ABSTRACT
Cardiometabolic risk factors increase the likelihood of cardiovascular disease development by 2-fold. Lycopene, a potent lipophilic antioxidant, may be able to mediate oxidative stress, a mechanism underpinning metabolic syndrome (MetS) and its risk factors. This is, to our knowledge, the first systematic review of the literature with the purpose of investigating the relation between circulating lycopene or dietary intake of lycopene and MetS as well as its risk factors. The review was conducted using PubMed and EBSCOhost databases with the search terms “lycopene” and “metabolic syndrome.” Inclusion criteria included human studies published in English in a scholarly, peer-reviewed journal and evaluation of lycopene in relation to ≥3 of the 5 MetS risk factors as defined by the National Cholesterol Education Program's Adult Treatment Panel III (ATP III) report. The process identified 11 studies, including 8 cross-sectional and 3 intervention studies. Cross-sectional studies were grouped into 3 categories, with several studies falling into >1 category, based on results reporting associations of lycopene with the prevalence and outcomes of MetS (5 studies), presence of ATP III risk factors (4 studies), and variables mediating lycopene's influence on MetS risk (3 studies). All studies in each category reported significant protective associations. Of the 3 intervention studies, all reported significant protective effects from a lycopene-rich beverage, despite varying doses and durations of intake. Although a protective relation between lycopene and MetS was generally supported, different MetS components appeared to be influenced by lycopene rather than demonstrating consistent improvement in a single component. Thus, additional research is needed to elucidate the mechanistic effects of lycopene on MetS, as well as to determine evidence-based recommendations concerning dose-durational effects of lycopene and MetS risk reduction. In conclusion, the evidence of lycopene's benefit exists such that lycopene status or lycopene consumption may be associated with favorable alterations to the components of MetS.
Keywords: lycopene, metabolic syndrome, ATP III, cardiometabolic, phytochemicals
Introduction
Currently, cardiovascular disease (CVD) affects 36.6% of American adults and its prevalence continues to rise, with an estimated 43.9% to be affected by 2030 (1). The AHA developed goals to be accomplished by 2020 that emphasize the improvement of cardiometabolic health and reduction in related deaths. The AHA recognizes that to maximize the outcomes of this undertaking, special attention should be given to metabolic syndrome (MetS), a condition characterized by cardiometabolic risk factors that, together, increase the likelihood of CVD development by 2-fold (2). Although multiple definitions of MetS exist, the National Cholesterol Education Program's Adult Treatment Panel III (ATP III) report is the most commonly used in the assessment of MetS (3). According to the report's criteria, a diagnosis of MetS is made if 3 of the following 5 factors are present: abdominal obesity demonstrated via waist circumference >102 cm for males and >88 cm for females, TG concentrations ≥150 mg/dL, HDL-cholesterol concentrations <40 mg/dL for males and <50 mg/dL for females, blood pressure ≥130/≥85 mm Hg, or fasting blood glucose ≥110 mg/dL. In applying these criteria, ∼35% of American adults have MetS, a statistic that demonstrates the significance of this condition that is derived largely from an unhealthy lifestyle (4, 5). Multiple influences can be targeted for improvement, such as tobacco abuse, excess alcohol consumption, and sedentary lifestyles; however, prevention and management through dietary mechanisms should not be underestimated.
The exact pathogenesis of MetS remains under investigation, but oxidative stress is acknowledged as being a key process underpinning this condition (6). This takes place when there is a homeostatic imbalance—reactive oxygen species production surpasses the capacity of endogenous and exogenous neutralization mechanisms—resulting in cellular damage. In such instances, exogenous methods of restoring oxidative balance, by dietary antioxidants, are imperative.
Carotenoids are a class of lipophilic compounds, found predominantly in fruits and vegetables, that have been demonstrated to positively affect human health (7). Epidemiological observations suggest that these compounds are protective against CVD, certain cancers, and ocular diseases, among others (7, 8). Their antioxidant functionality and mediating effects on chronic disease progression have been widely researched as a collective pigment family; however, the physiological functions and benefits derived from individual carotenoid compounds are less studied. This has resulted in the attribution of positive effects to a limited number of compounds, primarily β-carotene, because this carotenoid has been the focus of most studies (9–11). Nevertheless, lycopene, a potent antioxidant within this class, has demonstrated superior singlet oxygen–quenching abilities compared with the other carotenoids, such as β-carotene, lutein, and zeaxanthin (12, 13). This heightened antioxidant efficiency warrants further research regarding its independent relation with physiological measures related to MetS. As such, this is the first systematic review of the literature, to our knowledge, with the purpose of investigating the relation between circulating lycopene or dietary intake of lycopene and MetS as well as its risk factors.
Methods
Literature search
Relevant studies were identified for potential inclusion in this systematic review by database searches utilizing PubMed and EBSCOhost. Using the Boolean search terms “lycopene” and “metabolic syndrome,” articles published in English from scholarly, peer-reviewed journals were flagged for further review. Only human studies were considered, and date of publication did not serve as an exclusion criterion. After removing duplicate articles, the titles and abstracts of the 50 identified studies were examined by the lead author and a list of articles for full-text review was compiled. The reference list of each article was also examined to identify any additional studies for inclusion that might not have appeared in the search results. This led to the identification of 3 more publications for further review.
Application of inclusion/exclusion criteria
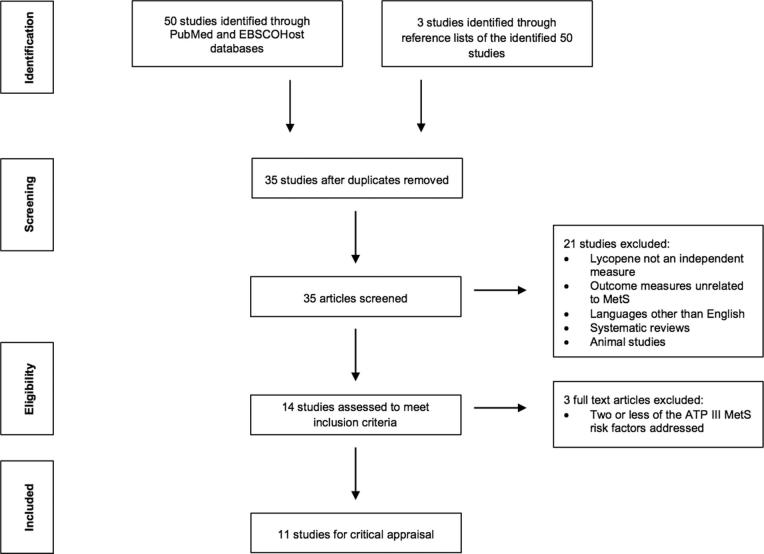
To be included in this systematic review, the studies must have evaluated lycopene and its relation to ≥3 of the 5 risk factors of MetS as defined by the ATP III criteria. Studies that utilized different criteria for assessing MetS, such as those of the International Diabetes Federation, were still included if ≥3 risk factors of the ATP III criteria were measured. Studies were omitted from this review based on the following exclusion criteria: ≤2 of the ATP III MetS components were addressed, assessment of general antioxidant status or carotenoid concentrations without lycopene as an independent measure, articles not published in peer-reviewed journals, and research presented in abstract or presentation format only. In total, the screening and eligibility process of this literature search led to the identification of 11 studies that were subjected to critical analysis (Figure 1).
FIGURE 1.
Search strategy flow diagram for research evaluating the relation between lycopene and MetS. ATP III, National Cholesterol Education Program's Adult Treatment Panel III; MetS, metabolic syndrome.
Data extraction and analysis
To assess quality and to minimize the risk of reporting bias, each author independently analyzed the final articles according to the Academy of Nutrition and Dietetics Evidence Analysis Library Worksheet (14). The checklist was originally created for evaluation of randomized controlled trials; however, this worksheet was adapted for cross-sectional analyses as well. Discussion was held among authors regarding the data extracted and differing interpretations were thoroughly considered before establishing consensus. Brief descriptions and summaries of results for the final articles included in this review are presented in Tables 1 and 2.
TABLE 1.
Cross-sectional studies assessing the relation between lycopene and MetS1
| Study (ref) | Groups | Final n | Sex, % male | Age,2 y | MetS definition | ATP III criteria assessed | Lycopene measurement |
|---|---|---|---|---|---|---|---|
| Ford et al. (15) | Divided based on MetS status | • 8466 (serum lycopene analysis)• 8554 (dietary intake analysis) | 49.6 | 40–55 | ATP III criteria (3) | • WC• TGs↑• Fasting blood glucose↓• HDL-C↓• Blood pressure | Serum lycopene concentration, NHANES III FFQ |
| Sluijs et al. (16) | Quartiles based on dietary carotenoid intake | 374 | 100.0 | 40–80 | ATP III criteria | • WC↓• TGs↓• Fasting blood glucose•HDL-C•Blood pressure | Self-administered FFQ |
| Yeo et al. (17) | Divided based on number of MetS risk factors | 299 | 100.0 | 48–50 | ATP III criteria adjusted for WC >90 cm and fasting blood glucose concentrations ≥100 mg/dL | • WC↓• TGs↓• Fasting blood glucose• HDL-C• Blood pressure | Serum lycopene concentration |
| Sugiura et al. (18) | Stratified by smoking status and MetS status | 958 | 31.6 | 30–70 | Japanese Committee for Diagnostic Criteria of Metabolic Syndrome adjusted for BMI in place of WC (19) | • TGs• Fasting blood glucose↓• HDL-C↑• Blood pressure↓• Smoking as a mediating variable such that significant relations between serum lycopene and alterations to MetS components were observed among nonsmokers. However, there was no significant correlation between any component of MetS and serum lycopene among current smokers. | Serum lycopene concentration |
| Han et al. (20) | Tertiles based on serum lycopene with mortality follow-up | 2499 | 48.4 | ≥20 | ATP III criteria | • Mortality↓3 | Serum trans-lycopene concentration |
| Liu et al. (21) | • Quartiles based on serum carotenoid concentration• Quartiles based on MetS components | 2148 | 28.0 | 50–75 | International Diabetes Federation (22) | • Prevalence↓3 | Serum lycopene concentration |
| Choi and Ainsworth (23) | Tertiles based on physical activity levels | • 1930 (MetS risk analysis)•1661 (serum lycopene analysis) | 49.1 | 40–70 | ATP III criteria | • Physical activity as a mediating variable suggesting that serum lycopene is significantly associated with an increased number of steps and a lower risk of developing MetS3 | Serum trans-lycopene concentration and total lycopene |
| Han et al. (24) | Tertiles based on serum lycopene | 13,196 | 48.0 | ≥20 | ATP III criteria | • BMI as a mediating variable such that the prevalence of MetS was significantly lower among normal-weight and overweight individuals in the higher serum lycopene tertiles compared with the lowest tertile. This relation was not corroborated for obese individuals.3 | Serum trans-lycopene concentration |
ATP III, National Cholesterol Education Program's Adult Treatment Panel III; HDL-C, HDL cholesterol; MetS, metabolic syndrome; WC, waist circumference; ↑significantly greater measure in relation to circulating lycopene status or intake; ↓significantly lower measure in relation to circulating lycopene status or intake.
Values are ranges unless not specified in the article.
This study required 3 of the 5 listed MetS components to be met for participant inclusion. However, the primary objective of this study was to evaluate either the overall prevalence of MetS, outcomes related to MetS (i.e., mortality), or variables mediating lycopene's influence on MetS risk (i.e., physical activity or obesity). As such, correlations between the individual ATP III components and serum lycopene were not reported and indicator arrows are not included for this. Instead, the primary findings of the study are reported with indicator arrows when necessary.
TABLE 2.
Intervention studies assessing the influence of lycopene on MetS1
| Study (ref) | Subjects | Intervention | Final n | Sex, % male | Age, y | MetS diagnosis | ATP III criteria assessed | Lycopene measurement |
|---|---|---|---|---|---|---|---|---|
| Tsitsimpikou et al. (25) | Pre-existing MetS diagnosis | Tomato juice supplementation 4 times/wk for 2 mo or placebo | 27 | 88.9 | 43–67 | AHA/National Heart, Lung, and Blood Institute (26) | • TGs• Fasting blood glucose• HDL-C↑ | No direct measure of lycopene in juice or circulation |
| Silveira et al. (27) | Clinically healthy, normal-weight, or overweight participants | 750 mL red orange juice daily for 8 wk | 35 | 54.3 | 26–46 | • ATP III (3)• WHO (28)• International Diabetes Federation (22) | • WC•TGs• Fasting blood glucose• HDL-C↓• Blood pressure↓ | No direct measure of lycopene in juice or circulation |
| Li et al. (29) | Healthy female students | 280 mL tomato juice daily for 2 mo | 25 | 0 | 20–30 | Not reported | • WC↓• TGs↑• Fasting blood glucose | Serum lycopene concentration |
Age values are ranges. ATP III, National Cholesterol Education Program's Adult Treatment Panel III; HDL-C, HDL cholesterol; MetS, metabolic syndrome; WC, waist circumference; ↑significant increase in outcome measure postintervention; ↓significant decrease in outcome measure postintervention.
Results
Study characteristics of the research identified
Of the articles identified and considered for systematic review, 11 articles met the inclusion criteria for critical analysis. The articles were published between 2003 and 2016 and originated from Brazil, China, Greece, Japan, Korea, Netherlands, Taiwan, and the United States. Eight of the 11 studies utilized a cross-sectional study design and the remaining 3 were intervention trials.
Studies varied widely not only in design but also in the definition of MetS and its components. Five of the studies used the ATP III criteria for diagnosis, whereas the remaining 6 studies utilized either a modified or separate set of criteria in which ≥3 risk factors of the ATP III criteria were evaluated. Although some studies assessed additional outcome measures, only the correlations between lycopene and components of MetS are highlighted throughout this review. Furthermore, if the studies satisfying inclusion criteria also assessed insulin resistance, LDL cholesterol, or total cholesterol, these significant measures were reported, as each is closely correlated with 2 formal ATP III risk factors (blood glucose and HDL cholesterol). In several studies, the sample sizes reported for outcomes of interest differed from the overall number of participants. As such, these unique sample sizes have been reported with the corresponding measure. Discussion of the 11 articles is separated by study design.
Cross-sectional studies
Results from the 8 cross-sectional studies reviewed were grouped according to the following categories: prevalence and outcomes of MetS, presence of ATP III risk factors, and variables mediating lycopene's influence on MetS. It should be noted that some studies were classified into >1 category.
Utilizing data from NHANES 2001–2006 and the 2001–2006 NHANES Linked Mortality File, with follow-up data through 2011, Han et al. (20) assessed the relation between lycopene and mortality among those living with MetS as defined by the ATP III criteria. Participants (n = 2499, 48.4% male, age ≥20 y, 55.9% non-Hispanic white, 13.5% non-Hispanic African American, 23.9% Mexican American, 6.7% other) were stratified into tertiles according to serum trans-lycopene concentrations. Compared with the lowest tertile's survival time (mean = 107.4 mo), the survival time was significantly greater (P < 0.05) among individuals in the highest and middle tertiles (mean = 120.6 mo and 116.3 mo, respectively). Furthermore, an adjusted hazards regression model suggested that those in the highest and middle tertiles of serum trans-lycopene concentration showed significantly lower HRs of mortality [HR (95% CI): 0.61 (0.42, 0.89), P = 0.0113, and 0.67 (0.45, 0.99), P = 0.0497, respectively] than those in the lowest tertile (HR: 1.0).
In a cross-sectional study among elderly Chinese adults (n = 2148, 28.0% male, age 50–75 y), Liu et al. (21) reported an inverse relation between serum carotenoid concentrations and the prevalence of MetS (11.4% prevalence) as established by the International Diabetes Federation in 2005 (22). Total and individual carotenoid concentrations (α-carotene, β-carotene, β-cryptoxanthin, lycopene, and lutein/zeaxanthin) were measured in serum with results grouped into quartiles. In the fully adjusted model, the OR of MetS between the highest and lowest quartiles of serum lycopene was 0.39 with a significant P for trend (P < 0.001; 95% CI: 0.26, 0.58). In addition, the results suggested that with each increasing number of MetS components (0 through 4), there was a significant decrease in serum lycopene concentrations (P for difference <0.001).
Using data from NHANES III 1988–1994, Ford et al. (15) investigated the relation between antioxidant intake (n = 8554) and serum concentrations (n = 8466) among individuals with and without MetS (49.6% male, age ≥20 y, 76.7% white, 23.3% other). The study sample consisted of 21.9% participants with MetS as confirmed by the ATP III criteria. To assess antioxidant consumption, an FFQ and 24-h dietary recall were analyzed for each participant, and serum concentrations of 5 carotenoids (α-carotene, β-carotene, β-cryptoxanthin, lutein/zeaxanthin, and lycopene) were also measured. There was no significant difference in antioxidant intake between participants with MetS and without. In addition, circulating lycopene was not significantly lower in those with MetS. However, serum lycopene was inversely associated with hyperglycemia and HDL cholesterol (P = 0.039 and P < 0.001, respectively). In contrast, a positive association between lycopene and TG concentrations was observed (P = 0.035).
Sluijs et al. (16) studied the relation between consumption of carotenoid compounds (β-carotene, α-carotene, β-cryptoxanthin, lycopene, and lutein plus zeaxanthin) and MetS along with its risk factors among individuals from the Netherlands (n = 374, 100% male, age 40–80 y). To quantify dietary carotenoid intake, participants completed an FFQ. Anthropometrics and biochemical markers were compared to ATP III criteria to assess MetS components, and 22% of the study sample met the diagnosis criteria. There was a significant inverse association between carotenoid intake and MetS diagnosis (P ≤ 0.05). After adjustment for confounders, the risk of developing MetS remained significantly lower with increased lycopene intake (P-trend = 0.01). In addition, higher lycopene intake was associated with lower waist circumference, as well as lower visceral and subcutaneous fat mass and serum TG concentrations (P-trend = 0.04). Although a self-reported dietary measure of lycopene was used, the study was strengthened by inclusion of fat mass measurements rather than relying solely on waist circumference measures or BMI.
A cross-sectional study of 299 Korean men (age 48–50 y) was conducted by Yeo et al. (17) to investigate the interrelations among brachial-ankle pulse wave velocity—a measure of arterial stiffness—serum lycopene, and MetS risk. To define MetS, the ATP III criteria were used with adjustment of the waist circumference and fasting glucose thresholds (>90 cm and ≥100 mg/dL, respectively). Participants were divided according to the number of MetS risk factors exhibited, and 18.4% participants possessed ≥3 risk factors, equating to a MetS diagnosis. As the number of MetS risk factors increased, there was a significant decrease in lycopene concentrations (P = 0.004). A significant inverse relation (P < 0.05) between serum lycopene concentration and risk factors (waist circumference, blood pressure, TGs, and fasting blood glucose, as well as HOMA-IR) was established; however, after adjusting for covariates, these correlations only remained significant for waist circumference, TGs, and HOMA-IR (P < 0.01, P < 0.001, and P < 0.01, respectively). It must be acknowledged that the results’ generalizability may be limited owing to the population-based adjustment of 2 of the MetS diagnosis criteria.
Smoking induces an additional level of oxidative stress that may influence the development of MetS. To evaluate the association between serum carotenoids and MetS stratified by smoking status, Sugiura et al. (18) conducted a cross-sectional investigation with Japanese participants (n = 958, 31.6% male, age 30–70 y). MetS was defined by criteria adjusted from those of the Japanese Committee for Diagnostic Criteria of Metabolic Syndrome in which BMI was used as a risk factor instead of waist circumference (19). Participants were stratified on the basis of smoking habits and MetS status, resulting in 4 groups for analysis. Among smokers, ∼10.7% had MetS compared with only 5.6% among nonsmokers. Results of an adjusted regression analysis among nonsmokers showed significant inverse correlations of serum lycopene with BMI (P = 0.003), systolic and diastolic blood pressures (P = 0.048 and P = 0.024, respectively), and fasting glucose (P = 0.009); furthermore, a significant positive correlation was noted between serum lycopene and HDL cholesterol (P < 0.001). In contrast, there was no significant correlation between any component of MetS and serum lycopene among current smokers. In addition, a significant difference between serum lycopene and the OR of MetS was not observed among smokers or nonsmokers. An inherent limitation of this study was the inclusion of BMI in place of waist circumference, because BMI does not directly assess abdominal or visceral adipose tissue and, therefore, is not an ideal anthropometric replacement for waist circumference (30–32).
Choi and Ainsworth (23) evaluated associations among MetS risk, food consumption, and serum antioxidant status along with physical activity in high-risk, middle-aged adults participating in NHANES 2005–2006 (n = 1930, 49.1% male, 40–70 y, 33.3% white, 33.3% black, 33.3% Hispanic/others). Participants were divided into tertiles according to the number of daily steps reported by an accelerometer. For both genders (n = 1661), serum trans-lycopene was significantly associated with daily steps in a positive manner (P < 0.01). For example, the active group (tertile 3) had the highest serum lycopene and the sedentary group (tertile 1) had the lowest. In an OR comparison to the active group, sedentary men and women had a 1.9- and 2.5-fold greater risk, respectively, of developing MetS, as defined by the ATP III criteria. Additional results suggest that higher concentrations of serum carotenoids, specifically lycopene, are significantly associated with increased number of steps and a lower risk of developing MetS (P < 0.01). It must be acknowledged that participants falling into the intermediate and active categories also consumed a greater amount of nutrient-dense foods than did their study counterparts. Although a host of factors likely influenced the results, lower circulating lycopene was indirectly linked with MetS.
Han et al. (24) examined the influence of BMI on the relation between serum lycopene concentrations and MetS using data collected from NHANES 2001–2006. The study sample included 13,196 participants (48% male, age ≥20 y, 52.2% non-Hispanic white, 19.8% non-Hispanic African American, 20.7% Mexican American, 7.3% other) divided into tertiles based on serum trans-lycopene concentrations. After controlling for confounders, individuals in the first tertile (lowest serum trans-lycopene concentrations) had the greatest prevalence of MetS (OR: 38.6%; 95% CI: 36.9%, 40.3%), as diagnosed using the ATP III criteria, compared with individuals in the second and third tertiles (OR: 29.3%; 95% CI: 27.5%, 31.1%, P < 0.05; OR: 26.6%; 95% CI: 24.9%, 28.3%, P < 0.05, respectively). In addition, BMI was found to have a significant interaction effect on the relation between serum trans-lycopene and MetS prevalence. For example, the prevalence of MetS was significantly lower among normal and overweight individuals in the second and third tertiles of serum trans-lycopene compared with the first (P < 0.05). However, the data did not corroborate this relation for obese individuals. Results suggest that the protective effects exerted by lycopene may be mediated by body weight.
Intervention studies
Among the 3 intervention studies identified, each assessed the reported outcome measures pre- and postintervention, and each randomized controlled trial was parallel in design.
Tsitsimpikou et al. (25) reported effects of tomato juice consumption on MetS-related risk factors as defined by the AHA/National Heart, Lung, and Blood Institute among Greek individuals with MetS (n = 27, 88.9% male, age 43–67 y) (26). Fifteen participants were instructed to consume commercially available tomato juice once daily 4 times/wk for 2 mo. Although an exact dose of tomato juice was not reported, it was noted that 100 g of a tomato beverage contained a mean ± SD of 2.51 ± 0.351 mg lycopene. The control group was encouraged to maintain routine dietary habits and to avoid lycopene-dense food sources or supplements. After the intervention period, a significant decrease in LDL cholesterol (33.1% mean reduction, P < 0.001) was observed along with a significant increase in HDL cholesterol (7.6% mean increase, P = 0.049). In addition, a significant decrease in fasting insulin resistance index (32.9% mean reduction, P = 0.016) was observed in the treatment group. An inherent limitation of the study includes the lack of a standardized amount of tomato juice and its lycopene content.
In a primary intervention study conducted by Silveira et al. (27), 35 healthy participants (54.3% male, age 26–46 y) living in Brazil were instructed to drink 750 mL red orange juice, containing an unspecified amount of lycopene, daily for an 8-wk period while maintaining all other lifestyle factors. The study objective was to determine the effects of daily red orange juice consumption on MetS risk factors as defined by a combination of the ATP III, International Diabetes Federation, and WHO definitions (3, 22, 28). For statistical analysis, participants were separated on the basis of weight status (normal weight or overweight/obese), and no changes were observed in body weight, BMI, percentage body fat, or waist circumference as a result of the intervention. A significant decrease (P < 0.05) in total cholesterol was observed among normal-weight (12% reduction) and overweight or obese participants (7% reduction), as was a significant decrease (P < 0.05) in LDL cholesterol for both groups (10% reduction). Of interest, HDL cholesterol was significantly decreased in normal-weight individuals (14% reduction, P < 0.05). There were also significant decreases (P < 0.05) in systolic blood pressure among normal-weight participants (4% reduction) and diastolic blood pressure among overweight or obese participants (5% reduction). A 28% reduction (P < 0.05) in HOMA-IR was observed in normal-weight participants, as was a nonsignificant reduction (16%) among overweight or obese participants. Despite significant improvements in MetS risk factors, this study remains limited by its lack of a control group. Furthermore, daily consumption of 750 mL of juice may not be the optimum intervention vehicle owing to its caloric provision.
In an intervention trial, Li et al. (29) recruited 25 healthy Taiwanese females (mean ± SD age: 22.5 ± 0.6 y) to evaluate the supplementation effect of tomato juice on metabolic health and adipokines. For 2 mo, study participants were instructed to drink 280 mL tomato juice (32.5 mg lycopene) daily while maintaining their typical diet and physical activity regimen. Analysis revealed significant decreases in waist circumference and total cholesterol (P < 0.0001 and P < 0.0005, respectively). In contrast, a significant increase in TGs was observed (P < 0.05), although TG concentrations were still within the normal range. In addition, serum concentrations of lycopene significantly increased by the end of the intervention period (P < 0.0005). A subanalysis was performed to ensure that these results were not mediated by the significant reductions in body weight (P < 0.01), body fat (P < 0.05), and BMI (P < 0.01). Upon stratifying participants as responders (reduction in body fat) and nonresponders (no reduction in body fat), the results remained significant for variables of MetS. Despite the strength of the analysis, the design lacked a control group because the authors noted the impracticality of developing a realistic placebo beverage. In addition, the external validity of the results may be limited, as noted by the authors, because the study sample consisted of women of normal body weight only.
Discussion
This is, to our knowledge, the first systematic review to assess the correlation between serum lycopene or dietary intake of lycopene and MetS. Previous publications have established biological mechanisms for the potential positive role of lycopene on outcomes related to metabolic derangement, such that its heightened efficiency in neutralizing singlet oxygen radicals may influence the development of MetS (12, 13). The ATP III criteria were utilized as the primary reference for this systematic review. Of the included studies, 5 established their diagnoses of MetS on the basis of the ATP III criteria, allowing for better interpretation and comparison among studies. However, those studies that applied either a modified or different definition were still included for comparison if a minimum of 3 risk factors of the ATP III criteria were addressed.
Results from the 8 cross-sectional studies reviewed encompassed various aspects of MetS and were grouped according to reported associations of lycopene with the prevalence and outcomes of MetS, presence of ATP III risk factors, and variables mediating lycopene's influence on MetS risk. In assessment of MetS prevalence and outcomes, 5 studies (15, 16, 20, 21, 24) evaluated this relation, and of these, 4 (16, 20, 21, 24) observed a significant inverse correlation. Higher serum concentrations of lycopene were associated with a reduced number of MetS diagnoses, as well as a decreased mortality risk if already possessing the condition.
The relation between lycopene and individual ATP III risk factors was evaluated by 4 studies (15–18). All 4 revealed that ≥1 assessed risk factor was inversely associated with either increased serum or dietary lycopene; however, Ford et al. (15) also observed an inverse relation between serum lycopene measures and HDL cholesterol as well as a positive association with TGs.
In addition, 3 of the 8 studies (18, 23, 24) considered variables potentially mediating lycopene's influence on MetS risk, including physical activity, tobacco use, and BMI. Stratification of these factors affected each study's findings, such that lycopene's association with the outcome measures varied depending on the classification of the participants. For example, Choi and Ainsworth (23) observed a positive association between serum lycopene concentrations and physical activity, and an inverse association between the latter and a MetS diagnosis. Although an indirect connection between serum lycopene and MetS diagnosis was established, physical activity as a mediating variable should not be underestimated. The results suggest an interplay among several variables that will lower the risk of MetS; thus, consumption of a nutrient-dense diet, containing lycopene, paired with an active lifestyle may be an efficacious intervention. Furthermore, serum lycopene was found only to be protective in nonsmoking participants and those with a normal or overweight BMI classification, but not among obese participants (18, 24). These findings are not unexpected, because elevated levels of oxidative stress have been observed in the presence of tobacco use and obesity (33, 34). The prooxidant–antioxidant imbalance that ensues may result in the increased utilization of endogenous and exogenous antioxidants, depleting circulating antioxidant concentrations; therefore, any protective association that exists between serum lycopene and MetS in these populations may be attenuated (35). Additional mechanisms proposed to explain reduced serum concentrations, notably in obese populations, include the deposition of fat-soluble antioxidants in adipose tissue or simply a lower dietary antioxidant intake (36–38). Although the mechanisms underpinning reduced lycopene in obesity and tobacco use are not fully elucidated, other studies have implicated greater amounts of adiposity and exposure to cigarette smoke in the reduction of serum carotenoid concentrations (39, 40).
Overall, the 8 cross-sectional studies reported a protective relation between lycopene and MetS to varying extents. Results from cross-sectional studies, though supportive, preclude the ability to ascribe causality because of both potential confounding and a lack of knowledge about the temporal relation between variables of interest.
Three intervention studies were identified which better elucidate this carotenoid's effect on MetS risk and outcomes. Despite the similar study objectives, each one varied in either its study design (controlled compared with noncontrolled), type of intervention, dose administered, and subject characteristics. These differences in methodology and study design make a direct comparison and evaluation of the target outcomes complex, because the significant findings for each of the 3 studies encompassed different combinations of MetS components. Nevertheless, all studies (25, 27, 29) revealed positive impacts of interventions containing lycopene-rich foods on the evaluated components of MetS, aside from TG concentrations and HDL-cholesterol concentrations. Increased TGs in young females were observed after a tomato juice intervention by Li et al. (29); however, this was not necessarily negative, as TG concentrations still remained within normal limits. In addition, Silveira et al. (27) reported a significant decrease in HDL-cholesterol concentrations among normal-weight participants (P < 0.05); however, the mean HDL-cholesterol concentration was still greater than the minimum value established as a MetS risk factor by the ATP III criteria. In a recent systematic review of 100% fruit juice interventions on blood lipid concentrations, a significant HDL-cholesterol–lowering effect was not observed; however, significantly greater TG concentrations were noted (41). The studies (n = 5) analyzed in the aforementioned review (41) were also interventions of short duration or cross-sectional designs. Thus, evaluation over an extended time period, in combination with careful appraisal of other confounding lifestyle factors, is needed to fully understand the potential health effects of fruit juice interventions.
Although insulin resistance is not one of the 5 formal MetS components of the ATP III criteria, it is referred to as an emerging risk factor because its presence is associated with other metabolic derangements and risk of CVD (3). Oxidative stress has been implicated in the onset of insulin resistance, because the increased concentrations of reactive oxygen species activate stress-induced pathways such as NF-κB and MAPK, attenuating the insulin response and subsequent glucose uptake (42, 43). Furthermore, pancreatic β-cells may be exceptionally susceptible to free radicals owing to their decreased endogenous antioxidant defense mechanisms, consequently impairing insulin secretion (43, 44). As such, the oxidative stress underpinning insulin resistance may be mitigated by lycopene-containing foods, thus positively affecting the ATP III risk factor of blood glucose. Two of the 3 studies (25, 27) showed a significant decrease in insulin resistance, whereas all 3 did not observe significant differences in blood glucose concentrations after the intervention. The noted null findings in blood glucose concentrations are interesting in that the supplemented juices were rich in carbohydrates and also the dosages ranged from 280 to 750 mL/d. The results from these intervention studies suggest that using a lycopene-rich tomato or red orange juice can exert beneficial effects on MetS risk factors without adversely contributing to blood glucose concentrations. However, it should be acknowledged that the impact a fruit juice will have on blood glucose is dependent upon the amount of digestible carbohydrates present, thus these findings should not be extrapolated to other fruit juices. The intervention studies revealed another interesting finding in regard to total cholesterol. Total cholesterol is a measure that encompasses both HDL cholesterol and LDL cholesterol as well as TG concentrations, thus, if one of these components is affected, the others will follow suit (45). This interdependence warrants the mention of significant findings in 2 of the 3 intervention studies (27, 29) in which a decrease in total cholesterol was reported independently of changes in body weight. These findings are consistent with hypothesized mechanisms regarding lycopene's influence on hypercholesterolemia. This carotenoid has been demonstrated to reduce the activity and expression of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, the regulatory enzyme of endogenous cholesterol synthesis, while also inhibiting LDL-cholesterol receptors and acyl-CoA cholesterol acyl transferase (46, 47).
Among the intervention studies included in this review, each implemented a food-first intervention. Although previous research has observed beneficial effects of lycopene in its isolated form, the incorporation of a functional food with the compound of interest could potentially enhance these protective properties through the provision of an intact food matrix (48, 49). The matrix may offer a synergistic environment to promote the bioactivity of phytonutrients; however, this matrix also presents a challenge, because the direct effects of lycopene are unable to be teased apart from other bioactive compounds within the food (50, 51). As such, each intervention study is limited in that the protective findings cannot solely be attributed to lycopene and may have been influenced by other nutrients within the intervention beverages. In summary, all of the intervention studies observed effects of lycopene on MetS outcomes, although 2 of the 3 studies lacked a control group.
Strengths and limitations
Strengths of this review lie in the included studies’ use of serum lycopene measures. Circulating measures are superior for assessing relations, because self-reported measures of lycopene intake are subject to recall bias or memory error (52). Eight of the 11 studies (15, 17, 18, 20, 21, 23, 24, 29) utilized a circulating measure for lycopene assessment and incorporated FFQs or dietary recalls to supplement the data. Only 1 study (16) relied solely on self-reported measures to evaluate the associations among variables of interest. In addition, the overall generalizability and data in support of the relation between lycopene and MetS are strengthened by the consistency of findings from multiple countries, as well as the implementation of whole food interventions.
Despite these strengths, a paucity of intervention studies in the literature must be acknowledged because 8 of the 11 articles meeting the inclusion criteria for this review were cross-sectional in design.
Implications for future research and conclusions
Although a generally protective relation between lycopene and MetS was reported in each of the included studies, different MetS risk factors appeared to be influenced by lycopene, rather than demonstrating the consistent improvement of a single MetS component. Thus, a mechanistic understanding of the science behind lycopene and MetS would be strengthened by intervention studies utilizing a single, universally accepted definition for MetS, as well as the implementation of control groups and dose-duration studies. In summary, the evidence of lycopene's benefit exists such that lycopene status or lycopene consumption may be associated with favorable alterations to the components of MetS. The majority of studies included in this review support a protective relation between lycopene and MetS. Nevertheless, additional research is needed to determine evidence-based recommendations concerning specific dose-durational effects of lycopene and MetS risk reduction.
Acknowledgments
All authors read and approved the final manuscript.
Notes
The authors reported no funding received for this study.
Author disclosures: KES, LT, and KMC-W, no conflicts of interest.
Abbreviations used: ATP III, National Cholesterol Education Program's Adult Treatment Panel III; CVD, cardiovascular disease; MetS, metabolic syndrome.
References
- 1. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, Floyd J, Fornage M, Gillespie C, Isasi C. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation 2017;135(10):e146–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, Rinfret S, Schiffrin EL, Eisenberg MJ. The metabolic syndrome and cardiovascular risk: a systematic review and meta-analysis. J Am Coll Cardiol 2010;56(14):1113–32. [DOI] [PubMed] [Google Scholar]
- 3. Grundy SM, Brewer HB, Cleeman JI, Smith SC, Lenfant C. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 2004;109(3):433–8. [DOI] [PubMed] [Google Scholar]
- 4. Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States. Natl Health Stat Report 2009;13:1–7. [PubMed] [Google Scholar]
- 5. Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA 2015;313(19):1973–4. [DOI] [PubMed] [Google Scholar]
- 6. Mahjoub S, Masrour-Roudsari J. Role of oxidative stress in pathogenesis of metabolic syndrome. Caspian J Intern Med 2012;3(1):386–96. [PMC free article] [PubMed] [Google Scholar]
- 7. Tapiero H, Townsend D, Tew K. The role of carotenoids in the prevention of human pathologies. Biomed Pharmacother 2004;58(2):100–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Krinsky NI, Johnson EJ. Carotenoid actions and their relation to health and disease. Mol Aspects Med 2005;26(6):459–516. [DOI] [PubMed] [Google Scholar]
- 9. Li F-J, Shen L, Ji H-F. Dietary intakes of vitamin E, vitamin C, and β-carotene and risk of Alzheimer's disease: a meta-analysis. J Alzheimers Dis 2012;31(2):253–8. [DOI] [PubMed] [Google Scholar]
- 10. Kasperczyk S, Dobrakowski M, Kasperczyk J, Ostałowska A, Zalejska-Fiolka J, Birkner E. Beta-carotene reduces oxidative stress, improves glutathione metabolism and modifies antioxidant defense systems in lead-exposed workers. Toxicol Appl Pharmacol 2014;280(1):36–41. [DOI] [PubMed] [Google Scholar]
- 11. Limon-Miro AT, Lopez-Teros V, Astiazaran-Garcia H. Dietary guidelines for breast cancer patients: a critical review. Adv Nutr 2017;8(4):613–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Di Mascio P, Kaiser S, Sies H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch Biochem Biophys 1989;274(2):532–8. [DOI] [PubMed] [Google Scholar]
- 13. Müller L, Caris-Veyrat C, Lowe G, Böhm V. Lycopene and its antioxidant role in the prevention of cardiovascular diseases—a critical review. Crit Rev Food Sci Nutr 2016;56(11):1868–79. [DOI] [PubMed] [Google Scholar]
- 14. Academy of Nutrition and Dietetics Evidence Analysis Manual [Internet] 2016[cited 2017 Jul]. Available from: https://www.andeal.org/evidence-analysis-manual. [Google Scholar]
- 15. Ford ES, Mokdad AH, Giles WH, Brown DW. The metabolic syndrome and antioxidant concentrations: findings from the third National Health and Nutrition Examination Survey. Diabetes 2003;52(9):2346–52. [DOI] [PubMed] [Google Scholar]
- 16. Sluijs I, Beulens JW, Grobbee DE, van der Schouw YT. Dietary carotenoid intake is associated with lower prevalence of metabolic syndrome in middle-aged and elderly men. J Nutr 2009;139(5):987–92. [DOI] [PubMed] [Google Scholar]
- 17. Yeo HY, Kim OY, Lim HH, Kim JY, Lee JH. Association of serum lycopene and brachial-ankle pulse wave velocity with metabolic syndrome. Metabolism 2011;60(4):537–43. [DOI] [PubMed] [Google Scholar]
- 18. Sugiura M, Nakamura M, Ogawa K, Ikoma Y, Matsumoto H, Ando F, Shimokata H, Yano M. Associations of serum carotenoid concentrations with the metabolic syndrome: interaction with smoking. Br J Nutr 2008;100(6):1297–306. [DOI] [PubMed] [Google Scholar]
- 19. Yamagishi K, Iso H. The criteria for metabolic syndrome and the national health screening and education system in Japan. Epidemiol Health 2017;39:e20017003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Han G-M, Meza JL, Soliman GA, Islam KM, Watanabe-Galloway S. Higher levels of serum lycopene are associated with reduced mortality in individuals with metabolic syndrome. Nutr Res 2016;36(5):402–7. [DOI] [PubMed] [Google Scholar]
- 21. Liu J, Shi W-Q, Cao Y, He L-P, Guan K, Ling W-H, Chen Y-M. Higher serum carotenoid concentrations associated with a lower prevalence of the metabolic syndrome in middle-aged and elderly Chinese adults. Br J Nutr 2014;112(12):2041–8. [DOI] [PubMed] [Google Scholar]
- 22. Alberti G, Zimmet P, Shaw J, Grundy SM. Metabolic syndrome—a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet Med 2006;23(5):469–80. [DOI] [PubMed] [Google Scholar]
- 23. Choi JE, Ainsworth BE. Associations of food consumption, serum vitamins and metabolic syndrome risk with physical activity level in middle-aged adults: the National Health and Nutrition Examination Survey (NHANES) 2005–2006. Public Health Nutr 2016;19(9):1674–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Han G-M, Soliman GA, Meza JL, Islam KM, Watanabe-Galloway S. The influence of BMI on the association between serum lycopene and the metabolic syndrome. Br J Nutr 2016;115(7):1292–300. [DOI] [PubMed] [Google Scholar]
- 25. Tsitsimpikou C, Tsarouhas K, Kioukia-Fougia N, Skondra C, Fragkiadaki P, Papalexis P, Stamatopoulos P, Kaplanis I, Hayes AW, Tsatsakis A. Dietary supplementation with tomato-juice in patients with metabolic syndrome: a suggestion to alleviate detrimental clinical factors. Food Chem Toxicol 2014;74:9–13. [DOI] [PubMed] [Google Scholar]
- 26. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation 2005;112(17):2735–52. [DOI] [PubMed] [Google Scholar]
- 27. Silveira JQ, Dourado GK, Cesar TB. Red-fleshed sweet orange juice improves the risk factors for metabolic syndrome. Int J Food Sci Nutr 2015;66(7):830–6. [DOI] [PubMed] [Google Scholar]
- 28. Alberti KGMM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabet Med 1998;15(7):539–53. [DOI] [PubMed] [Google Scholar]
- 29. Li Y-F, Chang Y-Y, Huang H-C, Wu Y-C, Yang M-D, Chao P-M. Tomato juice supplementation in young women reduces inflammatory adipokine levels independently of body fat reduction. Nutrition 2015;31(5):691–6. [DOI] [PubMed] [Google Scholar]
- 30. Park S-H, Choi S-J, Lee K-S, Park H-Y. Waist circumference and waist-to-height ratio as predictors of cardiovascular disease risk in Korean adults. Circ J 2009;73(9):1643–50. [DOI] [PubMed] [Google Scholar]
- 31. Lemieux S, Prud'homme D, Bouchard C, Tremblay A, Després J-P. A single threshold value of waist girth identifies normal-weight and overweight subjects with excess visceral adipose tissue. Am J Clin Nutr 1996;64(5):685–93. [DOI] [PubMed] [Google Scholar]
- 32. Frankenfield DC, Rowe WA, Cooney RN, Smith JS, Becker D. Limits of body mass index to detect obesity and predict body composition. Nutrition 2001;17(1):26–30. [DOI] [PubMed] [Google Scholar]
- 33. Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 2004;114(12):1752–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Langham MC, Zhou Y, Chirico EN, Magland JF, Sehgal CM, Englund EK, Mohler ER, Guo W, Barhoum S, Wehrli FW. Effects of age and smoking on endothelial function assessed by quantitative cardiovascular magnetic resonance in the peripheral and central vasculature. J Cardiovasc Magn Reson 2015;17(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Amirkhizi F, Siassi F, Minaie S, Djalali M, Rahimi A, Chamari M. Is obesity associated with increased plasma lipid peroxidation and oxidative stress in women? ARYA Atheroscler 2010;2(4):189–92. [Google Scholar]
- 36. Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 2000;72(3):690–3. [DOI] [PubMed] [Google Scholar]
- 37. Brouwer DJ, Van Beek J, Ferwerda H, Brugman AM, van der Klis FR, van der Heiden HJ, Muskiet FA. Rat adipose tissue rapidly accumulates and slowly releases an orally-administered high vitamin D dose. Br J Nutr 1998;79(6):527–32. [DOI] [PubMed] [Google Scholar]
- 38. Carrelli A, Bucovsky M, Horst R, Cremers S, Zhang C, Bessler M, Schrope B, Evanko J, Blanco J, Silverberg SJ. Vitamin D storage in adipose tissue of obese and normal weight women. J Bone Miner Res 2017;32(2):237–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Suzuki K, Inoue T, Hioki R, Ochiai J, Kusuhara Y, Ichino N, Osakabe K, Hamajima N, Ito Y. Association of abdominal obesity with decreased serum levels of carotenoids in a healthy Japanese population. Clin Nutr 2006;25(5):780–9. [DOI] [PubMed] [Google Scholar]
- 40. Handelman GJ, Packer L, Cross CE. Destruction of tocopherols, carotenoids, and retinol in human plasma by cigarette smoke. Am J Clin Nutr 1996;63(4):559–65. [DOI] [PubMed] [Google Scholar]
- 41. Crowe-White K, Parrott JS, Stote KS, Gutschall M, Benson-Davies S, Droke E, O'Neil CE, Wolfram T, Ziegler P. Metabolic impact of 100% fruit juice consumption on antioxidant/oxidant status and lipid profiles of adults: an evidence-based review. Crit Reviews Food Sci Nutr 2017;57(1):152–62. [DOI] [PubMed] [Google Scholar]
- 42. Henriksen EJ, Diamond-Stanic MK, Marchionne EM. Oxidative stress and the etiology of insulin resistance and type 2 diabetes. Free Radic Biol Med 2011;51(5):993–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Are oxidative stress–activated signaling pathways mediators of insulin resistance and β-cell dysfunction? Diabetes 2003;52(1):1–8. [DOI] [PubMed] [Google Scholar]
- 44. Tiedge M, Lortz S, Drinkgern J, Lenzen S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes 1997;46(11):1733–42. [DOI] [PubMed] [Google Scholar]
- 45. American Heart Association What your cholesterol levels mean [Internet] 2017[cited 2018 Feb 20]. Available from: http://www.heart.org/HEARTORG/Conditions/Cholesterol/AboutCholesterol/What-Your-Cholesterol-Levels-Mean_UCM_305562_Article.jsp.
- 46. Palozza P, Catalano A, Simone RE, Mele MC, Cittadini A. Effect of lycopene and tomato products on cholesterol metabolism. Ann Nutr Metab 2012;61(2):126–34. [DOI] [PubMed] [Google Scholar]
- 47. Fuhrman B, Elis A, Aviram M. Hypocholesterolemic effect of lycopene and β-carotene is related to suppression of cholesterol synthesis and augmentation of LDL receptor activity in macrophages. Biochem Biophys Res Commun 1997;233(3):658–62. [DOI] [PubMed] [Google Scholar]
- 48. Devaraj S, Mathur S, Basu A, Aung HH, Vasu VT, Meyers S, Jialal I. A dose-response study on the effects of purified lycopene supplementation on biomarkers of oxidative stress. J Am Coll Nutr 2008;27(2):267–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. McEneny J, Wade L, Young IS, Masson L, Duthie G, McGinty A, McMaster C, Thies F. Lycopene intervention reduces inflammation and improves HDL functionality in moderately overweight middle-aged individuals. J Nutr Biochem 2013;24(1):163–8. [DOI] [PubMed] [Google Scholar]
- 50. Raikos V. Food matrix: natural barrier or vehicle for effective delivery of carotenoids from processed foods? Insights Nutr Metab 2017;1(1):1–6. [Google Scholar]
- 51. Crowe KM. Designing functional foods with bioactive polyphenols: highlighting lessons learned from original plant matrices. J Hum Nutr Food Sci 2013;1(3):1018. [Google Scholar]
- 52. Baranowski T. 24-hour recall and diet record methods. In: Willet W, editor Nutritional Epidemiology. New York: Oxford University Press; 2013. p. 49–69. [Google Scholar]