and treatment availability.
2. Methods
This study was based on a Delphi design that included the following rounds: (1) systematic review of the literature; (2) formulation of recommendations by a panel of specialists formed by pain and/or neuromodulation professionals assigned by local and regional pain and neuromodulation societies (ie, Latin-American Pain Societies and Pain Societies from many LA countries), as well as researchers having published substantial research on NIBS in CP based in LA; (3) anonymous voting of the recommendations used as the basis for a consensus panel; (4) formulation of the final recommendation document; and (5) external review made by 3 specialists on pain and neuromodulation, located outside LA and Caribbean region. A patient having experienced NIBS treatment for CP was also invited to participate. The final report was based on the AGREE statement.
The reviewed interventions were: (1) repetitive TMS (rTMS); (2) tDCS; (3) transcranial alternating current stimulation (tACS); (4) transcranial random noise stimulation; (5) cerebellar tDCS; (6) transcutaneous vagus nerve stimulation; and (7) external trigeminal nerve stimulation.
For the purpose of this consensus paper, randomized double-blinded clinical trials were reviewed if they used a comparison group (treated by a sham or a second active NIBS procedure) and included as main outcome measures any of the following: (1) pain intensity; (2) pain-related quality of life; (3) pain impact on daily life; (4) use of pain medication; (5) number of days or hours without pain; and (6) frequency of migraine attacks.
For methods concerning group membership, and target population preferences and views according to the AGREE recommendations, please refer to supplementary file 1 (S1), available at http://links.lww.com/PR9/A35.
2.1. Search methods
A systematic review of clinical trials was performed on Medline (via PubMed) independently by 2 authors (A.F.B. and A.M.B.L.F.). Inconsistencies were resolved by a third author (D.C.A.). Descriptors and search strategy can be found at supplementary file 1 (S1), available at http://links.lww.com/PR9/A35.
2.2. Evidence selection criteria
The search was not delimited by sex, age, type of facility where the study was held, time, or language of publication. Double-blinded, sham-controlled studies with at least 10 CP patients per arm, treated by repeated sessions, were included. Exclusion criteria were: single case or case series reporting exclusively safety and tolerability data; single-session studies; literature reviews; and studies where pain was not the primary outcome, or where comorbidities included main psychiatric disorders (ie, major depression, schizophrenia, bipolar disorders, and drug addiction).
2.3. Strengths and limitations of the evidence
Strengths and limitations of the evidence were considered initially according to: (1) Study design—the study should have been designed to answer the clinical question regarding the effectiveness of neuromodulation in the control of pain. (2) Study methodology—the presence of randomization, blinding, allocation concealment, and appropriate data analysis was considered. (3) Appropriateness/relevance of primary and secondary outcomes were considered taking into account the items suggested by the IMMPACT recommendations for clinical trials involving interventions for patients with CP.26 To score the studies according to IMMPACT, the following items were evaluated in each selected study: pain intensity, pain quality and temporal characteristics, physical functioning, emotional functioning, self-perception of improvement and patient satisfaction, and occurrence of adverse events. Then, for the presence of outcome data for each of the 6 subitems above, a point scoring system was used by the writing committee and approved by all the authors: if one of the abovementioned items was contemplated in the study, the study received “one point,” and the sum of all points was calculated to assist consensus panel members in the task of providing recommendations.
2.4. Internal and external validity
The studies were also evaluated according to PEDro scale to assess external (item 1) and internal (items 2–11, score 0–10) validity.68 The scoring considered the following items: (1) Eligibility criteria were specified; (2) Subjects were randomly allocated to groups; (3) Allocation was concealed; (4) The groups were similar at baseline regarding the most important prognostic indicators; (5) There was blinding of all subjects; (6) There was blinding of all therapists who administered the therapy; (7) There was blinding of all assessors who measured at least one key outcome; (8) Measures of at least one key outcome were obtained from more than 85% of the subjects initially allocated to groups; (9) All subjects for whom outcome measures were available received the treatment or control condition as allocated or, where this was not the case, data for at least one key outcome were analyzed by intention to treat; (10) The results of between-group statistical comparisons are reported for at least one key outcome; (11) The study provided both point measures and measures of variability for at least one key outcome. As eligibility criteria (external validity) were established initially as inclusion/exclusion criteria, the final score was presented only for internal validity (maximum score of 10).
2.5. Classification of studies
Based on the data collected by the steering committee, and in accordance with the IMMPACT recommendations and the PEDro assessment, studies were then classified according to classes of evidence16 as:
Class I study was considered an adequately data-supported, prospective, randomized, sham-controlled clinical trial with masked outcome assessment in a representative population (n ≥ 25 patients receiving active treatment).57
It should include all 5 items below:
(1) Randomization concealment;
(2) Clearly defined primary outcomes;
(3) Clearly defined exclusion/inclusion criteria;
(4) Adequate accounting for dropouts and crossovers with numbers sufficiently low to have minimal potential for bias;
(5) Relevant baseline characteristics substantially equivalent among treatment groups or appropriate statistical adjustment for differences.
Class II: Prospective matched-group cohort study in a representative population (n ≤ 25 patients receiving active treatment) with masked outcome assessment that meets (1)–(5) mentioned above or a randomized, controlled trial in a representative population that lacks one criteria (1)–(5).
Class III studies included all other controlled trials.
Class IV studies are uncontrolled studies, case series, and case reports (which were not included in this study).
For methodological information on the formulation of the recommendation based on the systematic review and consideration of benefits, harms, infrastructure, and cost estimation of the recommended techniques, please refer to supplementary file 1 (S1), available at http://links.lww.com/PR9/A35. Recommendations were based on standardized criteria as follows16:
(1) Level A rating (established as effective, ineffective, or harmful) requires at least one convincing class I study or at least 2 consistent, convincing class II studies;
(2) Level B rating (probably effective, ineffective, or harmful) requires at least one convincing class II study or overwhelming class III evidence;
(3) Level C rating (possibly effective, ineffective, or harmful) requires at least 2 convincing class III studies.
3. Results
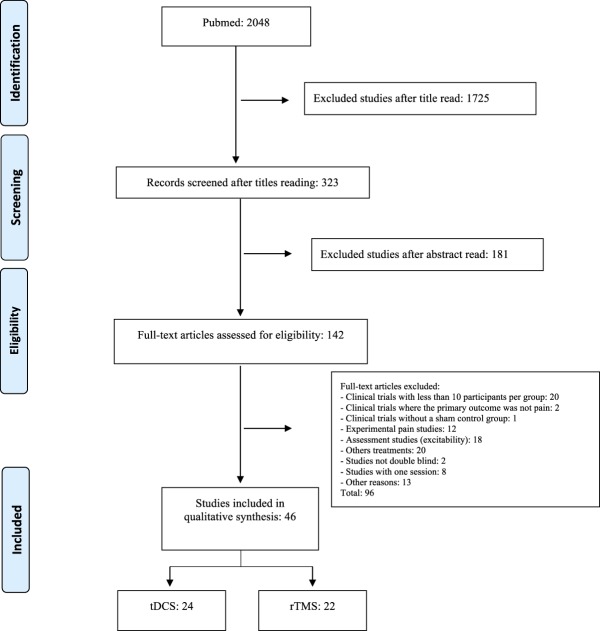
Search was developed from June 2016 to June 2017, yielding 2048 studies, from which 1999 studies were excluded (Fig. 1). The final analysis was made with 49 studies, 24 of tDCS3,4,7,8,17,24,29,33,36,41,42,54,65,66,75,78,83,90,94,105,108,110,113,116 and 22 of rTMS.1,6,15,22,23,35,44,51–53,63,64,67,69,73,76,77,84,85,87,101,103 Countries involved in these studies are shown in supplementary file 2 (S2), available at http://links.lww.com/PR9/A35.
Figure 1.
Study flowchart. rTMS, repetitive transcranial magnetic stimulation; tDCS, transcranial direct current stimulation.
3.1. Transcranial electrical stimulation (transcranial direct current stimulation)
We searched for 6 types of transcranial electrical stimulation: (1) tDCS; (2) tACS; (3) transcranial random noise stimulation; (4) cerebellar tDCS; (5) transcutaneous vagus nerve stimulation; and (6) external trigeminal nerve stimulation. Among the abovementioned types, only tDCS and tACS studies reached the standards to be included in the review. We included 24 parallel or crossover randomized controlled trial (RCT). Transcranial DCS was generally administered through a pair of 25- to 35-cm2 sponge electrodes, 1-2 mA of amplitude, current density 0.04 to 0.06 mA/cm2, for 20 minutes, during 5 sessions (range 3–18 sessions). High-density tDCS with 4 electrodes was investigated in only one study, as well as tACS. Those studies included 927 (38.62 ± 32.03/study) participants, with the maximum sample size of 135 participants.
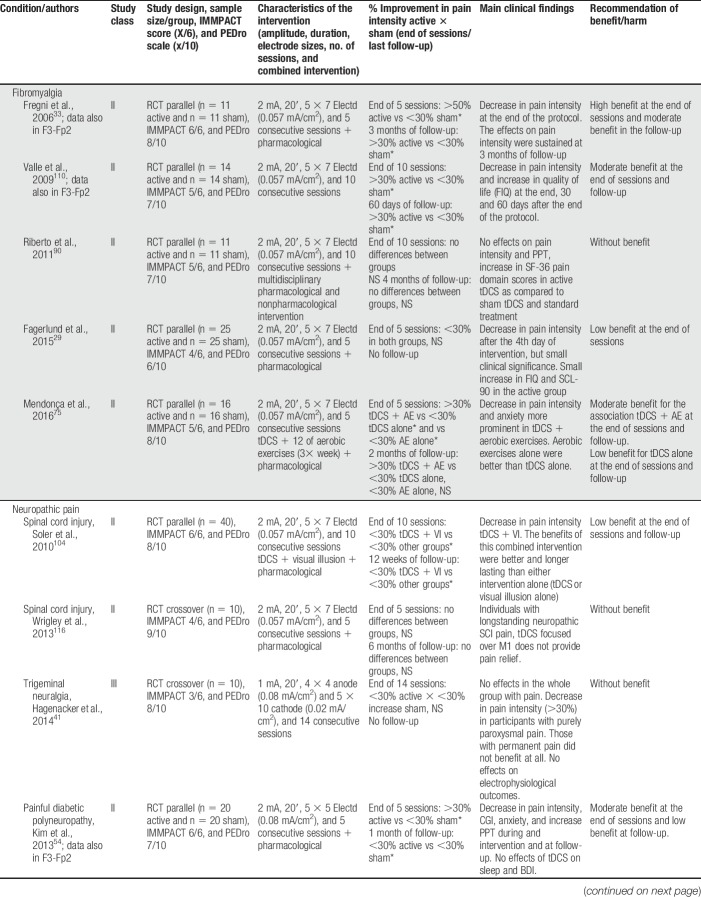
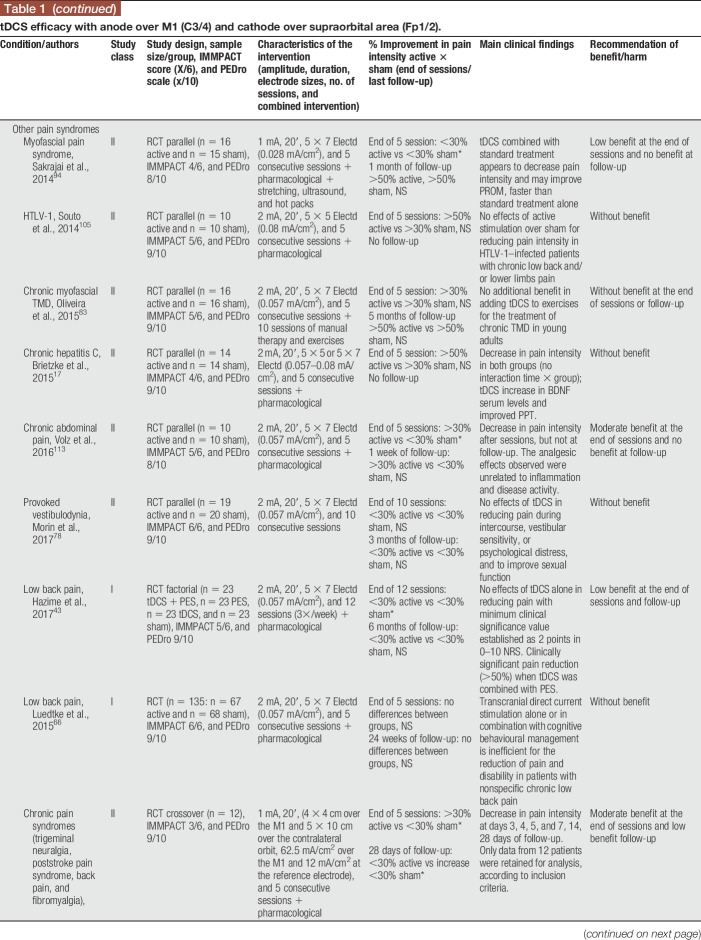
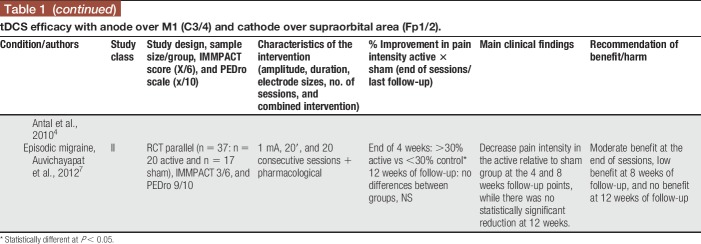
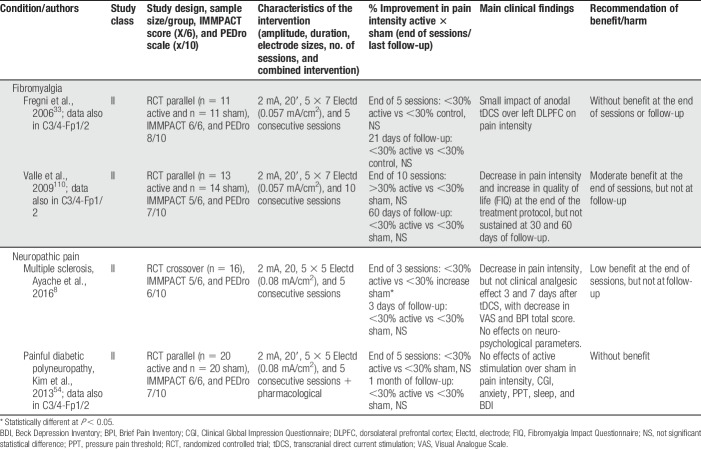
Anodal tDCS stimulation of the primary motor cortex (M1—C3, C4, or Cz positions of the 10/20 international EEG system) with the cathode over the contralateral supraorbital area (Fp1 or Fp2) was used in 19 of the 24 studies treating participants with fibromyalgia, neuropathic pain (spinal cord injury [SCI], trigeminal neuralgia, lumbar radiculopathy, and diabetic polyneuropathy), myofascial pain associated with or not with temporomandibular joint disorder, HTLV-1 infection–related pain, chronic hepatitis C, abdominal pain, vestibulodynia, and episodic migraine (Table 1). Some studies positioned the anode over the left prefrontal dorsolateral cortex (F3 of the 10/20 international EEG system) and the cathode over Fp2 (Table 2), but they were less frequently used. In some occasions, the montages of the primary motor cortex and dorsolateral prefrontal cortex (DLPFC) were assessed in the same study. From the 4 studies that used this F3/Fp2 montage, 2 included fibromyalgia and 2 neuropathic pain participants (multiple sclerosis and trigeminal neuralgia). Other montages were also found and are described in Table 3.
Table 1.
tDCS efficacy with anode over M1 (C3/4) and cathode over supraorbital area (Fp1/2).
Table 2.
tDCS efficacy with anode over left DLPFC (F3) and cathode over supraorbital area (Fp2).
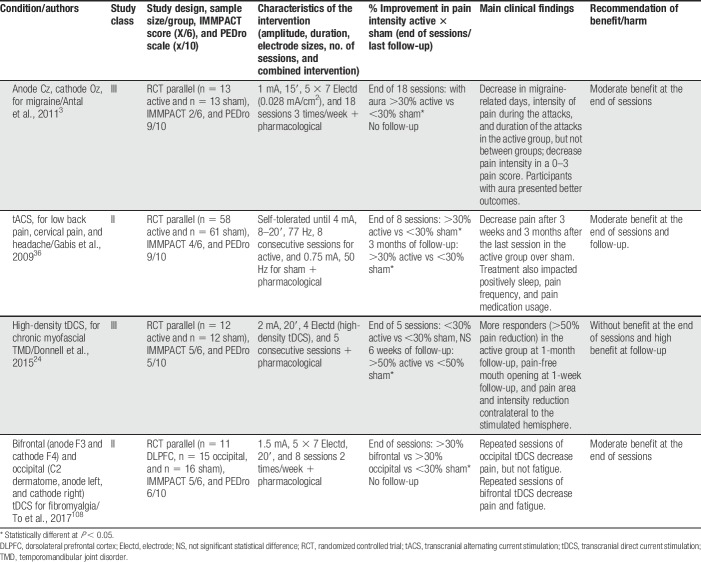
Table 3.
tDCS with other montages and tACS efficacy.
The studies were generally well designed and did not approach pain intensity only, but also affective dimensions of pain, and physical and emotional functions. Sixty-two percent of studies contemplated ≥5/6 IMMPACT items (Tables 1–3, supplementary file 3 [S3], available at http://links.lww.com/PR9/A35). The most frequently neglected item was “self-perception of improvement.” Stimulation was generally well tolerated, and none of the studies reported serious adverse events. PEDro classification of internal validity ranged from 6 to 9/10 (Tables 1–3; supplementary file 4 [S4], available at http://links.lww.com/PR9/A35), representing studies with adequate quality of evidence. Allocation concealment and blinding of the researchers who administered the techniques were the most common methodological limitation of the studies. This limitation could be mitigated in future studies by simply asking participants at the end of the study which group they participated using simple blinding assessment questionnaires.23 Some technological improvements may also improve this issue, by the use of devices with built-in solutions to perform active or sham stimulation according to predetermined blinded and coded protocols, so that the therapist will not know the type of stimulation delivered once the stimulator setup is performed.
In general, benefit of the montages addressing the primary motor cortex (M1) was low to moderate (>20 or >30% decrease in pain intensity) at the end of sessions and follow-up. These results were of moderate benefit when tDCS was applied to patients with fibromyalgia and of no benefit when other musculoskeletal or neurological problems were studied. Results for tDCS in neuropathic pain were not so as consistent as those for fibromyalgia, suggesting a lesser analgesic effect in patients with neuropathic pain. Several studies were classified as class II (supplementary file [S3], available at http://links.lww.com/PR9/A35). Two studies were classified as class I,42,66 both involving LBP participants. In one study,42 anodal tDCS to M1 was shown to have significant analgesic effects when associated with peripheral electric stimulation. However, both studies reported negative effects of stand-alone tDCS. This information was incorporated in the recommendations as class A for ineffectiveness of M1 anodal tDCS for this painful syndrome.
In general, montages stimulating the left prefrontal dorsolateral cortex were less commonly used and generally resulted in less benefit. Other tDCS montages and one tACS intervention showed to be beneficial, but the number of studies was small. Gabis et al.36 showed that a 77-Hz tACS for 8 days was moderately effective in reducing spinal pain and headache. Antal et al.3 showed that a tDCS montage with the cathode over Cz and the anode over Oz was also moderately effective in reducing migraine-related pain, but at the end of 18 sessions, and not at the end of 5 sessions of tDCS. Donnell et al.24 showed that a 2 × 2 multipolar tDCS montage targeting the motor cortex was not effective to control pain just after the end of 5 sessions, but was highly effective in the 6-week follow-up. Finally, To et al.108 showed that a bifrontal montage (anode over F3 and cathode over F4) and a montage targeting the C2 dermatome were moderately effective in reducing pain in fibromyalgia, whereas the first montage was also effective to reduce fatigue.
In the vast majority of studies, transcranial electrical stimulation was administered along with pharmacological treatment, frequently including CNS-acting medications such as tricyclic antidepressants and anticonvulsants. In 6 studies, tDCS was administered together with other interventions such as visual illusion,104 aerobic exercises,75 manual therapy,83 soft tissues stretching, hot packs, and low-level ultrasound,94 general rehabilitation procedures,90 and peripheral electrical stimulation (PES).43 In 3 of those studies,43,75,104 an additive effect of tDCS was shown, enhancing the overall effects on pain and other outcome measures.
3.2. Repetitive transcranial magnetic stimulation
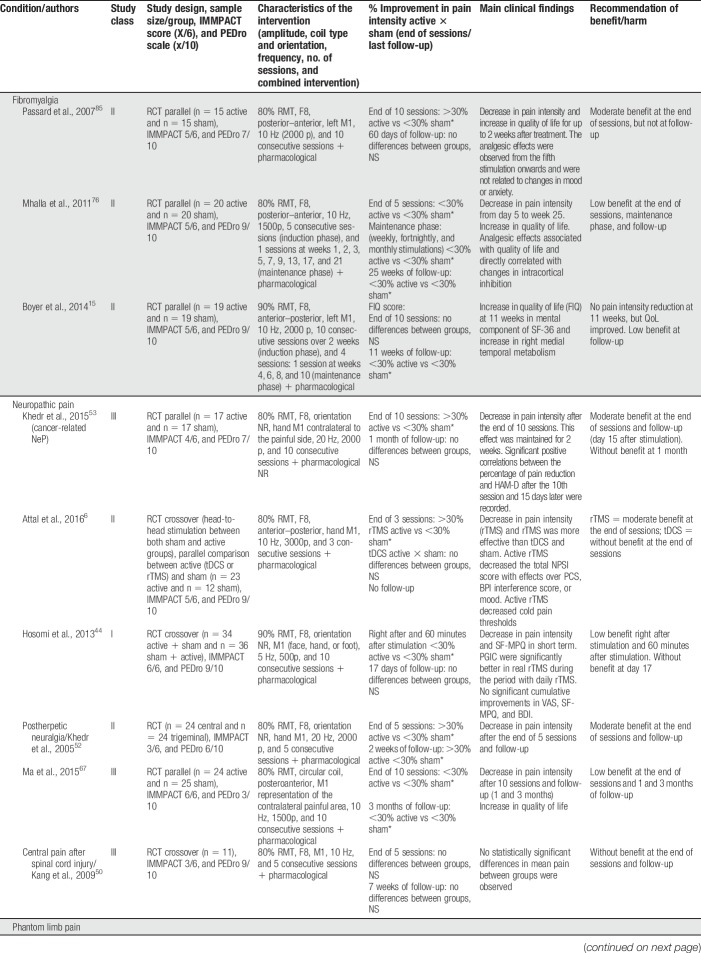
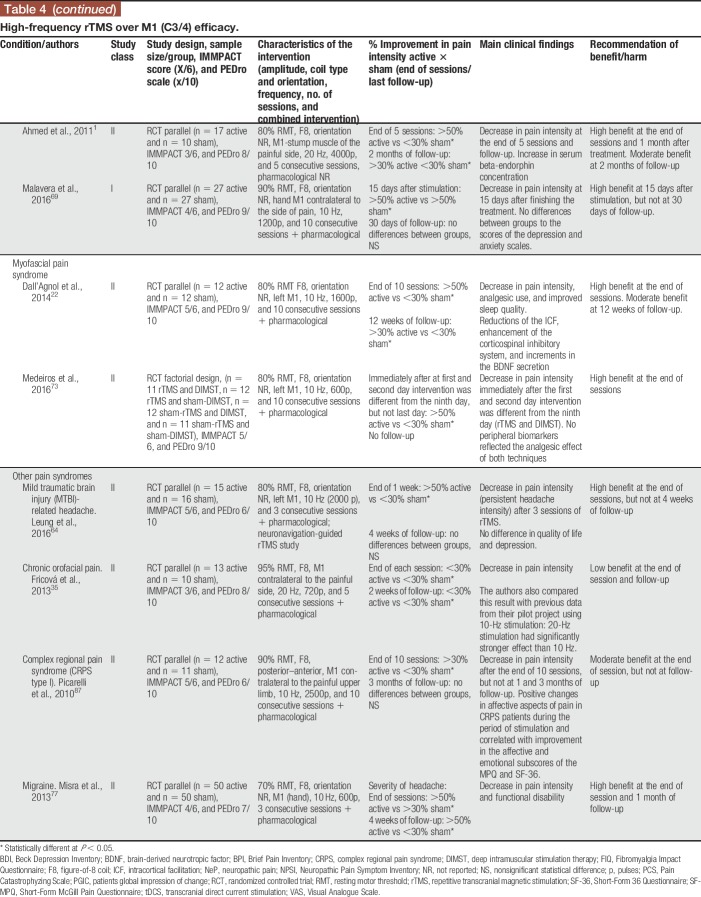
Our review distinguished between 2 types of TMS: (1) classic “superficial” rTMS; and (2) deep rTMS. Studies were also divided according to cortical target location: (1) primary motor cortex (M1); and (2) non-M1 (eg, DLPFC and primary sensory cortex). Twenty-two parallel or crossover RCTs were included, using multiple sessions of stimulation (Tables 4–6). Repetitive TMS was more frequently administered using superficial coils targeting M1, at high frequency (10–20 Hz) in sessions comprising 1500 to 3000 pulses. Repetitive TMS was also applied to DLPFC (Table 5) and with a deep rTMS technique (Table 6). Studies included 798 (36.27 ± 19.73/study) participants, with a maximal sample size of 100.67
Table 4.
High-frequency rTMS over M1 (C3/4) efficacy.
Table 6.
rTMS efficacy in other cortical areas.
Table 5.
rTMS efficacy over dorsolateral prefrontal cortex.
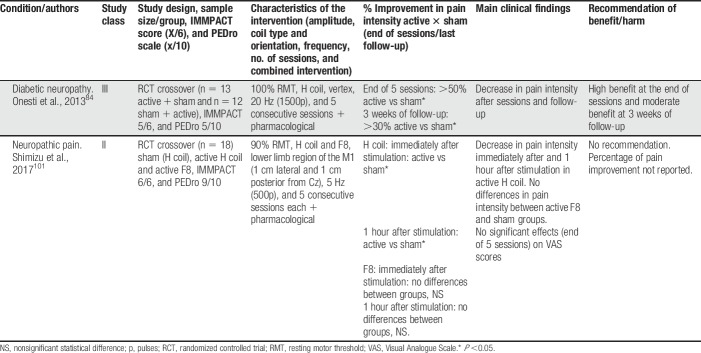
High-frequency rTMS over M1 was the most common approach, and it was more frequently compared with sham stimulation in parallel-design studies. Deep rTMS and superficial TMS to target outside M1 were rarely performed. In the vast majority of studies, rTMS was administered along with pharmacological treatment, frequently including CNS-acting medications such as tricyclic antidepressants and anticonvulsants. In some studies, physiotherapy was also performed during sessions, and in one study physiotherapy was performed as part of the protocol and was standardized in all patients.87 Head-to-head studies in NIBS were rare and only one has so far compared 10-Hz rTMS over M1 against anodal tDCS to the same target.6 In this study, which included 3 consecutive daily sessions of stimulation in patients with peripheral neuropathic pain due to radiculopathy, rTMS was superior to tDCS and sham, and its effects outlasted the stimulation session for a few days. Interestingly, in this same study, the placebo effect of sham-tDCS and sham-rTMS was similar and not significantly different.6 Few studies performed maintenance sessions of stimulation after an induction period (when sessions occur daily for 5–10 consecutive days). In these studies, it was shown that maintenance sessions performed weekly, fortnightly, and even monthly could maintain the effects triggered during the induction period.76 The induction/maintenance strategy is currently used for the treatment of major depression, and is sound and safe on clinical and practical basis; however, it cannot be fully recommended in the treatment of CP due to the still limited amount of data available using this strategy. Deep rTMS was only performed in 2 studies (Table 6), both targeted the leg area representation of M1 in peripheral neuropathic pain patients with the H (Hesed)—coil.84,101 In both, deep rTMS showed positive results, being short-lived (only present 1 hour after the stimulation) in one.101
The studies were generally well designed and did not approach pain intensity only but also how it influenced the affective dimension of pain, as well as physical and emotional function. Sixty-eight percent of studies contemplated ≥5/6 IMMPACT items (Tables 4–6; supplementary file 5 [S5], available at http://links.lww.com/PR9/A35). The item most frequently neglected was “self-perception of improvement.” PEDro classification for rTMS studies ranged from 3 to 9/10 (supplementary file 6 [S6], available at http://links.lww.com/PR9/A35). The major problems concerned blinding, especially of the therapist, and allocation concealment. Blinding the therapist to rTMS is virtually impossible, except for certain devices (TMS coils delivering active or placebo stimuli without an operator's knowledge), developed with this aim. However, many alternatives to patients' blinding are available and should be incorporated in the studies, the same for allocation concealment. Also, the use of a formal blinding assessment questionnaire is highly recommended and could overcome these potential biases as mentioned above for tDCS.
Two studies were ranked as class I, both on neuropathic pain.44,69 Virtually, all the superficial rTMS studies targeting M1 at high frequency (>5 Hz) were positive compared with placebo. All included studies targeting DLPFC were negative (Table 5). According to the systematic review at the end of stimulation, most studies found moderate/high effect for rTMS, whereas the effect was more frequently low/moderate after the maintenance sessions. After-effects assessed weeks to months after the end of treatment were variable and only performed in a few studies. Neuropathic pain and fibromyalgia were the pain syndromes more frequently assessed in all studies, and the effects of stimulation were overall moderate to high at the end of treatment, decreasing pain intensity and improving other pain-related factors such as fatigue, catastrophism,76 and quality of life.15 Patients with central neuropathic pain (mostly SCI and central poststroke pain) were more frequently mixed with neuropathic pain of peripheral origin44 in most trials. Trials with exclusive central neuropathic pain patients were the exception.23 Other (prevalent) pain syndromes such as musculoskeletal pain, migraine, and CRPS were underrepresented in rTMS studies. These studies suggest that the analgesic effects could be maintained in the long term with intermittent (ie, weekly; fortnightly) sessions of treatment, as evidenced in the treatment of major depression. Interestingly, the sham effect of rTMS was relatively low in most CP studies, being usually below 15% pain reduction, which is different from rTMS studies for major depression where both pharmacological and neuromodulatory treatments had significant placebo effects.18
Based on the data methodological steps above, the consensus panel provided specific recommendations for NIBS for CP. Details are shown in Boxes 1 and 2.
Box 1. tDCS recommendations for chronic pain relief.
Electrode position:
Unilateral pain or bilateral pain with unilateral predominance—anode over the contralateral M1 and cathode over the ipsilateral supraorbital area.
Electrodes' characteristics:
5 × 5 or 5 × 7 cm;
Sponge electrodes embedded with saline solution.
Amplitude:
2 mA.
Duration of stimulation and number of induction sessions:
20 to 30 minutes;
5 to 10 consecutive sessions (once daily).
Indications:
Low to moderate benefit to decrease pain intensity, without substantial risk of serious adverse event: anodal M1 tDCS for fibromyalgia;
Potential but still uncertain benefit, without substantial risk of serious adverse event: Anodal M1 tDCS for peripheral neuropathic pain, chronic abdominal pain, and migraine; bifrontal tDCS (anode F3 and cathode F4) and M1 HD-tDCS for fibromyalgia; Oz (cathode)/Cz (anode) tDCS for migraine.
Secondary benefits:
Possible improvement in quality of life aspects, anxiety, and pressure pain threshold.
Potential adverse events:
Itching, tingling, skin redness, somnolence, concentration issues, headache, fatigue, light headedness, and skin burning under the electrode (rare).
Precautions:
Prescription and follow-up by trained staff;
History of seizures;
Cranial bone defect;
Cranial skin scars.
Contraindications:
Head implants;
Tumor below the electrodes;
Hypertrophic skin scars below the electrodes.
Box 2. rTMS tDCS recommendations for chronic pain relief.
Coil type and positioning:
Figure-of-eight coil placed at M1, with the handle pointing forward or backwards to the sagittal plane.
Intensity:
80% to 90% RMT (when using 90%, refer to safety guidelines88).
Frequency:
10 to 20 Hz.
Number of pulses:
1500 to 3000 per session.
Interval between trains:
10 to 25′.
Number of induction sessions:
3 to 10.
Indications:
Low to moderate benefit, without substantial risk of serious adverse event: HF rTMS over M1 for fibromyalgia and neuropathic pain;
Potential but still uncertain benefit, but without substantial risk of serious adverse event: HF rTMS over M1 for myofascial or musculoskeletal pain, complex regional pain syndrome, and migraine;
So far without clear benefit: HF rTMS over left dorsolateral prefrontal cortex.
Potential adverse events:
Headache (up to 30% of patients report neck or head pain, usually after the first session of rTMS, which is usually mitigated by a proper and comfortable patient positioning during sessions);
Seizure (very rare when following the above recommendations; frequency <1/1000).
Precautions:
Prescription and follow-up by trained staff;
History of seizures;
Cranial bone defect;
History of substance abuse;
History of sleep deprivation.
Contraindications:
Intracranial metallic implants/electrodes (eg, cochlear implants and deep brain stimulation);
Presence of uncontrolled epilepsy.
4. Discussion
4.1. General recommendations
This study involves a consensus-based recommendation for the use of tDCS and rTMS in the control of CP. The consensus panel involved pain and/or neuromodulation specialists in LA and Caribbean region that voted in 2 rounds of discussion based on a systematic review of the literature and elaboration of recommendations based on the European Federation of Neurological Societies criteria for guidelines elaboration.16
According to these results, this consensus made a level A recommendation for efficacy of induction sessions (n = 5–10) of anodal tDCS, with 2-mA intensity over M1 (C3 and C4 of the 10/20 EEG international system or neuronavigated), with the cathode over the contralateral supraorbital area (Fp1 or Fp2 of the 10/20 EEG international system). Transcranial DCS might be used as an add-on analgesic treatment of patients who remain symptomatic, despite pharmacological and nonpharmacological treatment. These results are in agreement with previous reviews,45,59,70,119 and include not only the control of pain, but also increase in many aspects of quality of life, as well. A recent guideline60 made a level B recommendation for the use of anodal M1 tDCS, and this discrepancy is apparently due to the fact that they considered that trials coming from the same research group would be counted as one study. However, if we had followed the same criteria, we would again have 2 level II trials, which would increase the recommendation from level B to level A. It is important to highlight that although we input the higher level of recommendation to the use of tDCS in this condition, clinicians would expect only low (20%–30%) to moderate (30%–50%) pain intensity reduction.
Our results showed that the tDCS analgesic effects are somewhat less marked for patients with neuropathic pain, consistent with the guideline published by Lefaucheur et al.60 We did not classify HTLV-related pain105 as purely neuropathic, as those patients generally suffer from a mixture of nociceptive (low back) and neuropathic pain (lower limbs), and many of them have diffuse pain.95 Consequently, we included only 4 neuropathic pain studies,8,41,54,104 and only one of them54 showed a >30% reduction in pain intensity (Tables 1 and 2). This study involved 40 participants with diabetic polyneuropathy and showed a decrease in pain intensity and increase in pressure pain thresholds after consecutive sessions, but not at 1-month follow-up. Another study involving SCI pain showed <30% pain reduction,104 in accordance with recent meta-analyses.65,74 Even if the HTLV-related pain study has been included as a neuropathic pain study, the level of evidence for tDCS efficacy in neuropathic pain would not have increased, as there were no statistical differences between active and control groups in this study. Consequently, based on the diabetic polyneuropathy study, our level B recommendation for the use of tDCS in neuropathic pain only applies for peripheral neuropathy–related pain. Future studies should be developed to investigate efficacy of this approach, and clinicians would only expect a moderate decrease of pain.
Anodal tDCS over M1 was recommended as a level B in the treatment of chronic abdominal pain and migraine, as both have one class II study showing moderate benefits. We could not compare the results of the chronic abdominal pain study with other recommendations or meta-analyses. Our recommendation for migraine is supported by a meta-analysis102 showing that anodal M1 tDCS has a moderate to high effect size in the decrease of pain and reduction of pain killers intake. Two of the articles included in this study3,7 were also included in our review, but one of them is a class III study using cathodal tDCS over the visual cortex.3 The same recommendation was also achieved for bifrontal tDCS in the treatment of fibromyalgia, with one class II study. These approaches may be further investigated, as they involve unusual electrode montages that seem to be useful in the control of pain.
Regarding the position of the anode over the motor cortex, a location on the hemisphere contralateral to pain should be recommended for unilateral pain (C3 or C4). If pain is bilateral or diffuse, one may consider positioning the anode over the dominant hemisphere or contralateral to the worst pain. For axial or lower limbs pain, a reasonable position for the active electrode should be at the vertex (Cz). However, there is no study comparing these montages, which are being recommended based only on neuroanatomical characteristics of the M1.
A level A recommendation against the use of anodal tDCS over M1 was provided for LBP as a stand-alone treatment or when associated with cognitive behavioral therapy, as none of the included studies could show minimum benefits with tDCS only. It has to be highlighted that this recommendation was based on 2 class I studies, which reinforces this statement. A recent study suggested that patients with LBP do not respond to tDCS M1 stimulation.96 However, associating this approach with PES43 or exercises107 has been shown to be effective, but the exact mechanisms by which this additive effect happens are not known.
A recent comprehensive meta-analysis assessed efficacy of tDCS in the control of CP conditions,81 showing that the effects are below the clinical relevant analgesic effect (overall 17% decrease), but significant in the increase of quality of life. Although they compared studies across their 95% confidence intervals, which is a potentially better approach than ours, comparing ranges of decrease in pain intensity (<30%, >30%, and >50%), they did not involve a subgroup analysis by pain syndromes. They pooled the studies into neuropathic and non-neuropathic pain, which can hide specificities of different pain types. Also, they did not include migraine studies, nor did consider pain satisfaction or disability measures such as timed-up-and-go and mouth opening in temporomandibular joint pain participants. Another potential limitation was to consider all 2-mA studies at high risk of bias in the blinding assessment, an assumption that is subject to criticism given the literature showing that blinding can be effective in this setting, especially in parallel studies.19,20
Regarding rTMS, we recommended that high-frequency (10–20 Hz) rTMS should be used over the motor cortex area, using a figure-of-eight coil, with the handle pointing forward or backward to the sagittal plane, with intensities ranging from 80% to 90% of the resting motor threshold, 1500 to 3000 pulses per session, and intertrain interval of 10 to 25 seconds. Induction sessions would range from 3 to 10. The use of maintenance sessions is desirable, but there is still not a consensus on its effectiveness. This approach was recommended as an A level (low to moderate benefit) for the use of rTMS in the control of pain associated with fibromyalgia and neuropathic pain, and a level B recommendation for the treatment of myofascial pain, musculoskeletal pain, CRPS, and migraine. A level B recommendation was made to avoid the use of DLPFC rTMS in the control of pain.
The level A recommendation for the use of HF rTMS at M1 in the treatment of fibromyalgia is in accordance with recent studies showing that this approach may potentially decrease pain intensity and increase quality of life. Hou et al.45 demonstrated that HF rTMS over M1 could reduce pain, and fatigue, and improve general health and function. Their findings also show that HF rTMS over DLPFC could have the same effects but additionally influencing positively depression and sleep disturbances. In this study, rTMS and using M1 as a target yielded greater effect sizes than tDCS and using DLPFC as a target. This consensus' recommendations differ from those of Lefaucheur et al.,57 which did not recommend the use of rTMS in patients with fibromyalgia probably likely because 3 RCTs13,76,85 came from the same research group. However, we did not use such restraint that hindered us 2 positive and one negative class II RCT. Consequently, a level A recommendation was achieved but, again, clinicians should bear in mind that low to moderate decrease in pain intensity was provided by using HF rTMS at M1 as an add-on therapy, which was associated with improvement in quality of life, mood, and catastrophism. The attribution of a level A recommendation for the use of HF rTMS at M1 in the treatment of neuropathic pain was made taking into account 3 class III,50,53,67 3 class II,1,6,52 and 2 class I44,69 studies, showing low to moderate benefit in the decrease of pain and 1 class I study. This result is one of the most consistent and involves patients with central and peripheral neuropathic pain. It is consistent with a meta-analyses showing that HF rTMS to M1 is effective in the control of neuropathic pain,62 although they proposed that the central origin of pain was more prone to have better results, something that we could not observe in the current available data. The same recommendation has been attributed in a consensus guideline including quite different studies.57 However, this recommendation is in disagreement with 2 meta-analysis showing that rTMS is not effective in SCI neuropathic pain38 or in neuropathic pain in general.81 This last comprehensive meta-analysis made subgroup analysis separating non-neuropathic from neuropathic pain and showed that HF rTMS to M1 has a small but significant effect size in decreasing neuropathic pain, irrespective of its peripheral or central origin. Considering all these results, clinicians should expect low to moderate benefit of using HF rTMS to M1 in the control of neuropathic pain due to central or peripheral origin.
This consensus made a level B recommendation for the use of HF rTMS to M1 in the control of myofascial, musculoskeletal, CRPS, and migraine because for each type of pain, yielded at least a class II study. These pain syndromes were less frequently studied, had trials with smaller effect sizes, or represented studies that so far have not been widely replicated by different research groups.
4.2. Combination of neuromodulatory approaches
Among the studies reviewed for this consensus guideline, some tDCS studies investigated the association of NIBS with other nonpharmacological interventions such as PES,42 standardized physiotherapy,94 exercise,75,83,110 manual therapy,83 or visual illusion.96,104 These studies usually showed an additive effect, which raises the question of the clinical value of these combined strategies and their underlying mechanisms. For example, anodal tDCS of M1 has been beneficially combined with PES in individuals with chronic LBP,42 while the supposed effects of these procedures on cortical excitability are opposite, tDCS being excitatory and PES inhibitory. Noninvasive brain stimulation techniques could also be combined with cognitive training, such as mental practice and go-no-go tasks, which are known to be potentially beneficial to individuals with CP. The combination of NIBS techniques with other therapies is believed to be able to promote a variety of neural mechanisms related to synaptic plasticity such as metaplasticity (ie, the plasticity of synaptic plasticity).79
4.3. Relationship between benefits and harms and adverse events
The present estimation of the relationship between benefits and harms was based on the IMMPACT recommendations, which is a potential flaw of previous studies. Benefit was expressed in terms of percentage of pain relief in the active vs sham groups. Although our classification took into account a comparison between high (>50%), moderate (>30%), or small (<30%) pain relief in the active and control groups, we considered to only assume this difference if it was statistically different. This method could have a potential limitation because it did not take into account the net difference between the active and sham arms. For example, 2 studies with >50% pain reduction in the active arms, but one with sham effect <10% and the other with sham effect >30% would be both considered as “high benefit” given this difference was statistically different, and both would be scored as having a “high” treatment effect. However, considering the induction period, this type of situation only occurred for one study,77 where real rTMS caused significant >50% pain relief compared with sham, but the sham arm provided >30% pain relief. Except for this case, all other rTMS studies (n = 9) and all tDCS studies (n = 9) included in the present analysis provided sham effects that were <30% pain relief compared with baseline suggesting that the scoring system we used was minimally influenced by this potential classification bias. In fact, several reviews have shown that different from intuitive thinking, NIBS techniques have relatively low placebo effects in pain and depression trials.
Harm was assessed independently but scored together with benefits in our classification according to IMMPACT-based approach, which may also be another limitation. None of the included studies presented serious harmful effects for the participants, except a case of seizure, which is discussed below. This study did not involve an accurate reliability study of the benefits, but this aspect was indirectly approached through the regional experts' opinions, which also included availability of equipment and trained staff. In most of the tDCS and rTMS studies, adverse events had statistically equal frequency after active and sham sessions. Some tDCS studies did report adverse events in active groups.78,83,94,104,113 Only 2 rTMS1,50 and one tDCS studies failed to mention adverse events. In one tDCS study, it was not possible to state whether the adverse events were in the active or sham group.42 Treatments with tDCS were generally safe and well-tolerated, and there were no severe adverse events reported. The most frequent adverse events were itching, tingling, and skin redness. Somnolence, concentration issues, fatigue, light headedness, headache, and one little burning below the cathode were also reported,83 but participants nevertheless completed the studies. The use of rTMS is commonly associated with higher risks, but these were not seen in the results of the revised studies. Headache and neck pain can happen in around 30% of the patients and might be managed by properly positioning in the stimulation chair. A checklist of potential contraindications is available91 and should be used in the screening to rTMS utilization.
Transcranial DCS- and rTMS-related adverse events tended to resolve in minutes to hours after the end of sessions. Although the use of NIBS does not require advanced clinical facilities and specific cardiopulmonary resuscitation apparel, a trained staff is necessary to ensure proper use of equipment, electrode and coil positioning, and respect of indications, contraindications, and precautions of use. For results on the cost-estimation analysis, target population preferences, and views, please refer to supplementary file 2 (S2), available at http://links.lww.com/PR9/A35.
4.4. What is needed in a noninvasive brain stimulation facility?
This consensus work supports the use of NIBS neuromodulation for therapeutic purpose in patients with various CP syndromes using both tDCS and rTMS. For a detailed description on the recommendations of the infrastructure necessary to an NIBS facility, please refer to supplementary file 1 (S1), available at http://links.lww.com/PR9/A35.
4.5. Facilitators and barriers to application
Neuromodulation through tDCS or rTMS seems to be a fair option in the control of certain CP syndromes, as the benefits of those techniques are clearly higher than the risks. Potential facilitators to the implementation of NIBS approaches in the clinical setting also include the relative ease of training in low-complexity techniques and the possibility of combination and association with a variety of other pharmacological and nonpharmacological treatments. However, this implementation faces some important barriers that should be properly addressed in the way of further developing NIBS applications in clinical practice. Internal validity of included randomized clinical trials was good regarding selection, reporting, detection, and attrition bias, which was indirectly represented by high grades in PEDro score. However, internal validity was often compromised by the relatively low sample size of the studies, which is partially explained by difficulties in allocating participant of certain infrequent but important diseases, such as central pain syndromes. Hence, bigger studies should be encouraged, such as those with peripheral neuropathic pain, musculoskeletal pain, and migraine, which are more frequent in the population. Future studies need to address this problem through larger (>200) samples, including multicentric trials.
The cost of the devices, including maintenance, and the necessity of skilled supervision during treatment increase the requirements to set up neuromodulation clinical facilities. One can estimate approximately 40 minutes to perform a tDCS session, and up to 1 hour to perform an rTMS session, which will lead to approximately 10 to 15 patients/day using a single machine. As there is need of 5 to 10 daily sessions for each patient during the induction phase of treatment, this drastically reduces the number of patients that can be allocated to the practice of NIBS in a given center, unless several machines and a proper staff number are available. Regulatory policies are another issue in NIBS neuromodulation, regardless the amount of clinical and scientific evidence provided in this area57,60,82 because most countries have not so far officially regulated its use for pain relief.34
5. Summary of recommendations
This is the first regional consensus recommendation for the use of NIBS techniques for pain relief in LA and Caribbean region. This is an updated guideline supporting the use of tDCS and rTMS for pain and recommendations based on gathered scientific knowledge behind the use of these techniques. Based on this work, level A recommendation (low to moderate benefit) was provided for the use of anodal tDCS over M1 in the control of pain in fibromyalgia, and level B (potential, but still uncertain benefit) recommendation for its use in peripheral neuropathic pain, abdominal pain, and migraine. Bifrontal (F3/F4) tDCS has also received a level B recommendation for the treatment of fibromyalgia, as well as M1 HD-tDCS. A level B recommendation has also been attributed to Oz/Cz tDCS for migraine and for secondary benefits such as improvement in quality of life, decrease in anxiety, and increase in pressure pain threshold. Regarding rTMS, level A recommendation (low to moderate benefit) was provided for HF rTMS over M1 for fibromyalgia and neuropathic pain, and a level B recommendation (potential, but still uncertain benefit) for myofascial or musculoskeletal pain, CRPS, and migraine. Level A recommendation against the use of M1 tDCS for LBP and a level B recommendation against the use of HF rTMS over the left DLPFC in the control of CP were also recommended.
There are some limitations to this study. First, we did not perform a meta-analysis. Instead, we used guidelines to classify the evidence of studies. As yet, the number of studies is still low. In future, when number of studies has increased, a meta-analysis may be considered as part of our work to address our research question. Second, as in previous studies, studies with at least 25 participants receiving active treatment were classified as sufficiently statistically powered, considering previous recommendations. However, future studies or revision of this study may consider power calculations, instead. Classification of studies may also consider other instruments such as the GRADE system and also the problem of publication bias.
6. Conclusions
This study supports the use of tDCS and rTMS, but not other forms of NIBS, in the treatment of patients with certain CP conditions. Also, this is one of the few recommendations to argue against the use of some NINS techniques for some specific types of CP. We have also covered, in a systematic and AGREE-compliant manner, several crucial points that are frequently overlooked such as facilitators and barriers to the implementation of the recommendations. Likewise, we reported the first effort to provide a cost-estimation analysis for the use of NIBS techniques for pain in clinical practice in LA and Caribbean region. As all guideline recommendations, time will refine the current concepts and correct potential misinterpretations present in the actual study and, thus, periodic refreshing of this work will be scheduled.
Disclosures
The authors have no conflict of interest to declare.
Acknowledgments
The authors thank FEDELAT for its special contribution in joining this group of specialists and Mrs. Luana Bittencourt for her contribution in the final recommendations.
Part of this study was presented as a poster in the 2018 World Congress on Pain, at Boston, USA.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PR9/A35.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painrpts.com).
References
- [1].Ahmed MA, Mohamed SA, Sayed D. Long-term antalgic effects of repetitive transcranial magnetic stimulation of motor cortex and serum beta-endorphin in patients with phantom pain. Neurol Res 2011;33:953–8. [DOI] [PubMed] [Google Scholar]
- [2].Angulo J, Gonzalez R, Hernandez L, Hernandez-Ortiz A, Jaque J, Lara-Solares A, Robles San Roman M, Vacas J. Musculoskeletal chronic pain: Latin-American expert panel review based on scientific evidence [in Spanish]. Drugs Today (Barc) 2011;47(suppl C):1–31. [DOI] [PubMed] [Google Scholar]
- [3].Antal A, Kriener N, Lang N, Boros K, Paulus W. Cathodal transcranial direct current stimulation of the visual cortex in the prophylactic treatment of migraine. Cephalalgia 2011;31:820–8. [DOI] [PubMed] [Google Scholar]
- [4].Antal A, Terney D, Kuhnl S, Paulus W. Anodal transcranial direct current stimulation of the motor cortex ameliorates chronic pain and reduces short intracortical inhibition. J Pain Symptom Manage 2010;39:890–903. [DOI] [PubMed] [Google Scholar]
- [5].Araujo LQ, Macintyre CR, Vujacich C. Epidemiology and burden of herpes zoster and post-herpetic neuralgia in Australia, Asia and South America. Herpes 2007;14(suppl 2):40–4. [PubMed] [Google Scholar]
- [6].Attal N, Ayache SS, Ciampi De Andrade D, Mhalla A, Baudic S, Jazat F, Ahdab R, Neves DO, Sorel M, Lefaucheur JP, Bouhassira D. Repetitive transcranial magnetic stimulation and transcranial direct-current stimulation in neuropathic pain due to radiculopathy: a randomized sham-controlled comparative study. PAIN 2016;157:1224–31. [DOI] [PubMed] [Google Scholar]
- [7].Auvichayapat P, Janyacharoen T, Rotenberg A, Tiamkao S, Krisanaprakornkit T, Sinawat S, Punjaruk W, Thinkhamrop B, Auvichayapat N. Migraine prophylaxis by anodal transcranial direct current stimulation, a randomized, placebo-controlled trial. J Med Assoc Thai 2012;95:1003–12. [PubMed] [Google Scholar]
- [8].Ayache SS, Palm U, Chalah MA, Al-Ani T, Brignol A, Abdellaoui M, Dimitri D, Sorel M, Creange A, Lefaucheur JP. Prefrontal tDCS decreases pain in patients with multiple sclerosis. Front Neurosci 2016;10:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Baliki MN, Baria AT, Apkarian AV. The cortical rhythms of chronic back pain. J Neurosci 2011;31:13981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Baliki MN, Chialvo DR, Geha PY, Levy RM, Harden RN, Parrish TB, Apkarian AV. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci 2006;26:12165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Baliki MN, Schnitzer TJ, Bauer WR, Apkarian AV. Brain morphological signatures for chronic pain. PLoS One 2011;6:e26010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol 2010;9:807–19. [DOI] [PubMed] [Google Scholar]
- [13].Baudic S, Attal N, Mhalla A, Ciampi de Andrade D, Perrot S, Bouhassira D. Unilateral repetitive transcranial magnetic stimulation of the motor cortex does not affect cognition in patients with fibromyalgia. J Psychiatr Res 2013;47:72–7. [DOI] [PubMed] [Google Scholar]
- [14].Boulanger A, Clark AJ, Squire P, Cui E, Horbay GL. Chronic pain in Canada: have we improved our management of chronic noncancer pain? Pain Res Manag 2007;12:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Boyer L, Dousset A, Roussel P, Dossetto N, Cammilleri S, Piano V, Khalfa S, Mundler O, Donnet A, Guedj E. rTMS in fibromyalgia: a randomized trial evaluating QoL and its brain metabolic substrate. Neurology 2014;82:1231–8. [DOI] [PubMed] [Google Scholar]
- [16].Brainin M, Barnes M, Baron JC, Gilhus NE, Hughes R, Selmaj K, Waldemar G; Guideline Standards Subcommittee of the EFNS Scientific Committee. Guidance for the preparation of neurological management guidelines by EFNS scientific task forces—revised recommendations 2004. Eur J Neurol 2004;11:577–81. [DOI] [PubMed] [Google Scholar]
- [17].Brietzke AP, Rozisky JR, Dussan-Sarria JA, Deitos A, Laste G, Hoppe PF, Muller S, Torres IL, Alvares-da-Silva MR, de Amorim RF, Fregni F, Caumo W. Neuroplastic effects of transcranial direct current stimulation on painful symptoms reduction in chronic hepatitis C: a phase II randomized, double blind, sham controlled trial. Front Neurosci 2015;9:498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Brunoni AR, Lopes M, Kaptchuk TJ, Fregni F. Placebo response of non-pharmacological and pharmacological trials in major depression: a systematic review and meta-analysis. PLoS One 2009;4:e4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Brunoni AR, Schestatsky P, Lotufo PA, Bensenor IM, Fregni F. Comparison of blinding effectiveness between sham tDCS and placebo sertraline in a 6-week major depression randomized clinical trial. Clin Neurophysiol 2014;125:298–305. [DOI] [PubMed] [Google Scholar]
- [20].Brunoni AR, Valiengo L, Baccaro A, Zanao TA, de Oliveira JF, Goulart A, Boggio PS, Lotufo PA, Bensenor IM, Fregni F. The sertraline vs. electrical current therapy for treating depression clinical study: results from a factorial, randomized, controlled trial. JAMA Psychiatry 2013;70:383–91. [DOI] [PubMed] [Google Scholar]
- [21].Cruccu G, Garcia-Larrea L, Hansson P, Keindl M, Lefaucheur JP, Paulus W, Taylor R, Tronnier V, Truini A, Attal N. EAN guidelines on central neurostimulation therapy in chronic pain conditions. Eur J Neurol 2016;23:1489–99. [DOI] [PubMed] [Google Scholar]
- [22].Dall'Agnol L, Medeiros LF, Torres IL, Deitos A, Brietzke A, Laste G, de Souza A, Vieira JL, Fregni F, Caumo W. Repetitive transcranial magnetic stimulation increases the corticospinal inhibition and the brain-derived neurotrophic factor in chronic myofascial pain syndrome: an explanatory double-blinded, randomized, sham-controlled trial. J Pain 2014;15:845–55. [DOI] [PubMed] [Google Scholar]
- [23].de Oliveira RA, de Andrade DC, Mendonca M, Barros R, Luvisoto T, Myczkowski ML, Marcolin MA, Teixeira MJ. Repetitive transcranial magnetic stimulation of the left premotor/dorsolateral prefrontal cortex does not have analgesic effect on central poststroke pain. J Pain 2014;15:1271–81. [DOI] [PubMed] [Google Scholar]
- [24].Donnell A, D Nascimento T, Lawrence M, Gupta V, Zieba T, Truong DQ, Bikson M, Datta A, Bellile E, DaSilva AF. High-definition and non-invasive brain modulation of pain and motor dysfunction in chronic TMD. Brain Stimul 2015;8:1085–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Doth AH, Hansson PT, Jensen MP, Taylor RS. The burden of neuropathic pain: a systematic review and meta-analysis of health utilities. PAIN 2010;149:338–44. [DOI] [PubMed] [Google Scholar]
- [26].Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J; IMMPACT. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. PAIN 2005;113:9–19. [DOI] [PubMed] [Google Scholar]
- [27].Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, Haythornthwaite JA, Jensen MP, Kerns RD, Ader DN, Brandenburg N, Burke LB, Cella D, Chandler J, Cowan P, Dimitrova R, Dionne R, Hertz S, Jadad AR, Katz NP, Kehlet H, Kramer LD, Manning DC, McCormick C, McDermott MP, McQuay HJ, Patel S, Porter L, Quessy S, Rappaport BA, Rauschkolb C, Revicki DA, Rothman M, Schmader KE, Stacey BR, Stauffer JW, von Stein T, White RE, Witter J, Zavisic S. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 2008;9:105–21. [DOI] [PubMed] [Google Scholar]
- [28].Dy SM, Bennett WL, Sharma R, Zhang A, Waldfogel JM, Nesbit SA, Yeh HC, Chelladurai Y, Feldman D, Wilson LM, Robinson KA. Preventing complications and treating symptoms of diabetic peripheral neuropathy. Rockville, MD: Agency for Healthcare Research and Quality (US), 2017. Report No.: 17-EHC005-EF.AHRQ Comparative Effectiveness Reviews. [PubMed] [Google Scholar]
- [29].Fagerlund AJ, Hansen OA, Aslaksen PM. Transcranial direct current stimulation as a treatment for patients with fibromyalgia: a randomized controlled trial. PAIN 2015;156:62–71. [DOI] [PubMed] [Google Scholar]
- [30].Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpaa M, Hansson P, Jensen TS, Kamerman PR, Lund K, Moore A, Raja SN, Rice AS, Rowbotham M, Sena E, Siddall P, Smith BH, Wallace M. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 2015;14:162–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Flor H. Cortical reorganisation and chronic pain: implications for rehabilitation. J Rehabil Med 2003(41 suppl):66–72. [DOI] [PubMed] [Google Scholar]
- [32].Flor H. Maladaptive plasticity, memory for pain and phantom limb pain: review and suggestions for new therapies. Expert Rev Neurother 2008;8:809–18. [DOI] [PubMed] [Google Scholar]
- [33].Fregni F, Gimenes R, Valle AC, Ferreira MJ, Rocha RR, Natalle L, Bravo R, Rigonatti SP, Freedman SD, Nitsche MA, Pascual-Leone A, Boggio PS. A randomized, sham-controlled, proof of principle study of transcranial direct current stimulation for the treatment of pain in fibromyalgia. Arthritis Rheum 2006;54:3988–98. [DOI] [PubMed] [Google Scholar]
- [34].Fregni F, Nitsche MA, Loo CK, Brunoni AR, Marangolo P, Leite J, Carvalho S, Bolognini N, Caumo W, Paik NJ, Simis M, Ueda K, Ekhitari H, Luu P, Tucker DM, Tyler WJ, Brunelin J, Datta A, Juan CH, Venkatasubramanian G, Boggio PS, Bikson M. Regulatory considerations for the clinical and research use of transcranial direct current stimulation (tDCS): review and recommendations from an expert panel. Clin Res Regul Aff 2015;32:22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Fricova J, Klirova M, Masopust V, Novak T, Verebova K, Rokyta R. Repetitive transcranial magnetic stimulation in the treatment of chronic orofacial pain. Physiol Res 2013;62(suppl 1):S125–S134. [DOI] [PubMed] [Google Scholar]
- [36].Gabis L, Shklar B, Baruch YK, Raz R, Gabis E, Geva D. Pain reduction using transcranial electrostimulation: a double blind “active placebo” controlled trial. J Rehabil Med 2009;41:256–61. [DOI] [PubMed] [Google Scholar]
- [37].Galhardoni R, Correia GS, Araujo H, Yeng LT, Fernandes DT, Kaziyama HH, Marcolin MA, Bouhassira D, Teixeira MJ, de Andrade DC. Repetitive transcranial magnetic stimulation in chronic pain: a review of the literature. Arch Phys Med Rehabil 2015;96(4 suppl):S156–S172. [DOI] [PubMed] [Google Scholar]
- [38].Gao F, Chu H, Li J, Yang M, Du L, Li J, Chen L, Yang D, Zhang H, Chan C. Repetitive transcranial magnetic stimulation for pain after spinal cord injury: a systematic review and meta-analysis. J Neurosurg Sci 2017;61:514–22. [DOI] [PubMed] [Google Scholar]
- [39].Garcia JB, Hernandez-Castro JJ, Nunez RG, Pazos MA, Aguirre JO, Jreige A, Delgado W, Serpentegui M, Berenguel M, Cantemir C. Prevalence of low back pain in Latin America: a systematic literature review. Pain Physician 2014;17:379–91. [PubMed] [Google Scholar]
- [40].GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1545–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hagenacker T, Bude V, Naegel S, Holle D, Katsarava Z, Diener HC, Obermann M. Patient-conducted anodal transcranial direct current stimulation of the motor cortex alleviates pain in trigeminal neuralgia. J Headache Pain 2014;15:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hazime FA, Baptista AF, de Freitas DG, Monteiro RL, Maretto RL, Hasue RH, Joao SMA. Treating low back pain with combined cerebral and peripheral electrical stimulation: a randomized, double-blind, factorial clinical trial. Eur J Pain 2017;21:1132–43. [DOI] [PubMed] [Google Scholar]
- [43].Hazime FA, da Cunha RA, Soliaman RR, Romancini ACB, Pochini AC, Ejnisman B, Baptista AF. Anodal transcranial direct current stimulation (Tdcs) increases isometric strength of shoulder rotators muscles in handball players. Int J Sports Phys Ther 2017;12:402–7. [PMC free article] [PubMed] [Google Scholar]
- [44].Hosomi K, Shimokawa T, Ikoma K, Nakamura Y, Sugiyama K, Ugawa Y, Uozumi T, Yamamoto T, Saitoh Y. Daily repetitive transcranial magnetic stimulation of primary motor cortex for neuropathic pain: a randomized, multicenter, double-blind, crossover, sham-controlled trial. PAIN 2013;154:1065–72. [DOI] [PubMed] [Google Scholar]
- [45].Hou WH, Wang TY, Kang JH. The effects of add-on non-invasive brain stimulation in fibromyalgia: a meta-analysis and meta-regression of randomized controlled trials. Rheumatology (Oxford) 2016;55:1507–17. [DOI] [PubMed] [Google Scholar]
- [46].Hubbard CS, Khan SA, Keaser ML, Mathur VA, Goyal M, Seminowicz DA. Altered brain structure and function correlate with disease severity and pain catastrophizing in migraine patients. eNeuro 2014;1:e20.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Itz CJ, Geurts JW, van Kleef M, Nelemans P. Clinical course of non-specific low back pain: a systematic review of prospective cohort studies set in primary care. Eur J Pain 2013;17:5–15. [DOI] [PubMed] [Google Scholar]
- [48].Johnson MI, Elzahaf RA, Tashani OA. The prevalence of chronic pain in developing countries. Pain Manag 2013;3:83–6. [DOI] [PubMed] [Google Scholar]
- [49].Jutzeler CR, Curt A, Kramer JL. Relationship between chronic pain and brain reorganization after deafferentation: a systematic review of functional MRI findings. Neuroimage Clin 2015;9:599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kang BS, Shin HI, Bang MS. Effect of repetitive transcranial magnetic stimulation over the hand motor cortical area on central pain after spinal cord injury. Arch Phys Med Rehabil 2009;90:1766–71. [DOI] [PubMed] [Google Scholar]
- [51].Kang EK, Baek MJ, Kim S, Paik NJ. Non-invasive cortical stimulation improves post-stroke attention decline. Restor Neurol Neurosci 2009;27:645–50. [DOI] [PubMed] [Google Scholar]
- [52].Khedr EM, Kotb H, Kamel NF, Ahmed MA, Sadek R, Rothwell JC. Long lasting antalgic effects of daily sessions of repetitive transcranial magnetic stimulation in central and peripheral neuropathic pain. J Neurol Neurosurg Psychiatry 2005;76:833–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Khedr EM, Kotb HI, Mostafa MG, Mohamad MF, Amr SA, Ahmed MA, Karim AA, Kamal SM. Repetitive transcranial magnetic stimulation in neuropathic pain secondary to malignancy: a randomized clinical trial. Eur J Pain 2015;19:519–27. [DOI] [PubMed] [Google Scholar]
- [54].Kim YJ, Ku J, Kim HJ, Im DJ, Lee HS, Han KA, Kang YJ. Randomized, sham controlled trial of transcranial direct current stimulation for painful diabetic polyneuropathy. Ann Rehabil Med 2013;37:766–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol 2003;2:145–56. [DOI] [PubMed] [Google Scholar]
- [56].Leao Ferreira KA, Bastos TR, Andrade DC, Silva AM, Appolinario JC, Teixeira MJ, Latorre MD. Prevalence of chronic pain in a metropolitan area of a developing country: a population-based study. Arq Neuropsiquiatr 2016;74:990–8. [DOI] [PubMed] [Google Scholar]
- [57].Lefaucheur JP, Andre-Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH, Cantello RM, Cincotta M, de Carvalho M, De Ridder D, Devanne H, Di Lazzaro V, Filipovic SR, Hummel FC, Jaaskelainen SK, Kimiskidis VK, Koch G, Langguth B, Nyffeler T, Oliviero A, Padberg F, Poulet E, Rossi S, Rossini PM, Rothwell JC, Schonfeldt-Lecuona C, Siebner HR, Slotema CW, Stagg CJ, Valls-Sole J, Ziemann U, Paulus W, Garcia-Larrea L. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol 2014;125:2150–206. [DOI] [PubMed] [Google Scholar]
- [58].Lefaucheur JP, Andre-Obadia N, Poulet E, Devanne H, Haffen E, Londero A, Cretin B, Leroi AM, Radtchenko A, Saba G, Thai-Van H, Litre CF, Vercueil L, Bouhassira D, Ayache SS, Farhat WH, Zouari HG, Mylius V, Nicolier M, Garcia-Larrea L. French guidelines on the use of repetitive transcranial magnetic stimulation (rTMS): safety and therapeutic indications [in French]. Neurophysiol Clin 2011;41:221–95. [DOI] [PubMed] [Google Scholar]
- [59].Lefaucheur JP, Antal A, Ahdab R, Ciampi de Andrade D, Fregni F, Khedr EM, Nitsche M, Paulus W. The use of repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS) to relieve pain. Brain Stimul 2008;1:337–44. [DOI] [PubMed] [Google Scholar]
- [60].Lefaucheur JP, Antal A, Ayache SS, Benninger DH, Brunelin J, Cogiamanian F, Cotelli M, De Ridder D, Ferrucci R, Langguth B, Marangolo P, Mylius V, Nitsche MA, Padberg F, Palm U, Poulet E, Priori A, Rossi S, Schecklmann M, Vanneste S, Ziemann U, Garcia-Larrea L, Paulus W. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol 2017;128:56–92. [DOI] [PubMed] [Google Scholar]
- [61].Lembke A. Why doctors prescribe opioids to known opioid abusers. N Engl J Med 2012;367:1580–1. [DOI] [PubMed] [Google Scholar]
- [62].Leung A, Donohue M, Xu R, Lee R, Lefaucheur JP, Khedr EM, Saitoh Y, Andre-Obadia N, Rollnik J, Wallace M, Chen R. rTMS for suppressing neuropathic pain: a meta-analysis. J Pain 2009;10:1205–16. [DOI] [PubMed] [Google Scholar]
- [63].Leung A, Metzger-Smith V, He Y, Cordero J, Ehlert B, Song D, Lin L, Shahrokh G, Tsai A, Vaninetti M, Rutledge T, Polston G, Sheu R, Lee R. Left dorsolateral prefrontal cortex rTMS in alleviating MTBI related headaches and depressive symptoms. Neuromodulation 2018;21:390–401. [DOI] [PubMed] [Google Scholar]
- [64].Leung A, Shukla S, Fallah A, Song D, Lin L, Golshan S, Tsai A, Jak A, Polston G, Lee R. Repetitive transcranial magnetic stimulation in managing mild Traumatic brain injury-related headaches. Neuromodulation 2016;19:133–41. [DOI] [PubMed] [Google Scholar]
- [65].Luedtke K, Rushton A, Wright C, Geiss B, Juergens TP, May A. Transcranial direct current stimulation for the reduction of clinical and experimentally induced pain: a systematic review and meta-analysis. Clin J Pain 2012;28:452–61. [DOI] [PubMed] [Google Scholar]
- [66].Luedtke K, Rushton A, Wright C, Jurgens T, Polzer A, Mueller G, May A. Effectiveness of transcranial direct current stimulation preceding cognitive behavioural management for chronic low back pain: sham controlled double blinded randomised controlled trial. BMJ 2015;350:h1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Ma SM, Ni JX, Li XY, Yang LQ, Guo YN, Tang YZ. High-frequency repetitive transcranial magnetic stimulation reduces pain in postherpetic neuralgia. Pain Med 2015;16:2162–70. [DOI] [PubMed] [Google Scholar]
- [68].Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther 2003;83:713–21. [PubMed] [Google Scholar]
- [69].Malavera A, Silva FA, Fregni F, Carrillo S, Garcia RG. Repetitive transcranial magnetic stimulation for phantom limb pain in land mine victims: a double-blinded, randomized, sham-controlled trial. J Pain 2016;17:911–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Marlow NM, Bonilha HS, Short EB. Efficacy of transcranial direct current stimulation and repetitive transcranial magnetic stimulation for treating fibromyalgia syndrome: a systematic review. Pain Pract 2013;13:131–45. [DOI] [PubMed] [Google Scholar]
- [71].Masse-Alarie H, Beaulieu LD, Preuss R, Schneider C. The side of chronic low back pain matters: evidence from the primary motor cortex excitability and the postural adjustments of multifidi muscles. Exp Brain Res 2017;235:647–59. [DOI] [PubMed] [Google Scholar]
- [72].Masse-Alarie H, Schneider C. Revisiting the corticomotor plasticity in low back pain: challenges and perspectives. Healthcare (Basel) 2016;4:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Medeiros LF, Caumo W, Dussan-Sarria J, Deitos A, Brietzke A, Laste G, Campos-Carraro C, de Souza A, Scarabelot VL, Cioato SG, Vercelino R, de Castro AL, Araujo AS, Bello-Klein A, Fregni F, Torres IL. Effect of deep intramuscular stimulation and transcranial magnetic stimulation on neurophysiological biomarkers in chronic myofascial pain syndrome. Pain Med 2016;17:122–35. [DOI] [PubMed] [Google Scholar]
- [74].Mehta S, McIntyre A, Guy S, Teasell RW, Loh E. Effectiveness of transcranial direct current stimulation for the management of neuropathic pain after spinal cord injury: a meta-analysis. Spinal Cord 2015;53:780–5. [DOI] [PubMed] [Google Scholar]
- [75].Mendonca ME, Simis M, Grecco LC, Battistella LR, Baptista AF, Fregni F. Transcranial direct current stimulation combined with aerobic exercise to optimize analgesic responses in fibromyalgia: a randomized placebo-controlled clinical trial. Front Hum Neurosci 2016;10:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Mhalla A, Baudic S, Ciampi de Andrade D, Gautron M, Perrot S, Teixeira MJ, Attal N, Bouhassira D. Long-term maintenance of the analgesic effects of transcranial magnetic stimulation in fibromyalgia. PAIN 2011;152:1478–85. [DOI] [PubMed] [Google Scholar]
- [77].Misra UK, Kalita J, Bhoi SK. High-rate repetitive transcranial magnetic stimulation in migraine prophylaxis: a randomized, placebo-controlled study. J Neurol 2013;260:2793–801. [DOI] [PubMed] [Google Scholar]
- [78].Morin A, Leonard G, Gougeon V, Cyr MP, Waddell G, Bureau YA, Girard I, Morin M. Efficacy of transcranial direct-current stimulation in women with provoked vestibulodynia. Am J Obstet Gynecol 2017;216:584.e1–584.e11. [DOI] [PubMed] [Google Scholar]
- [79].Muller-Dahlhaus F, Ziemann U. Metaplasticity in human cortex. Neuroscientist 2015;21:185–202. [DOI] [PubMed] [Google Scholar]
- [80].Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basanez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabe E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, De Leo D, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, Diaz-Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fevre EM, Finucane MM, Flaxman S, Flood L, Foreman K, Forouzanfar MH, Fowkes FG, Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gonzalez-Medina D, Gosselin R, Grainger R, Grant B, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo JP, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Laden F, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Levinson D, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Mabweijano J, MacIntyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill N, McGrath J, Medina-Mora ME, Meltzer M, Mensah GA, Merriman TR, Meyer AC, Miglioli V, Miller M, Miller TR, Mitchell PB, Mock C, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta L, Montico M, Moradi-Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan KM, Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O'Donnell M, O'Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Phillips MR, Pierce K, Pion S, Polanczyk GV, Polinder S, Pope CA, III, Popova S, Porrini E, Pourmalek F, Prince M, Pullan RL, Ramaiah KD, Ranganathan D, Razavi H, Regan M, Rehm JT, Rein DB, Remuzzi G, Richardson K, Rivara FP, Roberts T, Robinson C, De Leon FR, Ronfani L, Room R, Rosenfeld LC, Rushton L, Sacco RL, Saha S, Sampson U, Sanchez-Riera L, Sanman E, Schwebel DC, Scott JG, Segui-Gomez M, Shahraz S, Shepard DS, Shin H, Shivakoti R, Singh D, Singh GM, Singh JA, Singleton J, Sleet DA, Sliwa K, Smith E, Smith JL, Stapelberg NJ, Steer A, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Syed S, Tamburlini G, Tavakkoli M, Taylor HR, Taylor JA, Taylor WJ, Thomas B, Thomson WM, Thurston GD, Tleyjeh IM, Tonelli M, Towbin JA, Truelsen T, Tsilimbaris MK, Ubeda C, Undurraga EA, van der Werf MJ, van Os J, Vavilala MS, Venketasubramanian N, Wang M, Wang W, Watt K, Weatherall DJ, Weinstock MA, Weintraub R, Weisskopf MG, Weissman MM, White RA, Whiteford H, Wiebe N, Wiersma ST, Wilkinson JD, Williams HC, Williams SR, Witt E, Wolfe F, Woolf AD, Wulf S, Yeh PH, Zaidi AK, Zheng ZJ, Zonies D, Lopez AD, AlMazroa MA, Memish ZA. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2197–223. [DOI] [PubMed] [Google Scholar]
- [81].O'Connell NE, Marston L, Spencer S, DeSouza LH, Wand BM. Non-invasive brain stimulation techniques for chronic pain. Cochrane Database Syst Rev 2018;4:CD008208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].O'Connell NE, Wand BM, Marston L, Spencer S, Desouza LH. Non-invasive brain stimulation techniques for chronic pain. Cochrane Database Syst Rev 2014:CD008208. [DOI] [PubMed] [Google Scholar]
- [83].Oliveira LB, Lopes TS, Soares C, Maluf R, Goes BT, Sa KN, Baptista AF. Transcranial direct current stimulation and exercises for treatment of chronic temporomandibular disorders: a blind randomised-controlled trial. J Oral Rehabil 2015;42:723–32. [DOI] [PubMed] [Google Scholar]
- [84].Onesti E, Gabriele M, Cambieri C, Ceccanti M, Raccah R, Di Stefano G, Biasiotta A, Truini A, Zangen A, Inghilleri M. H-coil repetitive transcranial magnetic stimulation for pain relief in patients with diabetic neuropathy. Eur J Pain 2013;17:1347–56. [DOI] [PubMed] [Google Scholar]
- [85].Passard A, Attal N, Benadhira R, Brasseur L, Saba G, Sichere P, Perrot S, Januel D, Bouhassira D. Effects of unilateral repetitive transcranial magnetic stimulation of the motor cortex on chronic widespread pain in fibromyalgia. Brain 2007;130:2661–70. [DOI] [PubMed] [Google Scholar]
- [86].Perrot S, Russell IJ. More ubiquitous effects from non-pharmacologic than from pharmacologic treatments for fibromyalgia syndrome: a meta-analysis examining six core symptoms. Eur J Pain 2014;18:1067–80. [DOI] [PubMed] [Google Scholar]
- [87].Picarelli H, Teixeira MJ, de Andrade DC, Myczkowski ML, Luvisotto TB, Yeng LT, Fonoff ET, Pridmore S, Marcolin MA. Repetitive transcranial magnetic stimulation is efficacious as an add-on to pharmacological therapy in complex regional pain syndrome (CRPS) type I. J Pain 2010;11:1203–10. [DOI] [PubMed] [Google Scholar]
- [88].Queiroz LP, Silva Junior AA. The prevalence and impact of headache in Brazil. Headache 2015;55(suppl 1):32–8. [DOI] [PubMed] [Google Scholar]
- [89].Reidler JS, Mendonca ME, Santana MB, Wang X, Lenkinski R, Motta AF, Marchand S, Latif L, Fregni F. Effects of motor cortex modulation and descending inhibitory systems on pain thresholds in healthy subjects. J Pain 2012;13:450–8. [DOI] [PubMed] [Google Scholar]
- [90].Riberto M, Marcon Alfieri F, Monteiro de Benedetto Pacheco K, Dini Leite V, Nemoto Kaihami H, Fregni F, Rizzo Battistella L. Efficacy of transcranial direct current stimulation coupled with a multidisciplinary rehabilitation program for the treatment of fibromyalgia. Open Rheumatol J 2011;5:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Rossi S, Hallett M, Rossini PM, Pascual-Leone A; Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 2009;120:2008–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Sa KN, Baptista AF, Matos MA, Lessa I. Chronic pain and gender in Salvador population, Brazil. PAIN 2008;139:498–506. [DOI] [PubMed] [Google Scholar]
- [93].Sa KN, Pereira Cde M, Souza RC, Baptista AF, Lessa I. Knee pain prevalence and associated factors in a Brazilian population study. Pain Med 2011;12:394–402. [DOI] [PubMed] [Google Scholar]
- [94].Sakrajai P, Janyacharoen T, Jensen MP, Sawanyawisuth K, Auvichayapat N, Tunkamnerdthai O, Keeratitanont K, Auvichayapat P. Pain reduction in myofascial pain syndrome by anodal transcranial direct current stimulation combined with standard treatment: a randomized controlled study. Clin J Pain 2014;30:1076–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].San-Martin DL, Santos DN, Baptista AF; Pain Study Group. Pain prevalence, characteristics and associated factors in human T-cell lymphotropic virus type 1 infected patients: a systematic review of the literature. Braz J Infect Dis 2016;20:592–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Schabrun SM, Burns E, Thapa T, Hodges P. The response of the primary motor cortex to neuromodulation is altered in chronic low back pain: a preliminary study. Pain Med 2018;19:1227–36. [DOI] [PubMed] [Google Scholar]
- [97].Schreiber KL, Loggia ML, Kim J, Cahalan CM, Napadow V, Edwards RR. Painful after-sensations in fibromyalgia are linked to catastrophizing and differences in brain response in the medial temporal lobe. J Pain 2017;18:855–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Seifert F, Maihofner C. Central mechanisms of experimental and chronic neuropathic pain: findings from functional imaging studies. Cell Mol Life Sci 2009;66:375–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Seifert F, Maihofner C. Functional and structural imaging of pain-induced neuroplasticity. Curr Opin Anaesthesiol 2011;24:515–23. [DOI] [PubMed] [Google Scholar]
- [100].Seymour B. The maladaptive brain: excitable pathways to chronic pain. Brain 2012;135:316–18. [DOI] [PubMed] [Google Scholar]
- [101].Shimizu T, Hosomi K, Maruo T, Goto Y, Yokoe M, Kageyama Y, Shimokawa T, Yoshimine T, Saitoh Y. Efficacy of deep rTMS for neuropathic pain in the lower limb: a randomized, double-blind crossover trial of an H-coil and figure-8 coil. J Neurosurg 2017;127:1172–80. [DOI] [PubMed] [Google Scholar]
- [102].Shirahige L, Melo L, Nogueira F, Rocha S, Monte-Silva K. Efficacy of noninvasive brain stimulation on pain control in migraine patients: a systematic review and meta-analysis. Headache 2016;56:1565–96. [DOI] [PubMed] [Google Scholar]
- [103].Short EB, Borckardt JJ, Anderson BS, Frohman H, Beam W, Reeves ST, George MS. Ten sessions of adjunctive left prefrontal rTMS significantly reduces fibromyalgia pain: a randomized, controlled pilot study. PAIN 2011;152:2477–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Soler MD, Kumru H, Pelayo R, Vidal J, Tormos JM, Fregni F, Navarro X, Pascual-Leone A. Effectiveness of transcranial direct current stimulation and visual illusion on neuropathic pain in spinal cord injury. Brain 2010;133:2565–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Souto G, Borges IC, Goes BT, de Mendonca ME, Goncalves RG, Garcia LB, Sa KN, Coutinho MR, Galvao-Castro B, Fregni F, Baptista AF. Effects of tDCS-induced motor cortex modulation on pain in HTLV-1: a blind randomized clinical trial. Clin J Pain 2014;30:809–15. [DOI] [PubMed] [Google Scholar]
- [106].Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. Neuroscientist 2011;17:37–53. [DOI] [PubMed] [Google Scholar]
- [107].Straudi S, Buja S, Baroni A, Pavarelli C, Pranovi G, Fregni F, Basaglia N. The effects of transcranial direct current stimulation (tDCS) combined with group exercise treatment in subjects with chronic low back pain: a pilot randomized control trial. Clin Rehabil 2018;32:1348–56. [DOI] [PubMed] [Google Scholar]
- [108].To WT, James E, Ost J, Hart J, Jr, De Ridder D, Vanneste S. Differential effects of bifrontal and occipital nerve stimulation on pain and fatigue using transcranial direct current stimulation in fibromyalgia patients. J Neural Transm (Vienna) 2017;124:799–808. [DOI] [PubMed] [Google Scholar]
- [109].Tsubokawa T, Katayama Y, Yamamoto T, Hirayama T, Koyama S. Chronic motor cortex stimulation for the treatment of central pain. Acta Neurochir Suppl (Wien) 1991;52:137–9. [DOI] [PubMed] [Google Scholar]
- [110].Valle A, Roizenblatt S, Botte S, Zaghi S, Riberto M, Tufik S, Boggio PS, Fregni F. Efficacy of anodal transcranial direct current stimulation (tDCS) for the treatment of fibromyalgia: results of a randomized, sham-controlled longitudinal clinical trial. J Pain Manag 2009;2:353–61. [PMC free article] [PubMed] [Google Scholar]
- [111].van Hecke O, Austin SK, Khan RA, Smith BH, Torrance N. Neuropathic pain in the general population: a systematic review of epidemiological studies. PAIN 2014;155:654–62. [DOI] [PubMed] [Google Scholar]
- [112].Vieira AS, Baptista AF, Mendes L, Silva KS, Gois SC, Lima FM, Souza I, Sa KN. Impact of neuropathic pain at the population level. J Clin Med Res 2014;6:111–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Volz MS, Farmer A, Siegmund B. Reduction of chronic abdominal pain in patients with inflammatory bowel disease through transcranial direct current stimulation: a randomized controlled trial. PAIN 2016;157:429–37. [DOI] [PubMed] [Google Scholar]
- [114].Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basanez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabe E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, De Leo D, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, Diaz-Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fevre EM, Finucane MM, Flaxman S, Flood L, Foreman K, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gosselin R, Grainger R, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo JP, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Ma J, Mabweijano J, MacIntyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill N, McGrath J, Medina-Mora ME, Meltzer M, Mensah GA, Merriman TR, Meyer AC, Miglioli V, Miller M, Miller TR, Mitchell PB, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta L, Montico M, Moradi-Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan KM, Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O'Donnell M, O'Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Phillips MR, Pierce K, Pion S, Polanczyk GV, Polinder S, Pope CA, III, Popova S, Porrini E, Pourmalek F, Prince M, Pullan RL, Ramaiah KD, Ranganathan D, Razavi H, Regan M, Rehm JT, Rein DB, Remuzzi G, Richardson K, Rivara FP, Roberts T, Robinson C, De Leon FR, Ronfani L, Room R, Rosenfeld LC, Rushton L, Sacco RL, Saha S, Sampson U, Sanchez-Riera L, Sanman E, Schwebel DC, Scott JG, Segui-Gomez M, Shahraz S, Shepard DS, Shin H, Shivakoti R, Singh D, Singh GM, Singh JA, Singleton J, Sleet DA, Sliwa K, Smith E, Smith JL, Stapelberg NJ, Steer A, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Syed S, Tamburlini G, Tavakkoli M, Taylor HR, Taylor JA, Taylor WJ, Thomas B, Thomson WM, Thurston GD, Tleyjeh IM, Tonelli M, Towbin JA, Truelsen T, Tsilimbaris MK, Ubeda C, Undurraga EA, van der Werf MJ, van Os J, Vavilala MS, Venketasubramanian N, Wang M, Wang W, Watt K, Weatherall DJ, Weinstock MA, Weintraub R, Weisskopf MG, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams SR, Witt E, Wolfe F, Woolf AD, Wulf S, Yeh PH, Zaidi AK, Zheng ZJ, Zonies D, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2163–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Waldfogel JM, Nesbit SA, Dy SM, Sharma R, Zhang A, Wilson LM, Bennett WL, Yeh HC, Chelladurai Y, Feldman D, Robinson KA. Pharmacotherapy for diabetic peripheral neuropathy pain and quality of life: a systematic review. Neurology 2017;88:1958–67. [DOI] [PubMed] [Google Scholar]
- [116].Wrigley PJ, Gustin SM, McIndoe LN, Chakiath RJ, Henderson LA, Siddall PJ. Longstanding neuropathic pain after spinal cord injury is refractory to transcranial direct current stimulation: a randomized controlled trial. PAIN 2013;154:2178–84. [DOI] [PubMed] [Google Scholar]
- [117].Xie YF, Huo FQ, Tang JS. Cerebral cortex modulation of pain. Acta Pharmacol Sin 2009;30:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Young NA, Sharma M, Deogaonkar M. Transcranial magnetic stimulation for chronic pain. Neurosurg Clin N Am 2014;25:819–32. [DOI] [PubMed] [Google Scholar]
- [119].Zhu CE, Yu B, Zhang W, Chen WH, Qi Q, Miao Y. Effectiveness and safety of transcranial direct current stimulation in fibromyalgia: a systematic review and meta-analysis. J Rehabil Med 2017;49:2–9. [DOI] [PubMed] [Google Scholar]
- [120].Ziemann U, Siebner HR. Modifying motor learning through gating and homeostatic metaplasticity. Brain Stimul 2008;1:60–6. [DOI] [PubMed] [Google Scholar]