Supplemental Digital Content is available in the text
Keywords: Major adverse cardiac events, nicorandil, perioperative complications, periprocedural myocardial injury
Abstract
Aims:
Nicorandil, which is a mitochondrial ATP-sensitive potassium channel opener, is believed to improve perioperative myocardial injury (PMI) in patients undergoing percutaneous coronary intervention (PCI), but recent studies have shown that nicorandil treatment did not improve functional and clinical outcomes in patients with angina pectoris who underwent elective PCI. We performed a meta-analysis to investigate the protective effect of nicorandil on perioperative injury in patients with angina pectoris who underwent elective PCI.
Methods:
The Medline, EMBASE, and Cochrane databases were searched for randomized clinical trials examining the effects of nicorandil. Two investigators independently selected suitable trials, extracted data, and assessed trial quality.
Results:
Seven studies of patients undergoing elective PCI, comprising a total of 979 patients, were included in this review. The results showed that nicorandil did not reduce the levels of markers of myocardial injury (standardized mean difference [SMD] 0.31 [95%CI −0.6, 1.22] for creatine kinase-MB [CK-MB] and 1.29 [95%CI −2.18, 4.76] for troponin I [TNI]), perioperative complications (relative risk [RR] 0.91 [95%CI 0.46–1.81]), target vessel revascularization (RR 0.79 [95%CI 0.50–1.25]) or major adverse cardiac events (MACE) (RR 0.83 [95%CI 0.49–1.43]). Nicorandil did reduce the corrected TIMI frame count (SMD-0.30 [95%CI −0.52, −0.09]).
Conclusion:
Although nicorandil did not reduce the overall incidence of perioperative complications and the incidence of major adverse cardiac events (MACE) in patients with angina pectoris who underwent elective PCI, it could still improve no reflow and slow coronary flow.
1. Introduction
Periprocedural myocardial injury (PMI) is a major complication that occurs after percutaneous coronary intervention (PCI) in patients with angina, and it is also considered to be a predictor of long-term adverse clinical events.[1,2] The PMI is often caused by distal thrombosis, collateral occlusion or stenosis, coronary artery spasm, and coronary dissection. Ischemic preconditioning (IPC) is a powerful form of endogenous myocardial protection for reducing perioperative myocardial injury, that is, transient ischemia reperfusion before long-term ischemia works well to reduce the area of myocardial infarction.[3–5] This protective effect is equivalent to a “myocardial injury vaccine”, but it must be triggered before the onset of myocardial ischemia; thus, its clinical application is greatly limited.
Some drugs have been proven to prevent PCI-induced PMI, and nicorandil works through dual anti-angina mechanisms. On one hand, nitrates can improve the symptoms of angina pectoris; on the other hand, as a mitochondrial ATP-sensitive potassium (mito-KATP) channel opener, nicorandil may mimic IPC to prevent oxidative stress injury and reduce the occurrence of arrhythmia and coronary spasm.[6,7] Wu et al[8] conducted a meta-analysis that included a large number of studies on the effect of nicorandil on acute myocardial infarction (AMI) intervention. The conclusion suggests that nicorandil has a protective effect on PMI after primary PCI in AMI. However, in recent years, some clinical trials have found that the protective effect of nicorandil during elective PCI in patients with angina pectoris was not significantly different compared with the effect in the control group.[9–11] Ye et al[12] published a meta-analysis in 2017 that aimed to evaluate the effects of nicorandil on PMI in patients (regardless of whether they presented with angina pectoris) undergoing PCI, and they found that nicorandil could not reduce the incidence of PMI in most of the population. However, that meta-analysis did not include the clinical trial of Kawai et al,[13] who found that nicorandil therapy can improve the slow coronary flow (SCF) phenomenon in the process of selective PCI for patients with stable angina. In addition, the meta-analysis did not include the study of Miyoshi et al[11] due to the time of publication. Moreover, Ye et al did not provide a clear data analysis for PCI perioperative complications, target vessel revascularization (TVR) and major adverse cardiac events (MACE).
For this reason, we performed a clinical meta-analysis to evaluate the protective effects of nicorandil on PMI in patients with angina pectoris who underwent elective PCI in non Chinese population.
2. Methods
2.1. Data sources
The Medline, EMBASE, and Cochrane databases and the reference lists found in original and review articles were searched independently by 2 reviewers (Zhu, Xu) using medical subject heading terms, key words, titles, and abstracts. The search terms were “percutaneous coronary intervention,” “PCI,” “coronary angioplasty,” “coronary stenting,” paired with “nicorandil.” All historical literature was searched up until July 2018. Language is limited to English only.
2.2. Study selection
An initial eligibility screen of all retrieved titles and abstracts was conducted, and original studies were included in our meta-analysis if they met the following criteria: First, participants were human subjects. Second, participants were patients with angina pectoris and underwent elective PCI. Angina pectoris was defined as typical precordial chest pain, angiographic evidence of a stenosis > 75% according to the American Heart Association (AHA) classification[14] and no increase in serum creatine kinase (CK) activity. Third, participants were randomly assigned to receive either nicorandil or placebo. Fourth, nicorandil was 1st administered before PCI, and there was no limit to the duration of the drug delivery. Fifth, the study included sufficient data on myocardial injury biomarkers (data at baseline and at the end of the study and/or data on change in standardized mean difference (SMD) from baseline or appropriate data estimation), perioperative complications, TVR and MACE. Only fully published trials were included (abstracts and congress presentations were not included). The primary outcome is the occurrence of PMI, including 2 subgroups: myocardial injury biomarkers, including creatine kinase-MB (CK-MB) and troponin I (TNI); and perioperative complications, including no reflow (defined as an acute reduction in flow (at least 1 grade, as defined by the TIMI trial) in the absence of mechanical obstruction) or SCF (defined as corrected TIMI frame count [cTFC] > 20 frames in the absence of mechanical obstruction), thrombolysis in myocardial infarction (TIMI) flow < 3, acute thrombosis, coronary dissection, coronary artery spasm, collateral stenosis or occlusion, and ventricular arrhythmias requiring cardioversion. Secondary endpoints were cTFC, TVR, and MACE during follow-up, including all causes of death, cardiac death, acute coronary syndrome (ACS), heart failure, and cerebrovascular accident. Two investigators (Zhu, Xu) independently reviewed all full-text articles that potentially met the inclusion criteria according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.[15] In cases of disagreement, a consensus was obtained by discussion with the 3rd author (Chen or Huang).
The exclusion criteria were as follows: First, non-randomized controlled trial (RCT); Second, patients with AMI and/or experienced primary PCI; Third, articles with incomplete or erroneous data; and Fourth, included participants were not clearly identified as having angina pectoris.
2.3. Data extraction
The methods of data extraction referred to previous studies.[16,17] All selected papers were reviewed by 2 reviewers, who independently extracted data to a data sheet. Data extraction included year of publication, study design, sample size, patient characteristics, inclusion and exclusion criteria, control and intervention protocols, randomization, blinding, and follow-up, as well as the outcome parameters described above. With respect to biomarker data, we used the peak ratio from its baseline value as reported in the paper. The SMD was used for analysis as detection times and unit differed. Where data were presented in a graph but not in the text, we request the data from the corresponding author of the paper. If the data were not provided, we extrapolated them from the graph using a charting digital tool (GetData Graph Digitizer, http://getdata-graph-digitizer.com). Following the extraction of relevant data by the 2 authors, data were examined for possible inconsistencies which were then resolved by discussion, and if consensus could not be reached, a 3rd author was consulted. Studies were not conducted directly on humans and ethical approval was therefore not necessary.
2.4. Quality assessment
Two authors used the 7 domains of the Cochrane risk of bias tool to evaluate the quality of the included studies, using the following criteria: randomization sequence generation, concealment of randomization sequence, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. Studies were classified as having low risk, high risk, or unclear risk of bias for each item, as suggested in the Cochrane Handbook.[18]
2.5. Statistical analysis
The verified data were analyzed using Stata software (version 13.0; Stata Corporation, College Station, TX) and REVMAN software (version 5.2; Cochrane Collaboration, Oxford, UK). One investigator entered the data and another investigator verified data entry. The relative risk (RR) and SMD, and their corresponding 95% confidence intervals (CI), were calculated for dichotomous or continuous outcome data, respectively. A fixed effects model was used to analyze data with values higher than 0.10 by heterogeneity testing (χ2-based Q-test), while a random effects model was used when values were less than 0.10. DerSimonian-Laird (D+L) method was used in random effects model, and inverse variance (I-V) method and Mantel-Haenszel (M-H) method were used in fixed effects model. The magnitude of heterogeneity was assessed by I2 test (I2 = 0–25%, no heterogeneity; I2 = 25–50%, moderate heterogeneity; I2 = 50–75%, large heterogeneity; I2 = 75–100%, extreme heterogeneity). An intervention was assumed to have had a significant if the 95% CI did not include the value 1 for RR or 0 for SMD. Sensitivity analysis was performed for large heterogeneous results, and it was completed by the method of changing the effect model and the method of investigating the influence of a single study. In the analysis for small-study effects, publication bias was assessed using funnel plot techniques and Harbord’ s test.[19]
3. Results
3.1. Literature search
The literature search identified 96 records of clinical trials in Medline, 196 records in EMBASE, and 93 records in the Cochrane databases (Fig. 1). After checking for duplicates, 196 unique references remained, and 174 of them were excluded for reasons such as patients were included in AMI and/or experienced emergency PCI or non-RCT; the remaining 22 full texts underwent further evaluation. Among these, 10 articles were excluded because they did not provide the necessary data for our meta-analysis: 1 article[20] was excluded because the standard deviation (SD) of its myocardial injury biomarkers could not be obtained, 2 articles[21,22] were excluded because there was no placebo group, and 2 articles[23,24] were excluded because participants were not clearly identified as having angina pectoris.
Figure 1.

Flow diagram of study search and selection. AMI = acute myocardial infarction, PCI = percutaneous coronary intervention, RCT = randomized controlled trial.
3.2. Nicorandil protocols
3.2.1. Nicorandil administration method
Nicorandil was administered by intravenous injection in 3 studies;[11,13,25] Isono et al[26] reported intravenous administration combined with oral administration. In 2 studies,[9,10] intracoronary administration was carried out; in 1 study, oral administration was reported[27] (Table 1).
Table 1.
Overview of included studies.

3.2.2. Nicorandil administration time
In Miyoshi et al's research, the time of administration was at least 1 hour before PCI to 8 hours after surgery. The time of Shehata et al's administration was 1 week before surgery and 6 months after surgery. In 3 studies, nicorandil was given before PCI. Isono et al administered nicorandil between the perioperative period and 3 to 6 months after the operation, and Murakami et al administered nicorandil in the perioperative period of PCI (Table 1).
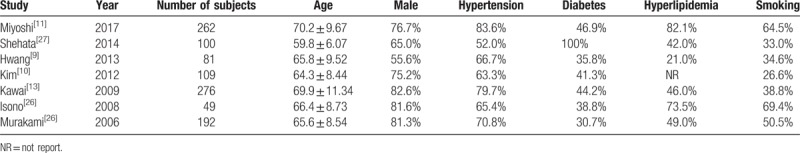
3.2.3. Patient characteristics
The mean ages of the included patients ranged from 59.2 to 79.2 years (Table 2); the percentages of males varied from 55.6% to 82.6%; and the percentages of smokers ranged from 26.6% to 69.4%. Patients with hypertension, diabetes, and hyperlipidemia, respectively, accounted for 52.0% to 83.6%, 30.7% to 100.0%, and 21.0% to 82.1%.
Table 2.
Patient characteristics.
3.2.4. Primary outcome
The primary outcome includes 2 indicators: myocardial injury biomarkers and perioperative complications. The CK-MB and TNI were not reduced by nicorandil treatment (SMD 0.31 [95%CI −0.6, 1.22] for CK-MB and 1.29 [95%CI −2.18, 4.76] for TNI) compared with the control (Fig. 2A and B), and significant statistical heterogeneity was observed (χ2 = 57.24, I2 = 94.8%, and Pheterogeneity = 0 and χ2 = 329.94, I2 = 99.1%, and Pheterogeneity = 0, respectively). The results of perioperative complications showed no difference between the nicorandil and placebo groups (RR 0.91 [95%CI 0.46–1.81]), and there was no statistical heterogeneity (χ2 = 11.82, I2 = 66%, and Pheterogeneity = .02) (Fig. 2C).
Figure 2.
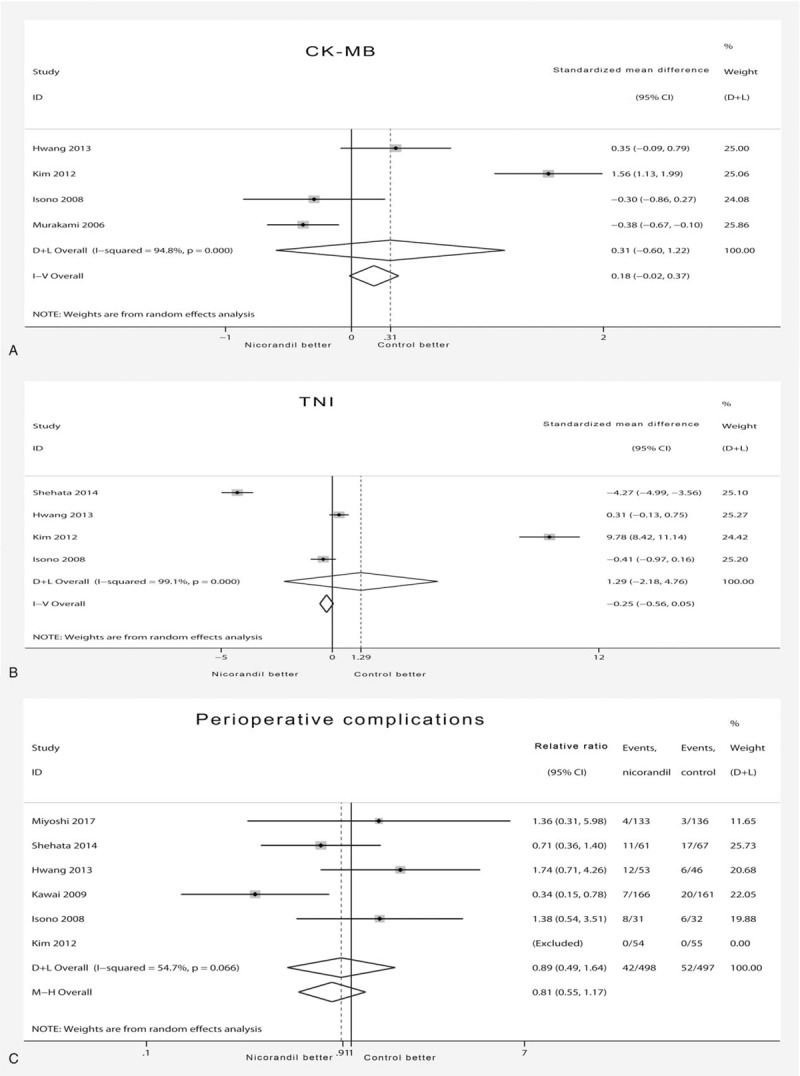
Forest plot of primary outcome. (A) Forest plot showing effects of nicorandil on change of CK-MB. A significant effect of nicorandil was assumed if the 95% CI did not include the value 0 for SMD. (B) Forest plot showing effects of nicorandil on change of TNI. A significant effect of nicorandil was assumed if the 95% CI did not include the value 0 for SMD. (C) Forest plot showing effects of nicorandil on incidence of perioperative complications. A significant effect of nicorandil was assumed if the 95% CI did not include the value 1 for RR. CI = confidence interval, CK-MB = creatine kinase-MB, D+L = DerSimonian-Laird, I-V = inverse variance, M-H = Mantel-Haenszel, RR = relative risk, TNI = troponin I, SMD = standardized mean difference.
3.2.5. Secondary outcome
Compared with TIMI blood flow classification, cTFC is a more objective index of continuous variables, so it is of great value to evaluate coronary blood flow after PCI. The CTFC was significantly decreased by nicorandil treatment (SMD −0.30 [95%CI −0.52, −0.09]) (Fig. 3A) compared with CTFC in the placebo group, and no statistical heterogeneity was found (χ2 = 0.18, I2 = 0%, and Pheterogeneity = .68). The TVR results showed no significant difference between the 2 groups (RR 0.79 [95%CI 0.50–1.25]) (Fig. 3B), with no statistical heterogeneity (χ2 = 3.51, I2 = 0%, and Pheterogeneity = .476). The results also showed that nicorandil did not significantly reduce the incidence of MACE (0.83 [95%CI 0.49–1.43]) (Fig. 3C) compared with the incidence in the placebo group, and there was no evidence for statistical heterogeneity (χ2 = 3.42, I2 = 0%, and Pheterogeneity = .490).
Figure 3.
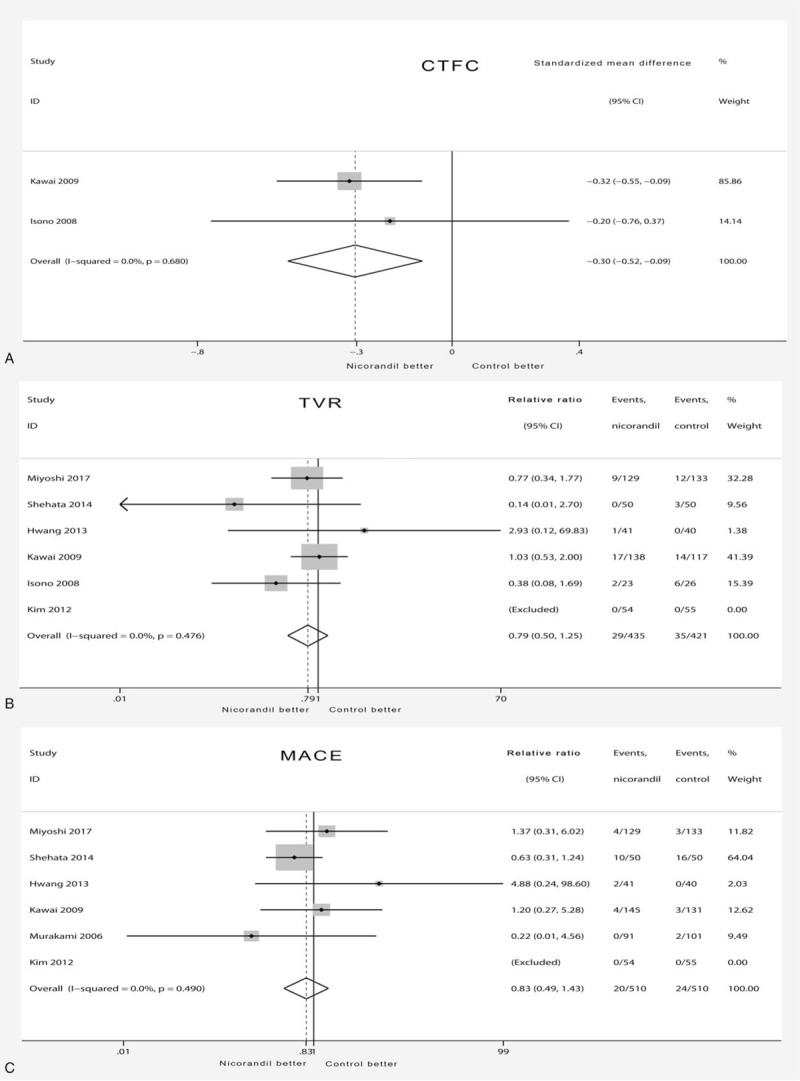
Forest plot of secondary outcome. (A) Forest plot showing effects of nicorandil on change of cTFC. A significant effect of nicorandil was assumed if the 95% CI did not include the value 0 for SMD. (B) Forest plot showing effects of nicorandil on incidence of TVR. A significant effect of nicorandil was assumed if the 95% CI did not include the value 1 for RR. (C) Forest plot showing effects of nicorandil on incidence of MACE. A significant effect of nicorandil was assumed if the 95% CI did not include the value 1 for RR. CI = confidence interval, cTFC = corrected TIMI frame count, MACE = major adverse cardiac events, RR = relative risk, SMD = standardized mean difference, TVR = target vessel revascularization.
3.2.6. Sensitivity analysis
Sensitivity analysis was performed for large heterogeneous results. Firstly, we evaluated the robustness of the outcomes of CK-MB, TNI and perioperative complications by the method of changing the effect model. The point estimation and interval of CK-MB were (SMD 0.31 [95% CI-0.60, 1.22]) by the D+L method and (0.18 [−0.02, 0.37]) by the I-V method. Similarly, the result of D+L method for TNI was (1.29 [2.18, 4.76]), while that of I-V method for TNI was (0.25 [−0.56, 0.05]). The result of D+L method for perioperative complications was (0.89 [0.49, 1.64]), while that of M-H method for perioperative complications was (0.81 [0.55, 1.17]). The results of the 2 models have no significant difference, suggesting that these results are robust. In addition, the method of investigating the influence of a single study was implemented, and sensitivity analysis charts showed good robustness of outcomes of CK-MB, TNI and perioperative complications (Supplementary Figs. 1–3).
3.2.7. Quality of studies
All studies referred to randomized groups; 2 of the studies were randomized by computers and are considered low risk[13,27] (Fig. 4); the remaining studies did not elaborate on the methods of randomization and are considered to have unclear risk. Miyoshi et al randomized participants by using a secure web site, and Kawai et al randomized participants by hiding group assignments in closed envelopes, so they are considered low risk; the remaining articles did not clarify how they randomized groups. Kawai et al conducted a double-blind study, and the blinding method was implemented through a colorless transparent bottle and solution, so its performance bias is considered to be low risk. Miyoshi et al conducted an open study, so the results are likely to be biased, and its performance bias is considered to be high risk. For the remaining studies, it is unclear whether effective blinding of participants and personnel was implemented. In the study of Kawai et al, the outcome evaluator is ignorant of the entire process of treatment allocation, so its detection bias is also considered to be low risk. Miyoshi et al conducted an open study, and the outcome evaluation is likely to be affected, so it is considered to be high risk. For the remaining articles, it is unclear whether effective blinding of outcomes assessment is implemented. There are data missing in 2 articles. In Kawail et al's research, 21 people treated with nicorandil and 23 people in the placebo group were lost to follow up, but the missing data do not affect the results, and thus, the study was defined as low risk. In Miyoshi et al's study, 17 people treated with nicorandil and 16 people in the placebo group did not complete the study, but the missing data do not affect the results, and thus, it was defined as low risk. For the remaining studies, the effects of attrition bias were unclear, and thus, they were considered to have unclear risk. No selectivity bias or other bias was found in any of the studies.
Figure 4.
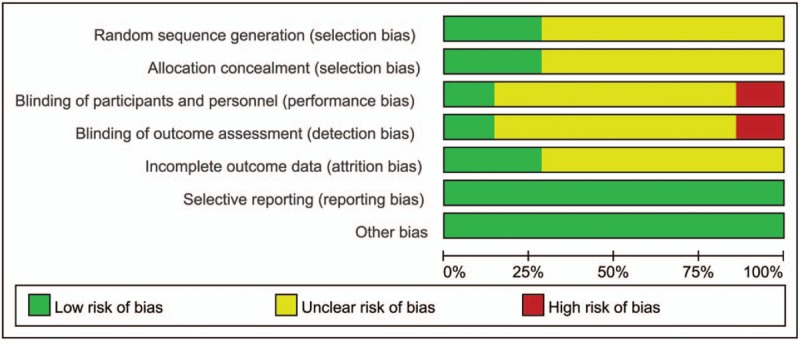
Risk of bias summary.
3.2.8. Publication bias
The funnel plot in the studies of perioperative complications was visually symmetric, and a statistical analysis of the funnel plot also suggested that no publication bias was present (Harbord's test, P = .137) (Fig. 5).
Figure 5.
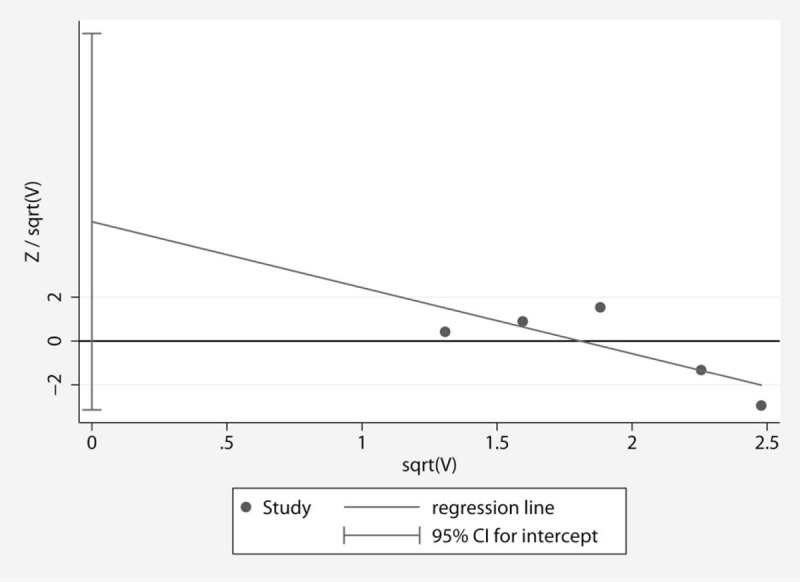
Harbord's funnel plot for evaluating the publication bias in the studies of perioperative complications.
4. Discussion
The main findings of this study were that nicorandil did not significantly improve PMI in patients with angina pectoris, including markers of myocardial injury and complications during the perioperative period of PCI. In addition, although nicorandil can reduce the cTFC compared to the placebo group, the occurrence of TVR and MACE has not been significantly improved in the nicorandil group. Myocardial injury biomarkers are relatively intuitive and simple indicators to evaluate PMI. In this meta-analysis, we included TNI and CK-MB, but these 2 results showed that nicorandil did not reduce the level of myocardial damage markers in patients with angina pectoris and undergoing elective PCI. The complications of PMI are more likely to assess the prognosis of the patients as a direct correlation with PCI than the biomarkers of myocardial injury, so it is also considered the primary outcome of this meta-analysis. However, our results suggest that perioperative complications have not been reduced with nicorandil treatment.
Compared with the TIMI flow classification, cTFC is a more objective continuous variable index, and it can be considered as a quantified indicator of no reflow and SCF. Our results indicate that it is the only index in our meta-analysis to indicate that nicorandil can improve PMI. For this purpose, we only merge the data of no reflow and slow flow phenomenon in PMI. Interestingly, nicorandil still can significantly improve the phenomenon of no reflow and slow flow (Supplementary Fig. 4). The reason that TVR and MACE are divided into 2 secondary endpoints is because they have some repeatability because MACE includes ACS, and the occurrence of ACS is likely to require TVR. The results of TVR and MACE suggest that nicorandil seem to improve perioperative myocardial injury, but there is no statistically significant difference.
Such results seem to be contrary to the conclusion of Wu et al, but the conclusion of our meta-analysis does not intend to deny the protection of nicorandil, which is the same as our starting point, that the IPC caused by recurrent angina pectoris may weaken the effect of nicorandil. The Lambiase et al[28] study of IPC caused by exercise showed that IPC had 2 time protection windows: the 1st protective window faded within 90 minutes, and the 2nd window appeared after 24 hours and could be maintained for 72 hours. Therefore, if patients suffer from angina pectoris just 4 days before PCI, the protective effect of IPC will be activated during PCI. In addition, one of the criteria for the exclusion of Matsuo et al's[29] study is the occurrence of angina 7 days before coronary angiography; the results suggest that nicorandil can improve PMI in patients undergoing percutaneous transluminal coronary angioplasty. Therefore, it is possible that the perioperative myocardial protection of nicorandil is weakened by sudden angina. In other words, angina patients undergoing selective PCI may be insensitive to the response of nicorandil, compared with AMI patients with primary PCI. However, nicorandil seems to retain a part of perioperative myocardial protection, that is, it can still reduce the occurrence of no reflow and SCF. In addition, whether the dosage of nicorandil needs to be increased in angina patients is still without any clinical evidence.
There are some limitations in this meta-analysis. First, in this analysis, in addition to patients with angina, the 2 studies[11,25] also included some patients with asymptomatic myocardial ischemia and old myocardial infarction who may have no IPC; however, in patients with angina, it is also difficult to determine if they suffered from angina pectoris just 4 days before PCI. Our results also showed that the possible existence of preconditioning has significantly affected the protective effect of nicorandil, so this limitation is not inconsistent with our conclusion. Second, the proportion of patients with diabetes is 30.7% to 100%, and oral sulfonylureas are thought to inhibit the effects of nicorandil.[30] However, the exclusion criteria for Miyoshi et al included patients taking oral sulfonylureas, and the conclusion still suggested nicorandil is ineffective. Several other studies[13,25,26] neglected to exclude patients taking oral sulfonylureas, and the final results still showed that nicorandil had a good protective effect. Therefore, we consider that the interference of oral sulfonylureas is not a key factor in the effectiveness of nicorandil.
An important limitation of most of these studies is that they had an inadequate blinding design for performance and outcome assessment, which is seemingly more difficult to achieve in RCTs than in trials investigating a pharmacological agent. In this meta-analysis, in addition to Kawai et al's research, other studies may have bias in performance and outcome evaluation.
Overall, our meta-analysis showed that the efficacy of nicorandil in patients with angina pectoris who underwent elective PCI is not as significant as that in patients with angina pectoris who underwent primary PCI, but it still has a certain myocardial protective effect, which is to reduce the occurrence of no reflow and SCF. The cause of this phenomenon may be that ischemic preconditioning weakens nicorandil's myocardial protection. The effects of these 2 protective measures are the same instead of synergism, so our results also suggest that nicorandil may share a protective pathway with ischemic preconditioning that plays the same or similar role as ischemic preconditioning. However, this study is based on a small number of subjects, and there are no clear data about the onset time of angina pectoris before PCI. Moreover, inadequate blinding designs may lead to bias in the data. Therefore, large-scale clinical trials still need to be carried out to further confirm the perioperative myocardial protective effect of nicorandil for patients with angina pectoris undergoing elective PCI.
Acknowledgments
This study was supported by Zhejiang Natural Science Foundation (LY15H020001), Science Technology Department of Zhejiang Province (2017C37130), Health and Family Planning Commission of Zhejiang Province (2016ZDB010), Hangzhou Health Science and Technology Project (2017Z10), and Hangzhou Science and Technology Bureau Project (20150733Q57, 20160533B63).
Author contributions
Designed the study: Jinyu Huang, Tielong Chen and Xiaojiang Fang. Performed the study: Houyong Zhu and Xiaoqun Xu. Analyzed the data: Houyong Zhu, Xiaoqun Xu and Jianwu Zheng. Wrote the paper: Houyong Zhu, Xiaoqun Xu. Revised the article: Jinyu Huang, Tielong Chen and Xiaojiang Fang. Houyong Zhu and Xiaoqun Xu contributed equally to this work.
Data curation: Jianwu Zheng.
Investigation: Houyong Zhu.
Methodology: Houyong Zhu, Jianwu Zheng.
Supervision: Xiaojiang Fang, Tielong Chen, Jinyu Huang.
Validation: Xiaojiang Fang, Tielong Chen, Jinyu Huang.
Writing – original draft: Houyong Zhu, Xiaoqun Xu.
Writing – review & editing: Tielong Chen, Jinyu Huang.
Houyong Zhu orcid: 0000-0001-6011-9810.
Supplementary Material
Footnotes
Abbreviations: CK-MB = creatine kinase-MB, cTFC = corrected TIMI frame count, D+L = DerSimonian-Laird, I-V = Inverse variance, MACE = major adverse cardiac events, M-H = Mantel-Haenszel, PCI = percutaneous coronary intervention, PMI = Periprocedural myocardial injury, SCF = slow coronary flow, TNI = troponin I, TVR = target vessel revascularization.
HZ and XX contributed equally to this work.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Cuculi F, Lim CC, Banning AP. Periprocedural myocardial injury during elective percutaneous coronary intervention: is it important and how can it be prevented? HEART 2010;96:736–40. [DOI] [PubMed] [Google Scholar]
- [2].Lim CC, Van Gaal WJ, Banning AP. Periprocedural myocardial injury: not a benign entity. J Am Coll Cardiol 2010;55:503–4. [DOI] [PubMed] [Google Scholar]
- [3].Giblett JP, Hoole SP. Remote ischemic conditioning in elective PCI? J Cardiovasc Pharmacol Ther 2017;22:310–5. [DOI] [PubMed] [Google Scholar]
- [4].Schmidt MR, Rasmussen ME, Bøtker HE. Remote ischemic conditioning for patients with STEMI. J Cardiovasc Pharmacol Ther 2017;22:302–9. [DOI] [PubMed] [Google Scholar]
- [5].McLeod SL, Iansavichene A, Cheskes S. Remote ischemic perconditioning to reduce reperfusion injury during acute ST-segment-elevation myocardial infarction: a systematic review and meta-analysis. J Am Heart Assoc 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Asakura M, Kitakaze M. Cardioprotection in the clinical setting-lessons from J-WIND studies. Cardiovasc Drugs Ther 2010;24:289–95. [DOI] [PubMed] [Google Scholar]
- [7].Kajimoto K, Shimamura K. Acute efficacy of combined therapy of carperitide and nicorandil for acute decompensated heart failure with left ventricular systolic dysfunction. Int J Cardiol 2011;149:e55–8. [DOI] [PubMed] [Google Scholar]
- [8].Wu M, Huang Z, Xie H, et al. Nicorandil in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention: a systematic review and meta-analysis. PLoS One 2013;8:e78231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hwang J, Lee HC, Kim BW, et al. Effect on periprocedural myocardial infarction of intra-coronary nicorandil prior to percutaneous coronary intervention in stable and unstable angina. J Cardiol 2013;62:77–81. [DOI] [PubMed] [Google Scholar]
- [10].Kim SJ, Kim W, Woo JS, et al. Effect of myocardial protection of intracoronary adenosine and nicorandil injection in patients undergoing non-urgent percutaneous coronary intervention: a randomized controlled trial. Int J Cardiol 2012;158:88–92. [DOI] [PubMed] [Google Scholar]
- [11].Miyoshi T, Ejiri K, Kohno K, et al. Effect of remote ischemia or nicorandil on myocardial injury following percutaneous coronary intervention in patients with stable coronary artery disease: a randomized controlled trial. Int J Cardiol 2017;236:36–42. [DOI] [PubMed] [Google Scholar]
- [12].Ye Z, Su Q, Li L. The clinical effect of nicorandil on perioperative myocardial protection in patients undergoing elective PCI: a systematic review and meta-analysis. Sci Rep 2017;7:45117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kawai Y, Hisamatsu K, Matsubara H, et al. Intravenous administration of nicorandil immediately before percutaneous coronary intervention can prevent slow coronary flow phenomenon. Eur Heart J 2009;30:765–72. [DOI] [PubMed] [Google Scholar]
- [14].Austen WG, Edwards JE, Frye RL, et al. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation 1975;51:5–40. [DOI] [PubMed] [Google Scholar]
- [15].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Xu X, Zhu H, Lv H. Safety of Staphylococcus aureus four-antigen and three-antigen vaccines in healthy adults: a meta-analysis of randomized controlled trials. Hum Vaccin Immunother 2018;14:314–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhu H, Xu X, Ding Y, et al. Effects of prostaglandin E1 on reperfusion injury patients: a meta-analysis of randomized controlled trials. Medicine (Baltimore) 2017;96:e6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Higgins J, Altman D, Sterne J. Chapter 8: assessing risk of bias in included studies. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (Updated March 2011): The Cochrane Collaboration. 2011. http://handbook.cochrane.org/. [Google Scholar]
- [19].Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med 2006;25:3443–57. [DOI] [PubMed] [Google Scholar]
- [20].Hirohata A, Yamamoto K, Hirose E, et al. Nicorandil prevents microvascular dysfunction resulting from PCI in patients with stable angina pectoris: a randomised study. EuroIntervention 2014;9:1050–6. [DOI] [PubMed] [Google Scholar]
- [21].Isobe N, Oshima S, Taniguchi K, et al. Nicorandil infusion leads to good recovery from ischemia of left ventricular regional work in comparison with nitroglycerin. Circ J 2002;66:943–8. [DOI] [PubMed] [Google Scholar]
- [22].Matsubara T, Minatoguchi S, Matsuo H, et al. Three minute, but not one minute, ischemia and nicorandil have a preconditioning effect in patients with coronary artery disease. J Am Coll Cardiol 2000;35:345–51. [DOI] [PubMed] [Google Scholar]
- [23].Kuwabara Y, Watanabe S, Nakaya J, et al. Postrevascularization recovery of fatty acid utilization in ischemic myocardium: a randomized clinical trial of potassium channel opener. J Nucl Cardiol 2000;7:320–7. [DOI] [PubMed] [Google Scholar]
- [24].Pang Z, Zhao W, Yao Z. Cardioprotective effects of nicorandil on coronary heart disease patients undergoing elective percutaneous coronary intervention. Med Sci Monit 2017;23:2924–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Murakami M, Iwasaki K, Kusachi S, et al. Nicorandil reduces the incidence of minor cardiac marker elevation after coronary stenting. Int J Cardiol 2006;107:48–53. [DOI] [PubMed] [Google Scholar]
- [26].Isono T, Kamihata H, Sutani Y, et al. Nicorandil suppressed myocardial injury after percutaneous coronary intervention. Int J Cardiol 2008;123:123–8. [DOI] [PubMed] [Google Scholar]
- [27].Shehata M. Cardioprotective effects of oral nicorandil use in diabetic patients undergoing elective percutaneous coronary intervention. J Interv Cardiol 2014;27:472–81. [DOI] [PubMed] [Google Scholar]
- [28].Lambiase PD, Edwards RJ, Cusack MR, et al. Exercise-induced ischemia initiates the second window of protection in humans independent of collateral recruitment. J Am Coll Cardiol 2003;41:1174–82. [DOI] [PubMed] [Google Scholar]
- [29].Matsuo H, Watanabe S, Segawa T, et al. Evidence of pharmacologic preconditioning during PTCA by intravenous pretreatment with ATP-sensitive K+ channel opener nicorandil. Eur Heart J 2003;24:1296–303. [DOI] [PubMed] [Google Scholar]
- [30].Reimann F, Ashcroft FM, Gribble FM. Structural basis for the interference between nicorandil and sulfonylurea action. Diabetes 2001;50:2253–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


