Abstract
Rationale:
Epithelial ovarian carcinoma (EOC) is the most common type of ovarian carcinoma, and the leading cause of female genital tract cancer-related deaths. However, brain metastasis (BM) of EOC is rare, with an incidence of only 1% to 2%. Ovarian clear cell carcinoma (OCCC), accounting for 5% to 25% of all EOC cases, has a poor prognosis compared with other epithelial cell type carcinomas.
Patient concerns:
We retrospectively analyzed the clinical data of a 62-year-old female, who was hospitalized with the main complaint of BM detection for 1 month. She was first diagnosed with ovarian cancer in 2004, and underwent a left oophorectomy. Three years later, the cancer metastasized to the other side, and she underwent a right oophorectomy, followed by 7 courses of platinum-based chemotherapy. She received regular follow-up, and tumor markers and pelvic imaging did not show any signs of progression until July 2012.
Diagnosis:
Combining the clinical manifestations with the results of radiological and pathological examinations, the findings were consistent with a diagnosis of BM from OCCC.
Interventions:
She received more than 20 courses of chemotherapy since July 2012. The BM was detected in 2016, and she underwent an intracranial lesion resection.
Outcomes:
Unfortunately, the patient went into a coma after the surgery, and passed away 1 month later.
Lessons:
For early detection of BM in long-term ovarian cancer, emphasis should be placed on the patient's neurological symptoms and signs as well as serum tumor marker changes. The combination of surgery, radiology, and chemotherapy may achieve long overall survival.
Keywords: brain metastasis, diagnosis, ovarian clear cell carcinoma, treatment
1. Introduction
Brain metastasis (BM) usually originates from lung cancer, breast cancer, or malignant melanoma, and rarely from ovarian cancer. In ovarian cancer, tumors usually prefer to metastasize to the pelvic and abdominal cavity, uterus, fallopian tube, lung, and liver, with the brain as a rare site accounting for only 1% to 2% of all ovarian cancers and fewer than 600 cases reported in the literature. The most common cases with BM are patients with epithelial serous carcinoma. BM has hardly been reported in ovarian clear cell carcinoma (OCCC), which has a poor prognosis compared with other epithelial cell type carcinomas. To our knowledge, only 12 cases have been specifically reported for OCCC, comprising 9 in a multicentric retrospective analysis from the Multicenter Italian Trials in Ovarian cancer group (MITO 19),[1] 2 in a Canadian retrospective analysis,[2] and 1 in a Japanese case report.[3] The present report is the 13th case of BM from OCCC. Informed consent for this case report was obtained from the patient's son.
2. Case report
The patient was a 62-year-old female who was admitted to Xiang Ya Hospital Oncology Ward in July 2016 because of BM detection for 1 month. She was first diagnosed with ovarian cancer in 2004, and underwent a left oophorectomy. Three years later, the cancer metastasized to the other side, and she underwent a right oophorectomy, followed by 7 courses of platinum-based chemotherapy. After finishing the adjuvant chemotherapy, she received regular follow-up. Her tumor markers and pelvic imaging did not show any signs of progression until July 2012, when the tumor marker CA125 began to increase, reaching 408.45 U/ml in January 2013. She restarted platinum-based chemotherapy and received more than 20 courses, but this time the tumor markers such as CA125, CA242, and CA19–9 increased repeatedly (Fig. 1). Considering the resistance to chemotherapy, she underwent a pathology consultation at Fudan University Shanghai Cancer Center, resulting in presentation of a high-grade adenocarcinoma, tending toward OCCC. Immunohistochemistry (IHC) showed EMA (+), CK7 (+), WT1 (-), Calretinin (-), PAX8 (+), P53 (+), P40 (-), Arg-1 (-), AFP (-), HNF1β(+), and ER (-) (Fig. 4). The patient had a headache, and a positron emission tomography/computed tomography (PET/CT) examination detected a brain mass on 17 May 2016 (Fig. 2). Head magnetic resonance imaging (MRI) revealed a mass of about 4.5 × 3.7 cm in the right cerebellar hemisphere (Fig. 3). An intracranial lesion resection was performed in July 2016. During the operation, the neurosurgeon found that the tumor located in the right cerebellum was about 3 × 4 × 5 cm, and involved the dura mater. The tumor had an unclear boundary, abundant blood supply, red color, and soft texture. The postoperative pathological examination revealed a low-differentiation metastatic carcinoma in the cerebellum considered to be OCCC, with IHC findings of GATA3 (+), P53 (+), PR (-), ER (-), P40 (-), CDX-2 (-), CK-Pan (+), and PAX8 (+) (Fig. 4). Unfortunately, the patient went into a coma after the surgery, and passed away 1 month later.
Figure 1.
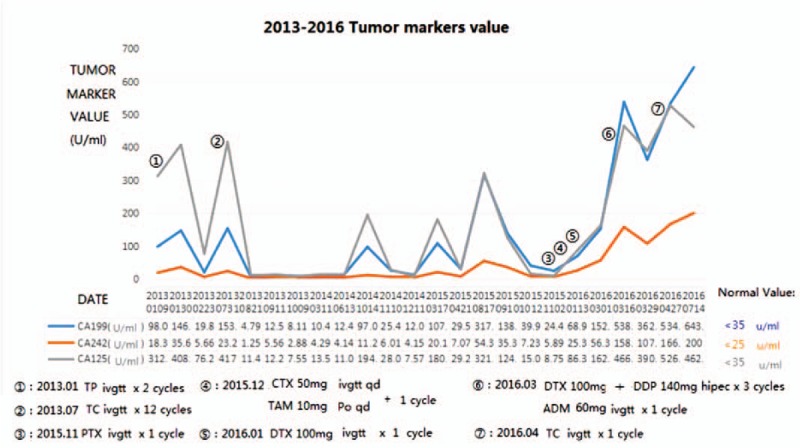
Values of tumor markers and chemotherapy regimens from 2013 to 2016.
Figure 4.
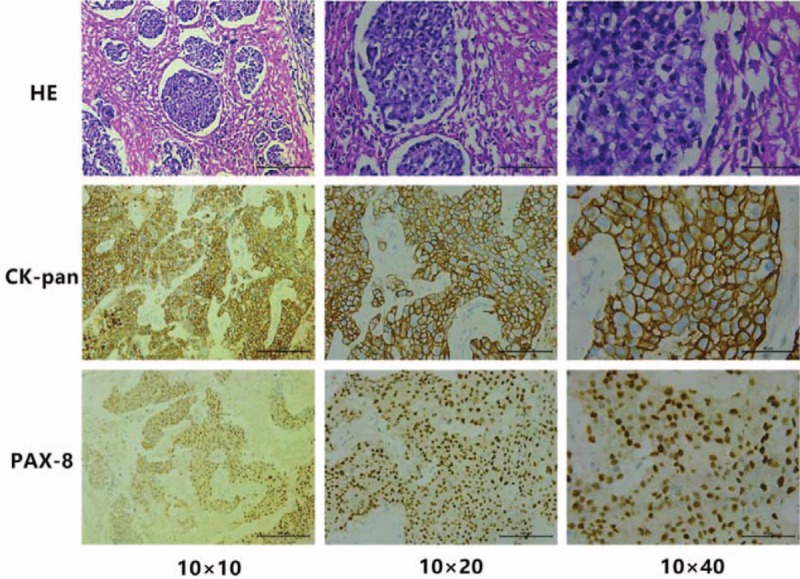
The pathological results of brain BM (HE + IHC): clear cell, CK-Pan (+), PAX-8 (+).
Figure 2.
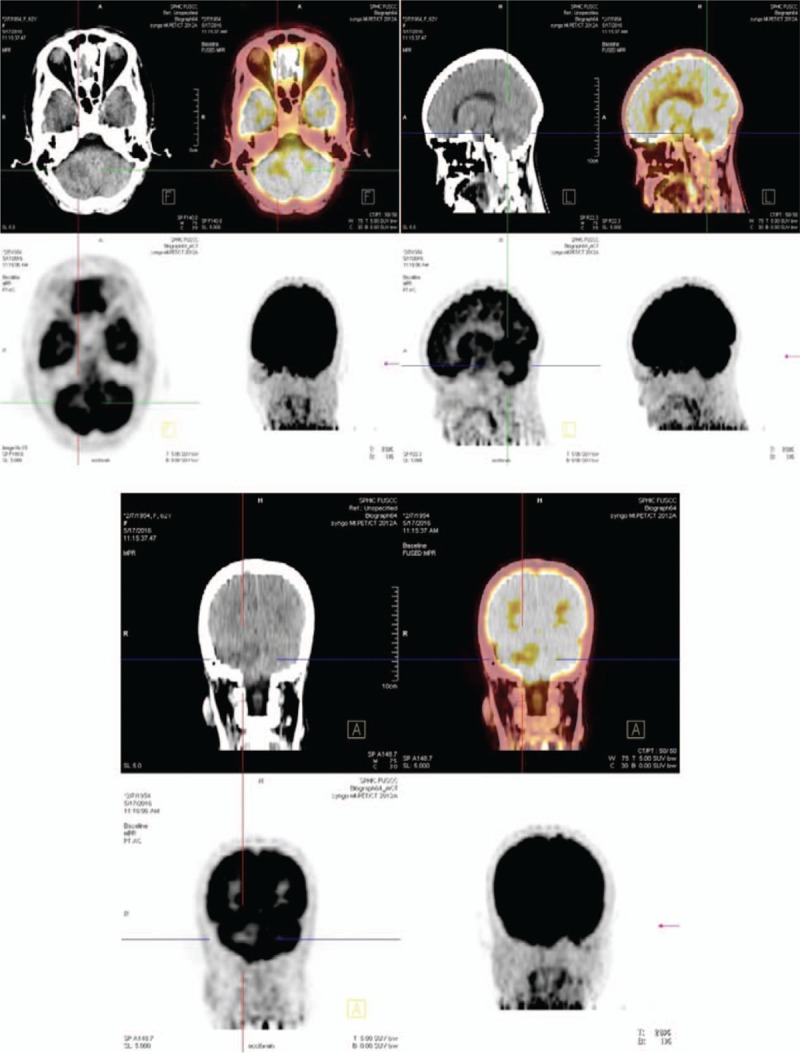
The images of PET/CT: There is a metabolic anomaly in the right cerebellar hemisphere. PET/CT = positron emission tomography/computed tomography.
Figure 3.
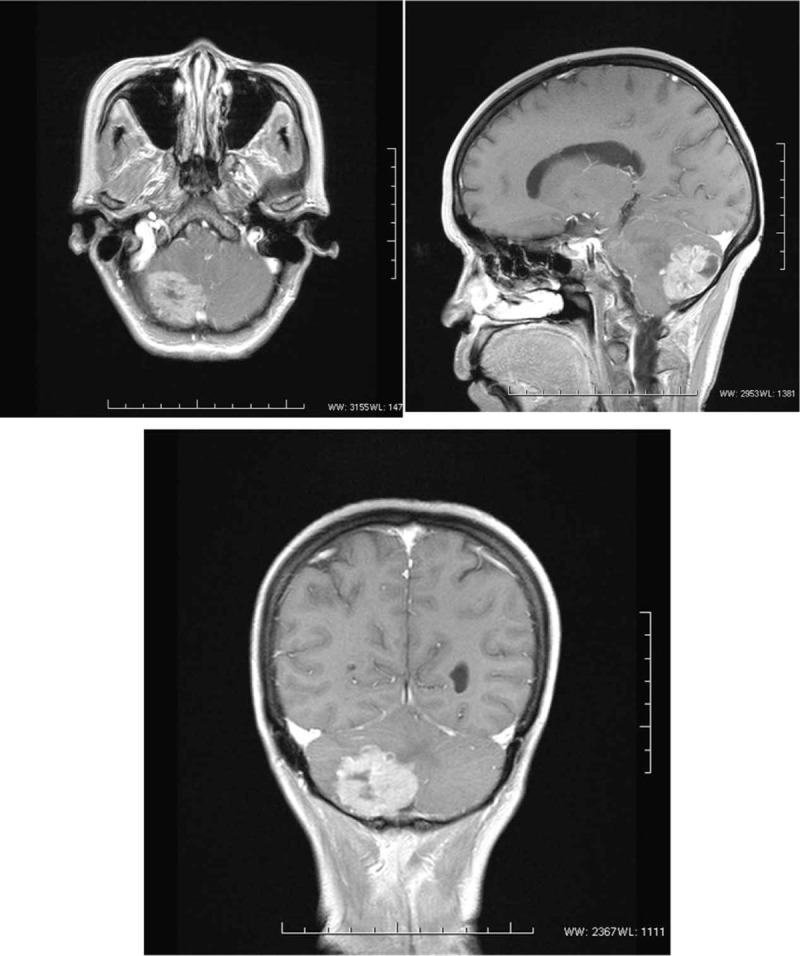
The images of MRI: In the right cerebellar hemisphere, a mass about 4.5∗3.7 cm was seen with a slightly longer T1 and slightly longer T2 signal.
3. Discussion
As a type of EOC, OCCC, which has Müller tube origin, has a worse prognosis than other EOCs through its resistance to platinum-based chemotherapy and frequent recurrence. It accounts for 5% to 25% of all EOCs, depending on geographic location and race.[4] BM is rare in ovarian cancer and has a poor prognosis. Shabnam et al[5] reported the median age of such patients was 54.3 years (range: 20–81 years). They further described that the interval from diagnosis of ovarian cancer to BM ranged from 0 to 133 months (median: 24 months) and the median survival was 8.2 months. The interval for the present patient was more than 10 years, and she passed away at 3 months after detection of BM.
3.1. Diagnosis
BM is always ignored in ovarian cancer because of its rarity, leading to delays in diagnosis and treatment. Therefore, during long-term follow-up, close attention should be paid to the patient's neurological symptoms and signs, including epilepsy, disposition change, visual disturbance, and dizziness. Whenever these manifestations occur, head MRI, PET/CT, and biopsy are recommended for early detection of BM. The present patient was diagnosed with BM by PET/CT, MRI, and biopsy, after feeling dizzy for several days.
Among BM cases in ovarian cancer, high-grade serous adenocarcinoma origin is found in the majority, while clear cell carcinoma origin is rare.[5] Considering the poor prognosis of OCCC, the differential diagnosis of BM is very important. Regarding this patient, several immunohistochemical markers could contribute to her differential diagnosis. Pax-8 is highly expressed in primary ovarian adenocarcinoma, with high sensitivity and specificity, and plays an important role in the judgement of primary ovarian adenocarcinoma and CK-pan (+) indicates epithelial source (Fig. 4). Thus, combined with her OCCC history, she was diagnosed with BM of OCCC. The following methods involving IHC markers are used for assessment of suspected BM from ovarian carcinoma: Firstly, Müllerian origin can be established using CK7/CK20, ER, and PAX8, as most ovarian carcinomas other than OCCC express CK7, ER, and PAX8, and are usually negative for CK20; Secondly, strong, diffuse nuclear staining for WT1 favors serous differentiation; Thirdly, high grade serous carcinoma usually shows overexpression of p53 (strong, diffuse nuclear staining in at least 75% of tumor cells) and confluent staining (nuclear and/or cytoplasmic) for p16; Fourthly, lack of nuclear WT1 expression suggests either OCCC or endometrioid carcinoma.
Furthermore, lack of ER staining with diffuse and strong nuclear expression of HNF-1β suggests OCCC.[6]
3.2. Monitoring
Serum tumor markers play an important role in ovarian cancer monitoring. In this case, the patient underwent examination of 12 serum tumor markers from 2013 to 2016: CA125, CA19–9, CA242, NSE, CEA, Ferritin, AFP, FPSA, TPSA, GH, CA15–3, and β-HCG. Only 3 of these markers, CA125, CA19–9, and CA242, increased repeatedly and synchronously.
CA125 is a high-molecular-weight glycoprotein expressed in the coelomic epithelium during embryonic development, and is widely used in EOC diagnosis and monitoring.[7] According to a retrospective analysis, postoperative CA125 levels of 10 to 35 U/ml in EOC patients indicates a relative risk of recurrence. When the CA125 level exceeds 10 U/ml and continuously increases, it needs to be vigilantly monitored and combined with an imaging examination (PET-CT).[8] This is exactly what happened in our case.
It was reported that CA19–9 secretion is predominantly a property of more malignant or drug-resistant cancers.[9] CA242 is associated with adenocarcinomas and e-selectin-mediated metastatic risk.
All of these tumor markers can improve the prognosis of patients with recurrence through early detection of recurrent lesions and early retreatment.
3.3. Treatment
Surgery, whole-brain radiation therapy, stereotactic radiosurgery, gamma knife surgery, chemotherapy, and various combinations of these methods can be applied for BM lesions, as previously described in the literature.[10–12]
However, the blood–brain barrier (BBB) is a huge obstacle for chemotherapy, because it prevents effective drug delivery. There are 2 broad treatment categories for brain tumors: systemic therapy and local delivery. Currently, there are 2 types of systemically administered drugs that can overcome the BBB:
-
1.
small lipophilic drugs that are not substrates for efflux transporters (or concurrent inhibition of efflux); and
-
2.
drugs that can utilize an influx transport system to gain access to the brain.
In local delivery, drugs are placed directly into the brain to achieve higher brain concentrations and avoid dose-limiting toxicities in peripheral organs. This approach includes Gliadel wafer and convection-enhanced delivery, and BBB disruption (BBBD) techniques to transiently open the BBB in either one hemisphere (osmotic BBBD) or one region (focused-ultrasound BBBD). While osmotic BBBD has shown promising results in the treatment of chemosensitive brain tumors such as primary central nervous system lymphoma, the potential clinical use of focused-ultrasound BBBD remains to be tested in large clinical trials.[13]
4. Conclusions
BM of OCCC is very rare, and has a poor prognosis. In patients with ovarian cancer, their neurological symptoms and signs deserve close attention, and regular imaging examinations and serum tumor marker tests are good choices for monitoring. Regarding treatment, the combination of surgery, radiology, and chemotherapy may achieve long overall survival. For chemotherapy, the issue of how to break through the BBB to allow effective drug delivery is a focus for research in the future.
Acknowledgments
The authors thank Alison Sherwin, PhD, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac) for editing the English text of a draft of this manuscript.
Author contributions
Conceptualization: Wei Liu.
Data curation: Wei Liu.
Formal analysis: Wei Liu.
Software: Xiangyu Xiao.
Supervision: Yuhua Feng.
Validation: Yuhua Feng.
Visualization: Xiangyu Xiao.
Writing – original draft: Ping Liu.
Writing – review & editing: Meizuo Zhong.
Footnotes
Abbreviations: BBB = blood–brain barrier, BBBD = blood-brain barrier disruption, BM = brain metastasis, EOC = epithelial ovarian carcinoma, IHC = immunohistochemistry, MITO = Multicenter Italian Trials in Ovarian cancer, MRI = magnetic resonance imaging, OCCC = ovarian clear cell carcinoma, PET/CT = positron emission tomography/computed tomography.
The authors report no conflicts of interest
References
- [1].Marchetti C, Ferrandina G, Cormio G, et al. Brain metastases in patients with EOC: clinico-pathological and prognostic factors. A multicentric retrospective analysis from the MITO group (MITO 19). Gynecol Oncol 2016;143:532–8. [DOI] [PubMed] [Google Scholar]
- [2].Nafisi H, Cesari M, Karamchandani J, et al. Metastatic ovarian carcinoma to the brain: An approach to identification and classification for neuropathologists. Neuropathology 2015;35:122–9. [DOI] [PubMed] [Google Scholar]
- [3].Takami M, Kita E, Kuwana Y, et al. A case of brain metastasis from advanced ovarian clear-cell carcinoma during maintenance chemotherapy with irinotecan+cisplatin. Gan to Kagaku Ryoho Cancer Chemother 2008;35:1243. [PubMed] [Google Scholar]
- [4].Chan JK, Teoh D, Hu JM, et al. Do clear cell ovarian carcinomas have poorer prognosis compared to other epithelial cell types? A study of 1411 clear cell ovarian cancers. Gynecol Oncol 2008;109:370–6. [DOI] [PubMed] [Google Scholar]
- [5].Pakneshan S, Safarpour D, Tavassoli F, et al. Brain metastasis from ovarian cancer: a systematic review. J Neurooncol 2014;119:1–6. [DOI] [PubMed] [Google Scholar]
- [6].Nafisi H, Cesari M, Karamchandani J, et al. Metastatic ovarian carcinoma to the brain: an approach to identification and classification for neuropathologists. Neuropathology 2015;35:122–9. [DOI] [PubMed] [Google Scholar]
- [7].Bast RC Jr, Klug TL, John ES, et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med 1983;309:883. [DOI] [PubMed] [Google Scholar]
- [8].Guo N, Peng Z. Does serum CA125 have clinical value for follow-up monitoring of postoperative patients with epithelial ovarian cancer? Results of a 12-year study. J Ovarian Res 2017;10:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Canney PA, Wilkinson PM, James RD, et al. CA19-9 as a marker for ovarian cancer: Alone and in comparison with CA125. Br J Cancer 1985;52:131–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cohen ZR, Suki D, Weinberg JS, et al. Brain metastases in patients with ovarian carcinoma: prognostic factors and outcome. J Neurooncol V 66 2004;313–25. [DOI] [PubMed] [Google Scholar]
- [11].Anupol N, Ghamande S, Odunsi K, et al. Evaluation of prognostic factors and treatment modalities in ovarian cancer patients with brain metastases. Gynecol Oncol 2002;85:487–92. [DOI] [PubMed] [Google Scholar]
- [12].Lee YK, Park NH, Kim JW, et al. Gamma-knife radiosurgery as an optimal treatment modality for brain metastases from epithelial ovarian cancer. Gynecol Oncol 2008;108:505–9. [DOI] [PubMed] [Google Scholar]
- [13].Parrish K, Sarkaria J, Elmquist W. Improving drug delivery to primary and metastatic brain tumors: Strategies to overcome the blood-brain barrier. Clin PharmacolTher 2015;97:336. [DOI] [PubMed] [Google Scholar]


