Abstract
The aim of this study was to investigate the relationship between high-mobility group box 1 (HMGB1) and colorectal cancer (CRC).
In this prospective study, patients with CRC undergoing primary surgery and healthy subjects (control group) were enrolled from July 2013 to December 2014. The serum HMGB1 concentration and HMGB1 mRNA expression were determined using enzyme-linked immunosorbent assay reverse transcription-polymerase chain reaction, respectively. Immunohistochemical analysis was performed to determine HMGB1, pERK, and c-inhibitor of apoptosis protein 2 (c-IAP2) protein expression levels in the cancer tissues.
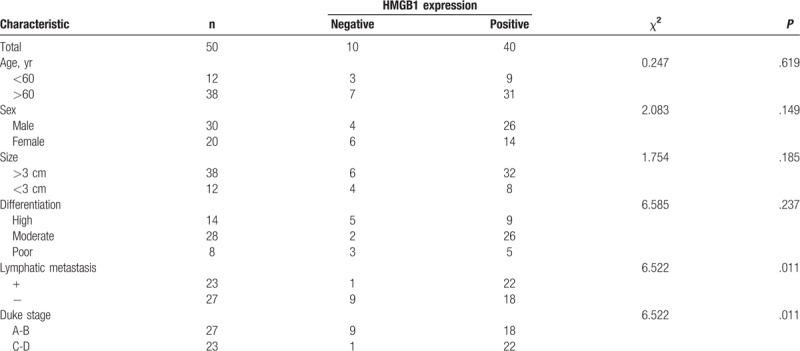
A total 144 patients with CRC and 50 healthy subjects underwent serum HMGB1 testing. Resected specimens of 50 patients were used for HMGB1 mRNA and protein expression analyses. Mean serum HMGB1 level in the patients with CRC was higher than that of the control group (8.42 μg/L vs 1.79 μg/L, P < .05). Mean serum HMGB1 level in the patients with CRC with distant metastasis was significantly higher than that of the controls (13.32 μg/L vs 7.37 μg/L, P < .05). The HMGB1 mRNA and protein expression levels in the CRC tissues were significantly higher than those in the adjacent normal mucosa. HMGB1 protein expression positively correlated with the lymph node metastasis. There were positive correlations between HMGB1 and c-IAP2 (r = 0.457, P < .05), HMGB1 and pERK (r = 0.461, P < .05), as well as pERK and c-IAP2 (r = 0.399, P < .05).
HMGB1 expression in CRC correlates with distant and lymph node metastasis. It may inhibit apoptosis by inducing activation of pERK and c-IAP2.
Keywords: c-inhibitor of apoptosis protein 2, colorectal cancer, high-mobility group box 1, pERK
1. Introduction
Colorectal cancer (CRC) is the 3rd most common type of cancer in the world, and its incidence continues to rise.[1,2] The probability of recurrence and subsequent death due to CRC is associated with its stage.[1,2] Because of its insidious onset, the diagnosis of CRC is usually delayed. However, serologic markers can be a relatively easier and cheaper alternative to colonoscopy for screening an average-risk population.[1] Several recent studies have shown that high-mobility group box 1 (HMGB1) plays a critical role in tumorigenesis, disease progression, and metastasis by activation of cancer cells and promotion of tumor angiogenesis, suggesting that HMGB1 may be useful as a new biomarker of cancer.[1–4]
Studies have shown that HMGB1 is overexpressed in various types of cancers, include CRC, and those cases with a higher expression of HMGB1 are associated with lymphatic metastasis, distant metastasis, and a poor prognosis.[5] Several reports have demonstrated that HMGB1 secreted by cancer cells may be involved in the occurrence of tumor metastasis.[6,7] In a study by Luo et al, the authors found that HMGB1 secreted by primary tumors had an apoptotic effect on Kupffer cells, thus promoting liver development.[6,7] Furthermore, some researchers have shown that increased levels of c-inhibitor of apoptosis protein 2 (c-IAP2) and pERK, the downstream effector molecules of HMGB1, are found in tumors.[8]
The current studies suggest that HMGB1 may be useful for the diagnosis and treatment of CRC.[1,3,4] However, whether HMGB1 has any role in the development of CRC metastasis is not clear. In this study, we investigated the effects of HMGB1 on CRC. In addition, the possible underlying mechanisms were examined.
2. Materials and methods
2.1. Ethics statement
The present study was approved by the Ethics Committee of Wuxi People's Hospital affiliated to Nanjing Medical University. All the patients and volunteers provided written informed consent for participation in this study.
2.2. Human CRC tissue and blood sample collection
Patients with histologically confirmed CRC on colonoscopic biopsies were enrolled from Wuxi People's Hospital affiliated to Nanjing Medical University (Wuxi, China) between July 2013 and December 2014. They will be selected according to the exclusion criteria: the patients underwent emergency operation, without preoperative colonoscope, and combined with multiple cancers or other malignant diseases. To test the serum HMGB1 levels, fresh blood samples were collected before and after surgery. These blood samples were transported to the laboratory within 30 minutes, and the serum was immediately separated. None of the patients with CRC had received any form of neoadjuvant therapy. All the tissue samples were collected just after surgical resection and were stored in liquid nitrogen until use. These tissue samples were sent to the laboratory within 30 minutes, and the samples were snap frozen upon acquisition and stored at −80°C until use. The histologic features of the specimens were evaluated by a senior pathologist.
For the control group, healthy volunteers with no evidence of CRC, precancerous colorectal tissues, or other tumors on colonoscopy received laboratory testing and follow-up examinations. The blood samples of these subjects were collected in the outpatient clinic.
2.3. Enzyme-linked immunosorbent assay testing
An HMGB1 enzyme-linked immunosorbent assay (ELISA) kit II (Shino-test, Tokyo, Japan) was used to measure the serum concentrations of HMGB1. ELISA was performed as per the manufacturer's recommendations. Purified anti-HMGB1 antibodies were coated in the wells of the microtiter strips. Samples were added to the wells, and the plate was incubated for 24 hours. After washing, a second antibody was added. After the next washing, a color solution was added, and the plate was incubated for 30 minutes. Subsequently, the HMGB1 concentration was determined by photometry using the color intensity at 450 nm and a reference wavelength of 600 to 650 nm.
2.4. Reverse transcription-polymerase chain reaction
Total RNA of the tissue samples was extracted using Trizol (Invitrogen, Carlsbad, CA), in accordance with the protocol provided by the manufacturer. The obtained RNA was 1st reversely transcribed into cDNA with the help of a RT reagent kit (Takara, Tokyo, Japan). Power SYBR Green polymerase chain reaction (PCR) master mix (Takara) and the ABI 7500 real-time PCR system (Applied Biosystems, Foster City, CA) were used to conduct real-time PCR in triplicate. All the procedures were carried out according to the manufacturer's recommendations. Briefly, the procedure included an initial denaturation cycle (10 minutes at 95°C) followed by 40 cycles of denaturation (each cycle of 15 seconds at 95°C), annealing, and elongation (each cycle of 60 seconds at 60°C). Subsequently, melting curve analysis was conducted. The relative standard curve method was used to calculate fold changes in gene expression. The primers for HMGB1 and the internal control glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were as follows: HMGB1, forward: 5′-TAACTGAATAGGGGCGTGGTCT-3′ and reverse: 5′-GAAAATGTGCTGGCTGTAGTGG-3′; GAPDH, forward: 5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse: 5′-TGGTGAAGACGCCAGTGGA-3′.
2.5. Immunohistochemical analyses
The HMGB1, c-IAP2, and pERK expression levels in the tissue samples were analyzed using immunohistochemistry. For antigen retrieval, the tissue sections were heated in a pressure cooker for 4 minutes in 0.05 M citrate buffer (pH 5.6). The sections were then incubated overnight at 4°C with primary antibody at an appropriate dilution, followed by washing and incubation with a biotinylated secondary antibody against rabbit IgG. Staining was visualized with a substrate solution containing diaminobenzidine and hydrogen peroxide. The rabbit monoclonal anti-HMGB1 antibody (1:200; Abcam, Cambridge, MA), anti-c-IAP2 antibody (1:50; Abcam), or anti-pERK (1:200; Cell Signaling Technology, Danvers, MA) was used as the primary antibody. As per the color intensity, the tissue samples were scored as 0, 1, 2, or 3 for no staining, light yellow, buffy, and brown color, respectively. Similarly, the percentage of positive cells was grouped as 0 (<5%), 1 (5–25%), 2 (26–50%), 3 (51–75%), and 4 (>76%). The final expression score was calculated by multiplying the color intensity with the percentage of positive cells. The score ranged from 0 to 12. The sum of the color intensity score and the percentage score was used to define the protein expression levels: 1 to 4, low expression, 5 to 8, moderate expression, and 9 to 12, high expression.
2.6. Statistical analysis
All the values were represented as the mean ± standard error of mean using GraphPad Prism 5 software. The Student t-test or 1-way analysis of variance was used to determine the statistical significance of continuous data. Pearson Chi-squared test was used for categorical data. The correlations were analyzed by Spearman correlation coefficients, and it is the results with P-values <.05.
3. Results
A total of 144 patients with CRC and 50 healthy subjects were recruited in this study for serum HMGB1 testing. Cancer tissues from resected specimens of 50 patients and adjacent normal mucosal tissues from 20 patients were used for HMGB1 mRNA testing and immunohistochemical studies.
3.1. Positive correlation of serum HMGB1 levels with CRC
Blood samples were collected from 144 patients with CRC and 50 healthy volunteers for testing serum HMGB1 levels (Table 1). The mean serum HMGB1 levels were significantly higher in the patients with CRC compared to the control group (8.42 ± 5.67 μg/L vs 1.79 ± 0.95 μg/L, P < .05) (Fig. 1). In addition, the preoperative serum HMGB1 concentrations were significantly higher than the postoperative values (8.42 ± 5.67 μg/L vs 1.64 ± 1.89 μg/L, P < .05). The serum HMGB1 levels in the patients with CRC with distant metastasis were significantly higher than those without metastasis (13.32 ± 6.12 μg/L vs 7.37 ± 5.17 μg/L, P < .05).
Table 1.
The correlation between the serum high-mobility group box 1 (HMGB1) level and the patients with colorectal cancer characteristics 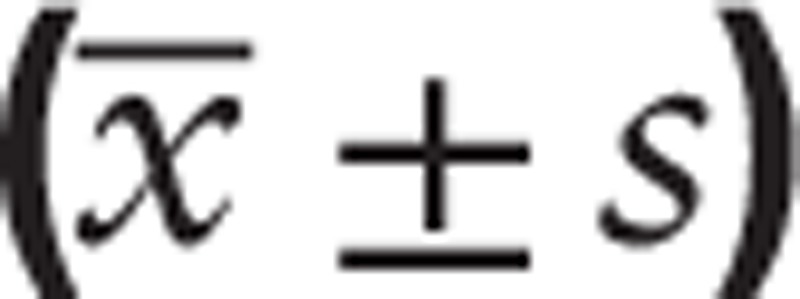 .
.
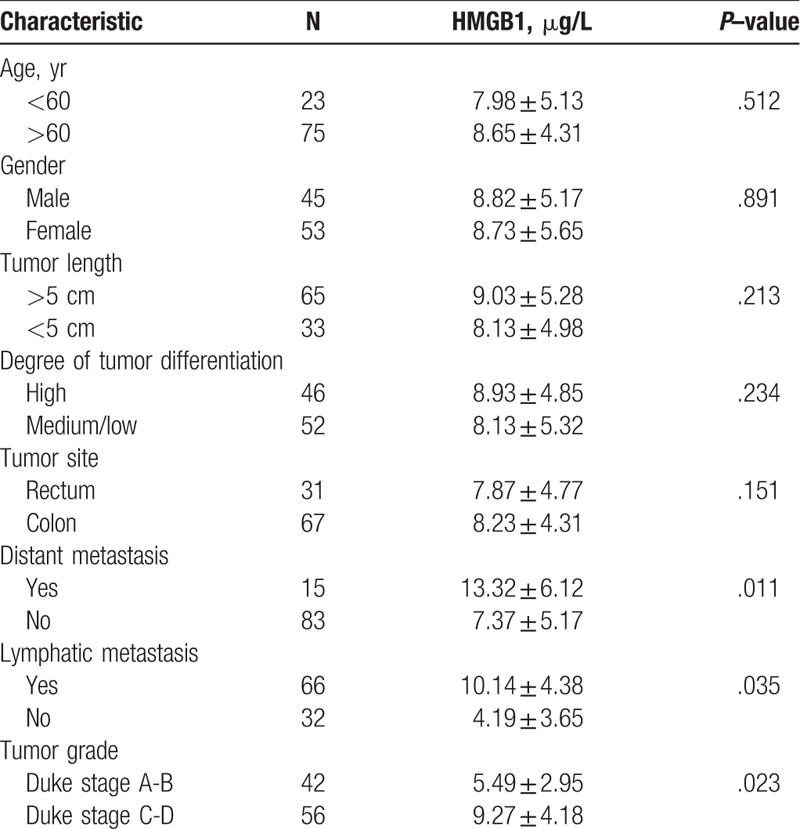
Figure 1.
Comparison of the serum high-mobility group box 1 (HMGB1) levels of patients with colorectal cancer (n = 149) with those of healthy controls (n = 50) using HMGB1 enzyme-linked immunosorbent assay (P < .05).
3.2. Positive correlation of HMGB1 mRNA levels with CRC
Real-time PCR analysis was performed in 50 tissue samples obtained after primary surgical resection of CRC. The mean HMGB1 mRNA levels were nearly 2.5-fold higher in the cancer tissues compared to the adjacent normal mucosa (P < .05) (Fig. 2A). According to the subgroup analysis of HMGB1 mRNA levels based on the stage of disease, Duke stage A-B and Duke stage C-D cancer tissues had significantly higher HMGB1 mRNA levels than the adjacent normal mucosa (P < .05), but the difference between the HMGB1 mRNA expression in Duke stage A-B and Duke stage C-D cancer tissues was insignificant (P = .061) (Fig. 2B).
Figure 2.
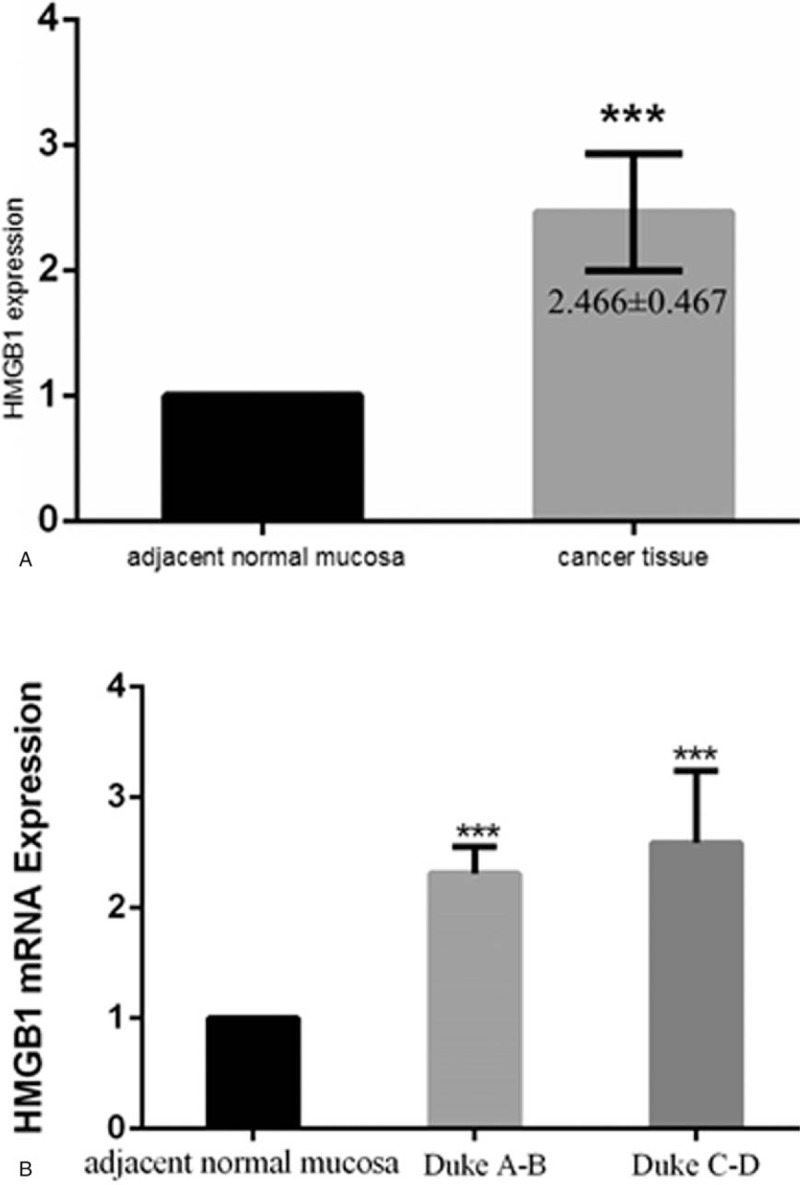
High-mobility group box 1 (HMGB1) mRNA expression in colorectal cancer. (A) HMGB1 mRNA expression was nearly 2.4-fold higher in the cancer tissues (P < .05). (B) Higher HMGB1 expression in Duke stage A-B and Duke stage C-D cancer tissues compared to the normal tissues (P < .05) was observed, but there was no significant difference between the Duke stage A-B and Dukes stage C-D groups (P > .05).
3.3. Positive correlation of HMGB1 protein expression with CRC
All the adjacent normal tissues showed negative immunohistochemical staining for HMGB1, while 80% of the cancer tissues stained positively for HMGB1 protein (χ2 = 37.33, P < .05). HMGB1 expression was statistically higher in the Duke stage C-D group than in the Duke stage A-B group (χ2 = 6.52, P < .05). HMGB1 was expressed either in the cellular cytoplasm or the nucleus. However, HMGB1 in the tumor cells was predominantly expressed in the cytoplasm. At higher magnification, numerous positive areas were actively proliferating in the cancer tissues (Fig. 3A). We also found that HMGB1 overexpression in the CRC tissues significantly correlated with the clinical stage and lymph node metastasis (P < .05) (Fig. 3B). In addition, there was no correlation between HMGB1 expression and age, sex, tumor size, or tumor differentiation degree (P > .05) (Table 2).
Figure 3.
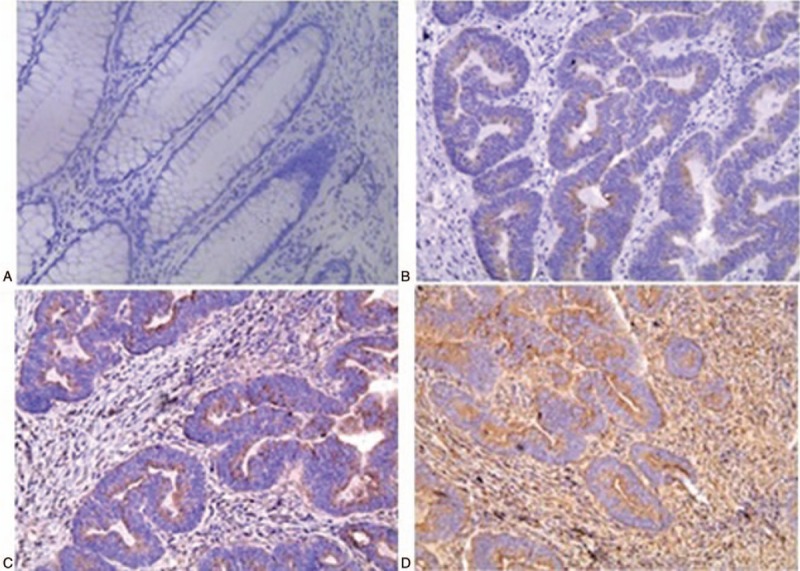
Immunohistochemical analyses showing significantly higher high-mobility group box 1 (HMGB1) protein expression in Dukes stages A, B, and C compared to normal tissue (magnification: 200× SP). (A) Normal tissue. (B–D) Tumor tissues in Dukes stages A, B, and C.
Table 2.
Association of high-mobility group box 1 (HMGB1) protein expression in colorectal cancer with clinical and pathologic features.
3.4. Positive correlation of pERK and c-IAP2 expression with CRC
Positive pERK expression was observed in 38 (76%) out of 50 cancer tissues, and weak staining for pERK was observed in the adjacent normal mucosa (Fig. 4A). pERK staining was mainly confined to the cytoplasm. Thus, positive staining for pERK was seen more frequently in cancer tissues for both Duke stage A-B and Duke stage C-D (χ2 = 33.25, P < .0001). But, there was no significant statistical difference between pERK positivity of Duke stage A-B and Duke stage C-D (χ2 = 0.1194, P = .7297).
Figure 4.
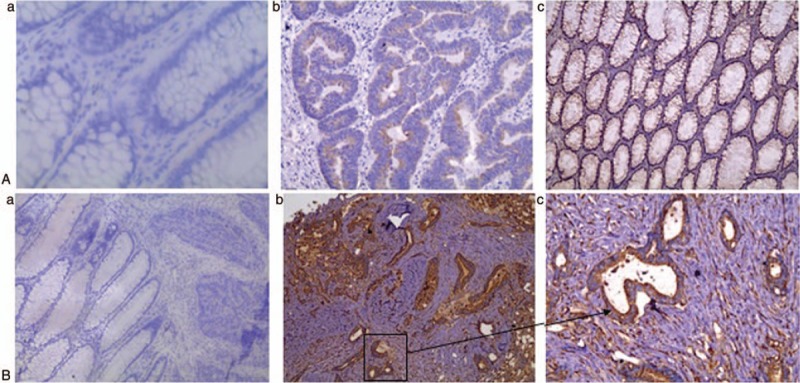
Immunohistochemical analyses of pERK and c-inhibitor of apoptosis protein 2 (c-IAP2) expression. Higher pERK (A) and c-IAP2 (B) protein expression was observed in colorectal cancer tissues, compared to the adjacent normal mucosa (magnification: a and b, 100×; c, 200× SP). (A-a and 4B-a) Normal tissue. (A-b, B-b) Tumor tissues in Dukes stage A-B. (A-c and B-c) Tumor tissues in Dukes stage C-D.
c-IAP2 positivity was observed in 45 (90%) out of 50 cancer tissues, but no positivity was found in the adjacent normal mucosa (χ2 = 50.40, P < .0001) (Fig. 4B). c-IAP2 staining was confined to the cytoplasm and was seldom observed in the membrane. The c-IAP2 expression in the adjacent normal mucosa was statistically lower than that in Duke stage A-B (χ2 = 33.36, P < .0001) and Duke stage C-D (χ2 = 39.17, P < .0001). In the pERK-negative staining group, the positivity for c-IAP2 was 10 and the negativity for c-IAP2 was 2. On using Spearman rank correlation, we found that there was a significant positive correlation between c-IAP2 and pERK, with a correlation coefficient of 0.399 (P = .0115) (Fig. 4C).
3.5. Correlation of HMGB1 expression with c-IAP2 and pERK expression
In the c-IAP2-positive staining group, negativity for HMGB1 was present in 9 samples. Only 1 sample had both HMGB1- and c-IAP2-negative staining. In the pERK-negative group, positivity for HMGB1 was observed in 7 samples, and negativity for HMGB1 was seen in 3 samples. Further correlation analysis revealed that HMGB1 had positive correlations with c-IAP2 and pERK, with correlation coefficient (R) values of 0.457 and 0.461, respectively (Fig. 5).
Figure 5.
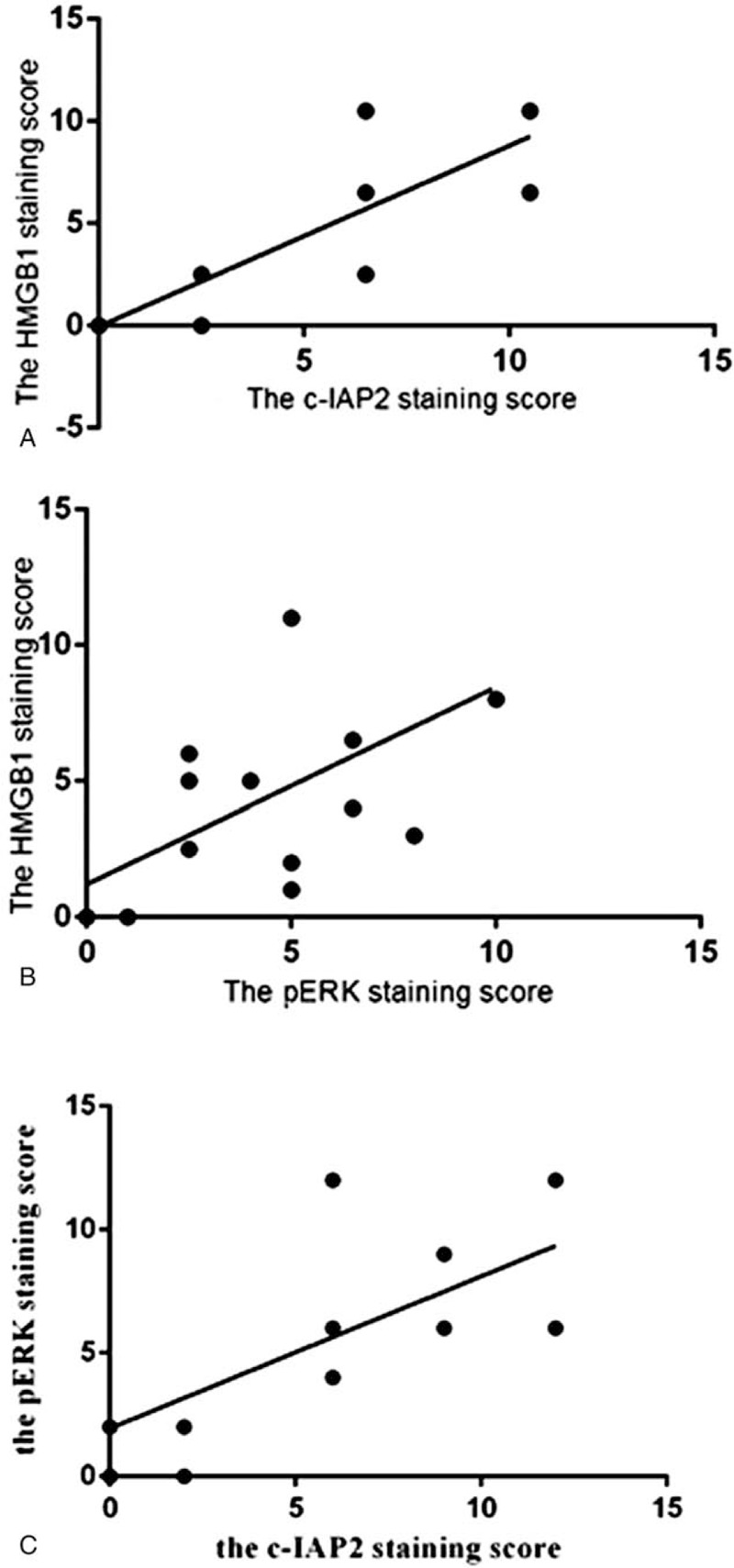
Graphical representation of the positive correlation between high-mobility group box 1 (HMGB1) and c-inhibitor of apoptosis protein 2 (c-IAP2) (R = 0.457) (A), HMGB1 and pERK (R = 0.461) (B), as well as c-IAP2 and pERK (R = 0.399) (C) in the colorectal cancer tissues.
4. Discussion
The HMGB1 has been suggested to play a role in the development of cancer metastasis, with downregulation of HMGB1 by siRNA leading to reduction of tumor migration and invasion.[9] Our study confirms that HMGB1 expression is closely related to the development of CRC, as suggested by a significantly higher serum concentration of HMGB1 in the patients with CRC as well as significantly higher HMGB1 mRNA and protein expression levels in the CRC tissues, compared to the normal mucosa.
The present study also showed that HMGB1 protein overexpression on immunohistochemical analysis significantly correlated with lymph node metastasis (P < .05). There was no significant correlation with tumor size or degree of tumor differentiation (P > .05). The HMGB1 protein expression was statistically lower in Duke stage A-B compared to Duke stage C-D (P < .05). However, there was no significant difference in HMGB1 mRNA levels between the Duke stage A-B and Duke stage C-D groups, probably due to the small sample size. For ethical reasons, we only chose 50 patients confirmed CRC after surgical resection. Adjacent normal mucosal tissues from 20 patients were randomly selected from these 50 patients. This is our limitation that we should collect both cancer tissues and adjacent normal mucosal tissues from these 50 patients, and conduct a comprehensive analysis. We will verify our result data in the next experiment.
C-IAP2, a member of the IAP family, is upregulated by the activation of nuclear factor κB (NF-κB) via HMGB1.[10,11] It is overexpressed in various cancer cells and seldom detected in normal tissues.[11] In the present study, the protein expression of both pERK and c-IAP2 was higher in cancer tissues than in the adjacent normal mucosa. But, there was no significant statistical difference between the pERK and c-IAP2 staining of Duke stage A-B and Duke stage C-D. Volp et al have studied the expression levels of HMGB1 and c-IAP2 in colon cancer and found them to be overexpressed.[12] They also demonstrated that HMGB1 inhibited apoptosis by suppressing the activity of caspase-9 and caspase-3.[12] Additionally, HMGB1 activates other signaling pathways, including the mitogen-activated protein kinase (MAPK) family involved in promoting tumor growth and metastasis.[13,14] Extracellular signal-regulated kinases 1 and 2 (ERK1/2) belong to the MAPK family.[7] Deletion of the c-IAP2 gene was determined to reduce the activity of pERK and the sensitivity enhancement of proapoptotic factors such as TNF-α.[10] Chen et al have reported that HMGB1 enhances ERK1/2 expression and promotes cancer progression.[8,15,16] Other studies also have found that extracellular HMGB1 facilitates cell migration, chemotaxis, and angiogenesis by ERK1/2 activation.[17]
In our study, using Spearman rank correlation, we demonstrated a significant positive correlation between HMGB1 and pERK, HMGB1 and c-IAP2, as well as c-IAP2 and pERK protein expression in cancer tissues. Bi et al also found the expression of HMGB1 and c-IAP2 proteins in cancer to be significantly associated with each other.[18] HMGB1 expression had a positive correlation with c-IAP2 and pERK, suggesting that HMGB1 may affect the expression of c-IAP2 via the activity of pERK. Recent research has shown that HMGB1 binds to CXCR4, which activates multiple signaling pathways, including ERK.[8,19,20] Activation of these pathways promotes overexpression of the downstream protein c-IAP2, which inhibits apoptosis.[11]
In conclusion, our results indicated that HMGB1 overexpression may trigger ERK activation, leading to the transcription of target genes such as NF-κB and overexpression of the downstream protein c-IAP2 to inhibit apoptosis. Further research is needed to understand the relationship between HMGB1, pERK, and c-IAP2, via silencing the target gene pERK or c-IAP2. We expect to find more association of HMGB1 with patients with CRC by this way to provide a more scientific reference for clinical decision-making.
Author contributions
Conceptualization: Min Xia.
Data curation: Fangmei An, Wenjia Zhang, Wenying Tian.
Formal analysis: Fangmei An.
Funding acquisition: Min Xia, Qiang Zhan.
Investigation: Wenjia Zhang, Wenying Tian, Fangmei An, Qiang Zhan.
Methodology: Min Xia, Qiang Zhan.
Project administration: Fangmei An, Wenjia Zhang.
Supervision: Fangmei An, Yang Jiao.
Validation: Wenying Tian.
Writing – original draft: Wenjia Zhang.
Writing – review & editing: Min Xia.
Footnotes
Abbreviations: CRC = colorectal cancer, ELISA = enzyme-linked immunosorbent assay, ERK1/2 = extracellular signal-regulated kinases 1 and 2, HMGB1 = high-mobility group box 1, IAP = inhibitor of apoptosis protein, PCR = polymerase chain reaction, MAPK = mitogen-activated protein kinase, NF-κB = nuclear factor κB.
WZ and FA contributed equally to this work.
This work was supported by the Wuxi Science and Technology Bureau (no: CSE01N1212 to MX), the National Natural Science Foundation of China (no: 81773227 to QZ), the Wuxi Medical Innovation Team (no: CXTD005 to QZ).
The authors have no conflicts of interest to disclose.
References
- [1].Lee H, Song M, Shin N, et al. Diagnostic significance of serum HMGB1 in colorectal carcinomas. PLoS One 2012;7:e34318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hirata Y, Kurobe H, Higashida M, et al. HMGB1 plays a critical role in vascular inflammation and lesion formation via toll-like receptor 9. Atherosclerosis 2013;231:227–33. [DOI] [PubMed] [Google Scholar]
- [3].Mou K, Liu W, Han D, et al. HMGB1/RAGE axis promotes autophagy and protects keratinocytes from ultraviolet radiation-induced cell death. J Dermatol Sci 2017;85:162–9. [DOI] [PubMed] [Google Scholar]
- [4].Pang X, Zhang Y, Zhang S. High-mobility group box 1 is overexpressed in cervical carcinoma and promotes cell invasion and migration in vitro. Oncol Rep 2017;37:831–40. [DOI] [PubMed] [Google Scholar]
- [5].Tang D, Loze MT, Zeh HJ, et al. The redox protein HMGB1 regulates cell death and survival in cancer treatment. Autophagy 2010;6:1181–3. [DOI] [PubMed] [Google Scholar]
- [6].Luo Y, Ohmori H, Fujii K, et al. HMGB1 attenuates anti-metastatic defence of the liver in colorectal cancer. Eur J Cancer 2010;46:791–9. [DOI] [PubMed] [Google Scholar]
- [7].Shen XJ, Wang HB, Ma XQ, et al. β,β-Dimethylacrylshikonin induces mitochondria dependent apoptosis through ERK pathway in human gastric cancer SGC-7901 cells. PLoS One 2012;7:e41773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chen Y, Lin C, Liu Y, et al. HMGB1 promotes HCC progression partly by downregulating p21 via ERK/c-Myc pathway and upregulating MMP-2. Tumour Biol 2016;37:4399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Xiao J, Ding Y, Huang J, et al. The association of HMGB1 gene with the prognosis of HCC. PLoS One 2014;9:e89097. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [10].Lee S, Challa-Malladi M, Bratton SB, et al. Nuclear factor-κB-inducing kinase (NIK) contains an amino-terminal inhibitor of apoptosis (IAP)-binding motif (IBM) that potentiates NIK degradation by cellular IAP1 (c-IAP1). J Biol Chem 2014;289:30680–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Varfolomeev E, Goncharov T, Vucic D. Roles of c-IAP proteins in TNF receptor family activation of NF-κB signaling. Methods Mol Biol 2015;1280:269–82. [DOI] [PubMed] [Google Scholar]
- [12].Volp K, Brezniceanu ML, Bosser S, et al. Increased expression of high mobility box l (HMGBI) is associated with an elevated level of the anti-apoptotic c-IAF2 protein inhuman colorectal carcinomas. Gut 2006;55:234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Herzog C, Lorenz A, Gillmann HJ, et al. Thrombomodulin's lectin-like domain reduces myocardial damage by interfering with HMGB1-mediated TLR2 signalling. Cardiovasc Res 2014;101:400–10. [DOI] [PubMed] [Google Scholar]
- [14].Nadatani Y, Watanabe T, Tanigawa T, et al. High-mobility group box 1 inhibits gastric ulcer healing through Toll-like receptor 4 and receptor for advanced glycation end products. PLoS One 2013;8:e80130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wang X, Xiang L, Li H, et al. The role of HMGB1 signaling pathway in the development and progression of hepatocellular carcinoma: a review. Int J Mol Sci 2015;16:22527–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Qiao J, Paul P, Lee S, et al. PI3K/AKT and ERK regulate retinoic acid-induced neuroblastoma cellular differentiation. Biochem Biophys Res Communi 2012;424:421–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Siegfried Z, Bonomi S, Ghigna C, et al. Regulation of the Ras. MAPK and P13K-roTOR signaling pathways by alter-native splicing in cancer. Int J Cell Biol 2013;2013:568931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bi MR, Zhu LY, Yan BZ, et al. Association of upregulated HMGB1 and c-IAP2 proteins with hepatocellular carcinoma development and progression. Hepat Mon 2014;14:e23552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Schiraldi M, Angela Raucci A, Muñoz LM, et al. HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with SDF-1α and signaling via CXCR4. J Exp Med 2012;209:551–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].He W, Wang Q, Xu J, et al. Attenuation of TNFSF10/TRAIL-induced apoptosis by an autophagic survival pathway involving TRAF2- and RIPK1/RIP1-mediated MAPK8/JNK activation. Autophagy 2012;8:1811–21. [DOI] [PMC free article] [PubMed] [Google Scholar]


