ABSTRACT
This is the first systematic review to examine the global prevalence of catch-up growth (CUG) in small for gestational age (SGA) infants who were born at full term (FT). Size at birth and subsequent growth is an important indicator of neonatal and adult health. Globally, 16% of infants are SGA at birth, ranging from 7% in industrialized countries to 41.5% in South Asia. SGA infants are at increased risk for negative developmental and adult health outcomes. Some achieve CUG but others do not. CUG has immediate and late health implications especially in low- and middle-income countries. This systematic review sought to determine the global prevalence of CUG among FT-SGA infants. We performed a literature search of MEDLINE, Pubmed, Embase, Web of Science, and Scopus, as well as grey literature databases, and identified 3137 studies. The final analysis included 11 studies. The median prevalence of CUG was 87.4% across all definitions of SGA and CUG. However, multiple definitions were used to classify SGA and CUG. Nine unique reference populations were used to classify SGA, and 6 to approximate CUG. Due to this heterogeneity, a meta-analysis could not be conducted. Program implementation for this vulnerable group of infants is dependent on proper classification. Given the wide range of definitions and reference standards used in the past, it is not possible to determine the global need for programs to address CUG for FT-SGA infants or to rationally plan any such programs. We highlight the need and propose standard definitions and references for SGA and CUG.
Keywords: small for gestational age, catch-up growth, systematic review, full-term infants, growth standards
Introduction
Size at birth is an important indicator of fetal, neonatal, and adult health. Increased size at birth and the corresponding reduction in neonatal mortality is frequently an indication of a country's improving social and economic status. Globally, ∼16% of all births are small for gestational age (SGA), ranging from 7% in industrialized countries to 41.5% in South Asia (1, 2). In 2010, 32.4 million infants (27% of live births) were born SGA in low- and middle-income countries (LMICs) of whom 29.7 million were full term (FT-SGA) (2). The disproportionate burden of SGA in LMICs may pose risks for future health outcomes among these populations.
Multiple classifications are used to identify infants born with insufficient growth. Historically, low birth weight (LBW) was the most common classification since it relied on a simple measurement, weight at birth. Infants born weighing <2500 g were automatically classified as LBW regardless of gestational age (3, 4). In utero, growth is measured indirectly through ultrasound assessments. Where fetal growth faltering has been identified, infants are classified as having intrauterine growth retardation; however, these measurements often lack precision and are not feasible in many developing countries where prenatal care is sporadic (3). Some infants weighing slightly >2500 g at birth also suffer from increased morbidity and mortality (5), and therefore have specific requirements for neonatal management (1). The classification of SGA helps identify infants at increased risk because it is based on gestational age and size according to standard sex-based birth-weight-for-gestational age standards (4). Although various criteria have been used to identify SGA infants, the following are the most common definitions: 1) children born below the 10th percentile for birth weight; or 2) children with a birthweight of <2 SDs below the mean (4). Children can also be further classified into full-term (37–42 wk) and preterm (<37 wk) gestational ages.
A landmark study by Karlberg et al. (6) in 1995 reported that 85% of children born SGA eventually reach a normal height. This finding was adopted by many healthcare organizations and presented in scientific reviews, leading to the widespread expectation that 85% of all SGA children catch-up regardless of predisposing factors (7, 8). However, no systematic review of the literature has been conducted to support this claim. Catch-up growth (CUG) is commonly defined as height velocity above the statistical limits of normal for age or maturity during a defined period of time following a period of growth inhibition (7). CUG is determined retrospectively over a course of months or years up to adult height. Although this definition is generally agreed upon, the empirical measurement of CUG has multiple cut-offs including a change in height-for-age z score (HAZ) of >0.67, achieving an HAZ of >–2 SD or >1.3 SD, or growth above the third percentile for height (for age) at any time during follow-up.
When growth is completed, adult height serves as a proxy for health and reflects the environment during growth. Some of the consequences of being born FT-SGA include lower psychologic and intellectual performance, and increased risk for precocious puberty, type 2 diabetes, metabolic syndrome, obesity, and cardiovascular disease (9–12). Given the developmental risks associated with SGA births, we believe that there is value in establishing standardized definitions for SGA and CUG, in addition to universally accepted birth-weight-for-gestation-age standards and growth standards, and that these should be be implemented globally. This systematic review investigates the prevalence of CUG among FT-SGA newborns.
Methods
We identified studies that described CUG among FT-SGA infants. Publications were obtained through 5 online databases: MEDLINE, Pubmed, Embase, Web of Science, and Scopus. Reference lists were reviewed for additional publications and a grey literature search was performed to identify unpublished literature. Duplicate studies were identified and removed prior to analysis.
All authors agreed on search terms prior to data collection to ensure accuracy and relevance. An initial search of MEDLINE was undertaken followed by a textual analysis of publications’ titles, abstracts, and index terms in order to further refine the appropriateness of search terms. Once search terms were finalized, we conducted a search based on the use of all identified search terms across all included databases until 26 February, 2016 (Supplementary Table 1). Relevant publications were exported and managed in Endnote citation manager.
The initial search was for infants born SGA, or with fetal growth retardation. Some keywords used were “small for gestational age,” “fetal growth retardation,” “SGA infant,” “SGA newborn,” “small for age,” “small for date,” “congenital hypotrophy,” and “intrauterine growth retardation.” Subsequent searches were used to identify publications that measured growth patterns during infancy and childhood. Keywords included: “catch up,” “catch up growth,” “caught up,” “body height,” “body weight,” and “body size.” Finally, we refined our search to focus on FT-SGA infants through the use of the terms “birth full-term,” “birth term,” “full-term birth,” “term infant,” “term birth,” “term neonate,” and “term newborn.” Supplementary Table 1 provides a full list of search terms for each database.
Inclusion criteria
We included prospective and retrospective birth cohort studies that measured the growth trajectory of healthy, singleton, FT (between 37–42 wk gestation) SGA newborns. Studies were required to meet the following criteria: 1) be written in English; 2) determine SGA based on a birth weight cut-off; 3) report growth outcomes after birth on ≥1 occasion; 4) report outcomes as length or height in median HAZs or prevalence; and 5) define SGA and CUG. Countries of origin and publication date were not limited.
Exclusion criteria
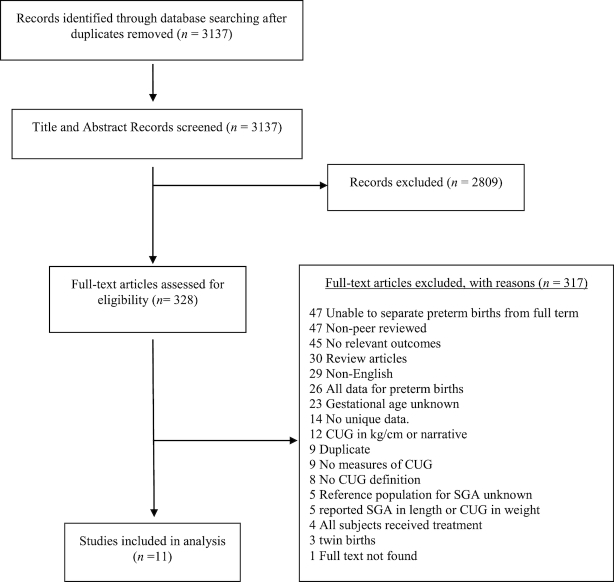
Duplicate studies and review papers were removed prior to analysis. Separate publications utilizing the same birth cohort were screened, and the less detailed publication was excluded. Experimental studies with growth hormone interventions were also excluded, including cases where measurements were provided for a control population. Figure 1 provides a flow diagram that depicts the flow of information through the different phases of our systematic review. It maps out the number of records identified, included and excluded, and the reasons for exclusions.
FIGURE 1.
PRISMA flow diagram illustrating the flow of number of articles identified, included, and excluded through the different phases of the systematic review. CUG, catch-up growth; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Two reviewers independently screened titles and abstracts for eligibility with Covidence online review software. Discrepancies were discussed by the reviewers and final decisions were agreed upon before proceeding to full-text screening. Each reviewer screened full texts, and independently extracted data from selected publications into duplicate extraction forms. Extraction forms were designed a priori based upon the Joanna Briggs Institute—Data Extraction Form for Prevalence and Incidence Studies, modified to meet study needs (36). All team members agreed upon modifications, and the forms were pilot tested by both reviewers with a small sample of articles (n = 10). Data were extracted onto the extraction forms independently and then matched.
Reviewers also completed an in-depth quality assessment of each study based on the 10 questions from the Critical Appraisal Checklist for Studies Reporting Prevalence Data by the Joanna Briggs Institute (Supplementary Table 2) (36). Any disagreements that arose between the reviewers were resolved through discussion, or with a third reviewer. No studies were excluded based upon the quality assessment.
Characterizing SGA and CUG in the included studies
Included studies reported a range of definitions to identify and to classify SGA infants. SGA was defined as weight at birth that met the following criteria: 1) <–2 SDs from the median; 2) less than the 10th percentile birth weight of a reference; and 3) less than the 5th percentile birth weight of a reference. Authors also used a wide variety of reference populations for their SGA definition. Reference populations for each included study are recorded in the table of included studies.
Similarly, definitions of CUG varied considerably among publications. Although birth weight is used to classify SGA infants, subsequent CUG is measured in length or height. Among included studies, CUG was defined in 4 different ways: 1) as length measurements that changed from <2 SD to >–2 SDs during a defined period of time; 2) as length measurements that increased by >0.67 SD during any time period; 3) as length measurements that were greater than the third percentile cut-off during any time period; 4) as length measurements that were >1.33 SDs.
In order to categorize CUG, researchers also utilized a wide variety of reference populations to which cohorts were compared. Reference populations were commonly drawn from the region or country where the study was being conducted. Consequently, expectations of “normal” growth varied substantially among publications, complicating the generalizability of CUG. Due to the heterogeneity of data, a meta-analysis could not be conducted, and thus descriptive statistics were used.
Methodologic quality
Based upon the quality appraisal, we identified very few sources of potential bias (Supplementary Table 3). In 2 studies, the small sample size was determined to contribute a high risk of bias, 2 studies were assessed as unclear bias regarding the representative nature of the sample, and 3 studies were assessed as unclear bias based upon standard criteria used for measurement of the condition (in this case the condition was SGA). No studies were excluded at this stage of the review.
Results
Our searches identified 3137 studies for review. After screening title and abstract, 328 studies required full-text evaluation. Of these, 11 studies met our inclusion criteria (Table 1). Of the included studies, 8 (73%) were prospective in design and 3 (27%) were retrospective. Almost all the studies (91%) were from countries classified as High Income by the World Bank, whereas 1 study was from an Upper Middle-Income country. Geographically, 1 study was from the East Asia and Pacific region, 2 studies from Latin America and the Caribbean, 1 study from North America, and 7 from Europe and Central Asia. Six (55%) studies collected data from 1959 to 2000, 2 studies (18%) from 2000 to 2010, and 3 did not identify a study period. The mean cohort size was 2229 children (range: 44–17,046). Only 2 studies followed children's growth trajectories to adulthood (Table 2).
TABLE 1.
Details of the included studies1
| Author, publication year | Country | Sample size (n) | Study period | Age follow-up, y | Retro/prospective study | SGA definition | SGA reference | CUG definition | CUG reference | CUG as HAZ or percent |
|---|---|---|---|---|---|---|---|---|---|---|
| Albertsson et al., 1994 (13) | Sweden | 3,650 | 1992 | 0.5–18 | R | <–2 SD | Own study AGA participants | >–2SD | Own study AGA participants | HAZ & Percent |
| Fitzhardinge et al., 1972 (14) | Canada | 96 | 1960–1966 | 6 | P | <3 PCTL | Streeter et al. (15) | >3 PCTL | Stuart et al. (16) | Percent |
| Itabashi et al., 2007 (17) | Japan | 449 | 1980–2000 | 1–5 | R | <10 PCTL | Nishisa et al. (18) | >–2SD | Suwa et al. (19) | Percent |
| Kramer et al., 2014 (20) | Belarus | 17,046 | 1996 | 11.5 | P | <10 PCTL | Kramer et al. (21) | ≥Δ 0.67 | CDC (22) | Percent |
| Luo et al., 1998 (23) | Sweden | 2,815 | Not reported | 18 | R | <–2 SD | Karlberg et al. (6) | >–2SD | Niklasson et al. (24) | Percent |
| Mericq et al., 2005 (25) | Chile | 108 | Not reported | 1, 3 | P | <5 PCTL | Juez et al. (26) | ≥Δ 0.67 | Youlton et al. (27) | HAZ |
| Perucchin et al., 2011 (28) | Italy | 44 | 2002, 2004 | 0.5-2 | P | <–2 SD | Gairdner et al. (29) | >3 PCTL | Unknown | HAZ & Percent |
| Sebastiani et al., 2015 (30) | Spain | 46 | 2005 | 3, 6 | P | <–2 SD | Unknown | >–2SD | Unknown | HAZ |
| Soto et al., 2003 (31) | Chile | 108 | Not reported | 1 | P | <5 PCTL | Juez (26) | ≥Δ 0.67 | Youlton et al. (27) | HAZ & Percent |
| Toumba et al., 2005 (32) | Greece | 104 | 1993 | 0.5-3 | P | <–2 SD | Ogden et al. (33) | >–2SD | CDC (22) | Percent |
| van Weissenbruch et al., 2005 (34) | Netherlands | 53 | 1980 | 9, 12 | P | <10 PCTL | Kloosterman (35) | >–1.3SD | Unknown | HAZ |
1AGA, appropriate for gestational age; CUG, catch-up growth; HAZ, height-for-age z score; P, prospective study design; PCTL, percentile; R, retrospective study design; SGA, small for gestational age.
TABLE 2.
Characteristics of the included studies (n = 11)1
| Study characteristics | n | % |
|---|---|---|
| Study design | ||
| Prospective | 8 | 73 |
| Retrospective | 3 | 27 |
| Study period (year) | ||
| Before 2000 | 6 | 55 |
| After 2000 | 2 | 18 |
| Unspecified | 3 | 27 |
| World Bank Income Groups | ||
| High Income | 10 | 91 |
| Upper Middle Income | 1 | 9 |
| World Bank Region | ||
| Europe and Central Asia | 7 | 64 |
| Latin America and Caribbean | 2 | 18 |
| North America | 1 | 9 |
| East Asia and Pacific | 1 | 9 |
| SGA definition | ||
| <–2 HAZ | 5 | 45 |
| <10th percentile | 3 | 27 |
| <5th percentile | 2 | 18 |
| <3rd percentile | 1 | 9 |
| Catch-up growth definition | ||
| >–2 HAZ | 5 | 45 |
| Δ0.67 HAZ change | 3 | 27 |
| >3rd percentile | 2 | 18 |
| >1.3 HAZ | 1 | 9 |
HAZ, height-for-age z score; SGA, small for gestational age; Δ, change in z score.
SGA classification
There was a lack of consensus of cut-off values to define SGA as well as the reference populations used to determine SGA. Researchers used 4 different cut-off points to define SGA. A birthweight of <–2 SDs was the most common in 5 (45%) studies, followed by a birthweight of less than the 10th percentile (27%), a birthweight of less than the 5th percentile (18%), and less than the 3rd percentile (9%). Only 1 reference population was used more than once (26).
CUG classification
CUG in height was determined in 2 ways: either as a positive change in HAZ or by achieving a value above a predetermined cut-off between birth and the follow-up period. Three studies defined CUG as a change in HAZ of >0.67. The remaining studies defined CUG as achieving an HAZ of >–2 SD (5 studies); >1.3 SDs (1 study); or greater than the 3rd percentile (2 studies). Again, there was little consensus on reference populations when determining CUG, and 3 studies did not specify any reference population for CUG.
Growth outcomes
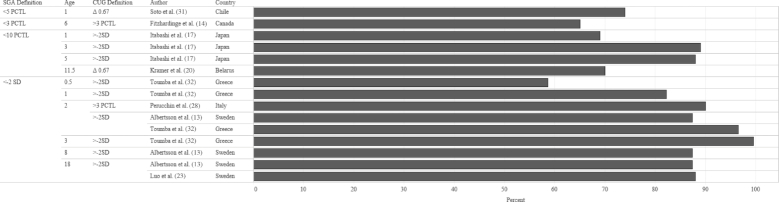
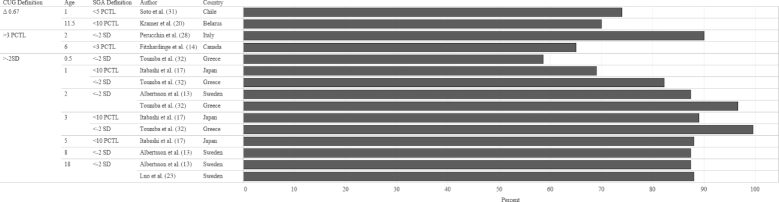
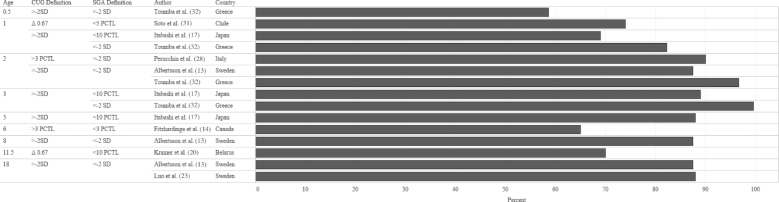
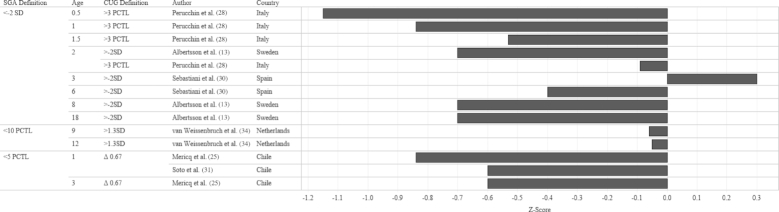
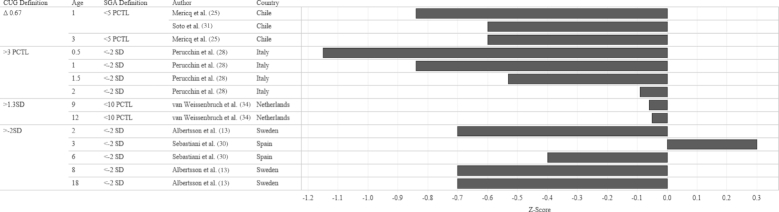
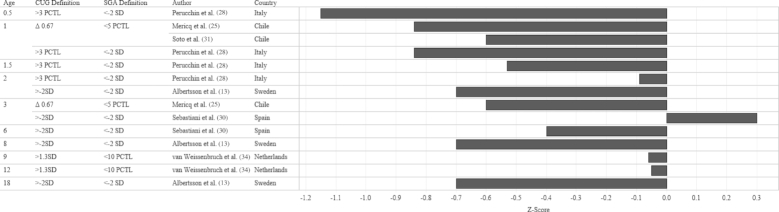
In order to identify the prevalence of CUG in infants born FT-SGA, we examined study outcomes according to 3 criteria: SGA definition at birth, CUG definition, and age when the outcome was measured. The median prevalence of CUG irrespective of SGA definition and CUG definition was 87.4% (Figures 2 and 3). Although a significant proportion of each study population appeared to achieve CUG, CUG did not clearly occur at a specific age. Growth measurements were collected at different follow-up intervals (Figure 4), which resulted in few overlapping data points. For example, Toumba et al. (32) collected data at 3 age points: 0.5, 1, and 3 years, whereas Luo et al. (23) only followed up at 18 y of age. Five of 7 studies that collected data from 2 y of age onward reported that >80% of the study population had achieved CUG. Rather than reporting the prevalence of CUG, some studies reported the median HAZ score of the cohort at specific follow-up intervals. When median HAZ scores were characterized by SGA definition or CUG definition, there were no discernable trends (Figures 5 and 6). Median HAZs improved from ages 0.5 to 2 years, after which they became inconsistent (Figure 7).
FIGURE 2.
Mean CUG reported as a percentage of study sample size by SGA definition, age, CUG definition, and study country. Values on the x-axis represent percentage CUG. CUG, catch-up growth; PCTL, percentile; SGA, small for gestational age.
FIGURE 3.
Mean CUG reported as a percentage of study sample size by CUG definition, age, SGA definition, and study country. Values on the x-axis represent percentage CUG. CUG, catch-up growth; PCTL, percentile; SGA, small for gestational age.
FIGURE 4.
Mean CUG reported as a percentage of study sample size by age, CUG definition, SGA definition, and study country. Values on the x-axis represent percentage CUG. CUG, catch-up growth; PCTL, percentile; SGA, small for gestational age.
FIGURE 5.
Mean CUG reported as mean z score of study sample size by SGA definition, age, CUG definition, and study country. Values on the x-axis represent percentage CUG. CUG, catch-up growth; PCTL, percentile; SGA, small for gestational age.
FIGURE 6.
Mean CUG reported as mean z score of study sample size by CUG definition, age, SGA definition, and study country. Values on the x-axis represent percentage CUG. CUG, catch-up growth; PCTL, percentile; SGA, small for gestational age.
FIGURE 7.
Mean CUG reported as mean z score of study sample size by age, CUG definition, SGA definition, and study country. Values on the x-axis represent percentage CUG. CUG, catch-up growth; PCTL, percentile; SGA, small for gestational age.
Discussion
To our knowledge, this is the first systematic review to examine the global prevalence of CUG in FT-SGA children. This review highlights the challenges associated with comparing outcomes where there is both a lack of consensus on definitions used for SGA and CUG as well as multiple growth reference standards for different populations and jurisdictions.
At least 3 research groups have noted that the use of different cut-offs and different reference standards markedly influences the prevalence of SGA and CUG. Karlberg et al. (6) were the first to show that the same cohort could yield variable prevalence rates for SGA if different definitions of SGA were used. Based on the use of 26 different reference populations, Katz et al. (5) calculated the prevalence of SGA based upon a birth weight less than the 10th percentile for 2 birth datasets: 1 from Nepal and 1 from South India. They found the prevalence of SGA to vary from 10.5% to 72.5% in Nepal and from 12.0% to 78.4% in South India among the 26 reference populations, and concluded that the prevalence of SGA and its association with neonatal mortality can vary significantly depending on the choice of the reference population. A systematic review by Chrestani et al. (37), examining associated factors for accelerated growth in childhood, also remarked on the lack of uniformity in CUG definitions and the need for standardization.
Despite the lack of uniformity in SGA and CUG definitions and reference populations, the findings of Karlberg et al. (6) that >85% of children born FT-SGA will achieve CUG in their first year is commonly accepted. We were unable to verify this outcome, given the great variability in definitions and reference populations in studies included in our review. Three studies in the review reported measurements of CUG at 1 year of age, none of which had achieved 85% CUG prevalence (range: 69–82.2%); however, by 2 years of age, 2 of 3 studies reported >85% prevalence of CUG (range: 87.4–96.6%) (Figure 4).
A key finding from this review is that without a standard definition for SGA and CUG, and a universally acceptable reference population standard, it will not be possible to obtain a true estimate of the prevalence of either SGA or CUG. In 2014, the International Fetal and Newborn Growth Consortium for the 21st Century (INTERGROWTH-21) published standards for newborn birth weight, length, and head circumference intended to help assess the newborn size and to complement the WHO Child and Adolescent Growth Standards (2006/2007) (38, 39). All 11 studies included in our review were conducted before the development and publication of the INTERGROWTH-21 birth weight for gestational age standards. Future studies, however, are more likely to use the WHO Growth Standards because 125 countries were reported to have adopted these standards by 2011 (40). It is more challenging to come up with globally accepted definitions of SGA and CUG because to a large extent these definitions remain subjective. Nevertheless, with the use of, for example, combinations of nominal group process, consensus development panels, and the Delphi technique it would be possible for researchers working in this field to come to an agreement on definitions (41). Until then, the “true" prevalence of SGA and CUG will remain elusive.
The most obvious limitation of this systematic review is our inability to complete a meta-analysis due to the high degree of heterogeneity of the included studies. This limited our ability to validate the finding of Karlberg et al. (6) of 85% CUG by 2 years of age. Additionally, the median prevalence values presented in each of the studies could not be tested statistically because of the different reference populations used. The wide range of study dates may also contribute to overestimating CUG when considering secular trends towards increased height. In collecting studies to be included in this systematic review, we noted a virtual absence of studies of CUG in children born FT-SGA in LMICs.
In conclusion, our review contributes to an understanding of CUG in FT-SGA infants, with the major caveat that a lack of common definitions and standard reference populations limits its true predictive value. Twenty-two years ago, the 1995 WHO report on “Physical Status: The Use and Interpretation of Anthropometry” recommended the use of a birth-weight-for-gestational-age cut-off at the 10th percentile according to the Williams curve as a reference for the classification of SGA (4). This recommendation was not wholly implemented but is based on reasonable statistical principles. Each tool used to define SGA or CUG may not be perfect but in order to make comparisons between future research in this area, they must be unified. To aid in this endeavour, we make the following proposals: 1) that SGA be defined based on the use of the 10th percentile as the cut-off; 2) that the INTERGROWTH-21 birth-weight-for-gestational-age standards be used to standardize the identification of infants born SGA; 3) that a change of >0.67 in z score represents a clinically significant response and should be used to define CUG (42); 4) that the WHO Growth Standards be used to calculate z scores.
The adverse consequences of being born SGA continue throughout the life cycle. A child's pattern of linear growth is an easily measured and comparable indicator of overall health (43). Knowing the optimal growth trajectory for FT-SGA children can assist the identification of growth problems and potential interventions.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—SCC and SZ: were involved in the concept and design of the systematic review; SCC and SC: screened abstracts, titles, and full-text articles, and undertook data extraction and quality assessment; all authors: interpreted the data; SCC: drafted initial versions of the manuscript which were revised by SC and SZ; and all authors: read and approved the final manuscript.
Notes
The authors reported no funding received for this study.
None of the authors have any conflicts of interest to disclose. None of the authors have any financial relationships relevant to this article to disclose.
Supplemental Tables 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: AGA, appropriate for gestational age; CUG, catch-up growth; FT-SGA, full-term small for gestational age; HAZ, height-for-age z score; LBW, low birth weight; LMIC, low- and middle-income country; SGA, small for gestational age.
References
- 1. Tudehope D, Vento M, Bhutta Z, Pachi P. Nutritional requirements and feeding recommendations for small for gestational age infants. J Pediatr. 2013;162(3 Suppl):S81–9. [DOI] [PubMed] [Google Scholar]
- 2. Lee AC, Katz J, Blencowe H, Cousens S, Kozuki N, Vogel JP, Adair L, Baqui AH, Bhutta ZA, Caulfield LE et al. National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010. Lancet Glob Health. 2013;1(1):e26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee PA, Chernausek SD, Hokken-Koelega AC, Czernichow P, International Small for Gestational Age Advisory Board. International Small for Gestational Age Advisory Board consensus development conference statement: management of short children born small for gestational age, April 24–October 1, 2001. Pediatrics. 2003;111(6 Pt 1):1253–61. [DOI] [PubMed] [Google Scholar]
- 4. WHO Physical Status: The Use and Interpretation of Anthropometry. WHO Technical Report Series Geneva: WHO; 1995. [Google Scholar]
- 5. Katz J, Wu LA, Mullany LC, Coles CL, Lee AC, Kozuki N, Tielsch JM. Prevalence of small-for-gestational-age and its mortality risk varies by choice of birth-weight-for-gestation reference population. PLoS One. 2014;9(3):e92074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Karlberg J, Albertsson-Wikland K. Growth in full-term small-for-gestational-age infants: from birth to final height. Pediatr Res. 1995;38(5):733–9. [DOI] [PubMed] [Google Scholar]
- 7. Wit JM, Boersma B. Catch-up growth: definition, mechanisms, and models. J Pediatr Endocrinol Metab. 2002;15(Suppl 5):1229–41. [PubMed] [Google Scholar]
- 8. Saenger P, Czernichow P, Hughes I, Reiter EO. Small for gestational age: short stature and beyond. Endocr Rev. 2007;28(2):219–51. [DOI] [PubMed] [Google Scholar]
- 9. van Wassenaer A. Neurodevelopmental consequences of being born SGA. Pediatr Endocrinol Rev. 2005;2(3):372–7. [PubMed] [Google Scholar]
- 10. Milovanovic I, Njuieyon F, Deghmoun S, Chevenne D, Levy-Marchal C, Beltrand J. SGA children with moderate catch-up growth are showing the impaired insulin secretion at the age of 4. PLoS One. 2014;9(6):e100337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hong YH, Chung S. Small for gestational age and obesity related comorbidities. Ann Pediatr Endocrinol Metab. 2018;23(1):4–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Verkauskiene R, Petraitiene I, Albertsson-Wikland K. Puberty in children born small for gestational age. Horm Res Paediatr. 2013;80(2):69–77. [DOI] [PubMed] [Google Scholar]
- 13. Albertsson-Wikland K, Karlberg J. Natural growth in children born small for gestational age with and without catch-up growth. Acta Paediatr Suppl. 1994;83(399):64–71. [DOI] [PubMed] [Google Scholar]
- 14. Fitzhardinge PM, Steven EM. The small for dates infant. Later growth patterns. Pediatrics. 1972;49(5):671–81. [PubMed] [Google Scholar]
- 15. Wislocki GB, Ingalls NW, Lineback PE, Streeter GL, Corner GW, Barry LW, Dantschakoff V. Contributions to Embryology, 11, USA: Carnegie Institution of America; 1920. [Google Scholar]
- 16. Stuart HC, Reed RB. Longitudinal studies of child health and development. Series 2. Description of project. Pediatrics. 1959;24(5):875–85. [PubMed] [Google Scholar]
- 17. Itabashi K, Mishina J, Tada H, Sakurai M, Nanri Y, Hirohata Y. Longitudinal follow-up of height up to five years of age in infants born preterm small for gestational age; comparison to full-term small for gestational age infants. Early Hum Dev. 2007;83(5):327–33. [DOI] [PubMed] [Google Scholar]
- 18. Nishisa H, Sakanoue M, Kurachi K, Asada M, Kubo S, Funakawa H. Fetal growth curves of Japanese. Acta Pediatr Jpn. 1984;20:90–7. [Google Scholar]
- 19. Suwa S, Tachibana K. Standard growth charts for height and weight of Japanese children from birth to 17 years based on a cross-sectional survey of national data. Clin Pediatr Endocrinol. 1993;2(2):87–97. [Google Scholar]
- 20. Kramer MS, Martin RM, Bogdanovich N, Vilchuk K, Dahhou M, Oken E. Is restricted fetal growth associated with later adiposity? Observational analysis of a randomized trial. Am J Clin Nutr. 2014;100(1):176–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kramer MS, Platt RW, Wen SW, Joseph K, Allen A, Abrahamowicz M, Blondel B, Bréart G; System FIHSGotCPS. A new and improved population-based Canadian reference for birth weight for gestational age. Pediatrics. 2001;108(2):e35. [DOI] [PubMed] [Google Scholar]
- 22. Kuczmarski RJ, Ogden CL. CDC Growth Charts. 2000, Advance Data, June, 314:1–27. [PubMed] [Google Scholar]
- 23. Luo ZC, Albertsson-Wikland K, Karlberg J. Length and body mass index at birth and target height influences on patterns of postnatal growth in children born small for gestational age. Pediatrics. 1998;102(6):E72. [DOI] [PubMed] [Google Scholar]
- 24. Niklasson A, Ericson A, Fryer J, Karlberg J, Lawrence C, Karlberg P. An update of the Swedish reference standards for weight, length and head circumference at birth for given gestational age (1977–1981). Acta Paediatr. 1991;80(8–9):756–62. [DOI] [PubMed] [Google Scholar]
- 25. Mericq V, Ong KK, Bazaes R, Pena V, Avila A, Salazar T, Soto N, Iniguez G, Dunger DB. Longitudinal changes in insulin sensitivity and secretion from birth to age three years in small- and appropriate-for-gestational-age children. Diabetologia. 2005;48(12):2609–14. [DOI] [PubMed] [Google Scholar]
- 26. Juez G. Intrauterine growth curve for the appropriate diagnosis of intrauterine growth retardation. Rev Med Chil. 1989;117(11):1311. [PubMed] [Google Scholar]
- 27. Youlton R, Valenzuela C. Growth patterns in height and weight in children aged 0 to 17 years and cranial circumference in children aged 0 to 2 years from medium-high and high socioeconomic status in Santiago. Comparison with growth in children from medium-low and low status in the northern area of Santiago. Rev Chil Pediatr. 1990;1–22. [PubMed] [Google Scholar]
- 28. Perucchin PP, Traggiai C, Calevo MG, Gastaldi R, Di Battista E, Amisano A, Lorini R. Auxological and metabolic study in small for gestational age children during 2 years follow-up. J Matern Fetal Neonatal Med. 2011;24(2):381–7. [DOI] [PubMed] [Google Scholar]
- 29. Gairdner D, Pearson J. A growth chart for premature and other infants. Arch Dis Child. 1971;46(250):783–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sebastiani G, Diaz M, Bassols J, Lopez-Bermejo A, De Zegher F, Ibanez L. The sequence of prenatal growth restraint and postnatal catch-up growth leads to a thicker intima media and more pre-peritoneal and hepatic fat by age 3–6 years. Hormone Res Paediatr. 2015;84:231. [DOI] [PubMed] [Google Scholar]
- 31. Soto N, Bazaes RA, Pena V, Salazar T, Avila A, Iniguez G, Ong KK, Dunger DB, Mericq MV. Insulin sensitivity and secretion are related to catch-up growth in small-for-gestational-age infants at age 1 year: results from a prospective cohort. J Clin Endocrinol Metab. 2003;88(8):3645–50. [DOI] [PubMed] [Google Scholar]
- 32. Toumba M, Hadjidemetriou A, Topouzi M, Savva SC, Demetriadou R, Kanaris C, Skordis N. Evaluation of the auxological and metabolic status in prepubertal children born small for gestational age. J Pediatr Endocrinol Metab. 2005;18(7):677–88. [DOI] [PubMed] [Google Scholar]
- 33. Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, Grummer-Strawn LM, Curtin LR, Roche AF, Johnson CL. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109(1):45–60. [DOI] [PubMed] [Google Scholar]
- 34. van Weissenbruch MM, Engelbregt MJ, Veening MA, Delemarre-van de Waal HA. Fetal nutrition and timing of puberty. Endocr Dev. 2005;8:15–33. [DOI] [PubMed] [Google Scholar]
- 35. Kloosterman G. Intrauterine growth and intrauterine growth curves. Ned Tijdschr Verloskd Gynaecol. 1969;69(5):349–65. [PubMed] [Google Scholar]
- 36. Joanna Briggs Institute. Joanna Briggs Institute Reviewers’ Manual: 2014 edition, Supplement. Australia: The Joanna Briggs Institute; 2014. [Google Scholar]
- 37. Chrestani MA, Santos IS, Horta BL, Dumith SC, de Oliveira Dode MA. Associated factors for accelerated growth in childhood: a systematic review. Matern Child Health J. 2013;17(3):512–9. [DOI] [PubMed] [Google Scholar]
- 38. Villar J, Cheikh Ismail L, Victora CG, Ohuma EO, Bertino E, Altman DG, Lambert A, Papageorghiou AT, Carvalho M, Jaffer YA et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014;384(9946):857–68. [DOI] [PubMed] [Google Scholar]
- 39. Group WHOMGRS. WHO child growth standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76–85. [DOI] [PubMed] [Google Scholar]
- 40. de Onis M. Update on the implementation of the WHO child growth standards. World Rev Nutr Diet. 2013;106:75–82. [DOI] [PubMed] [Google Scholar]
- 41. Waggoner J, Carline JD, Durning SJ. Is there a consensus on consensus methodology? Descriptions and recommendations for future consensus research. Acad Med. 2016;91(5):663–8. [DOI] [PubMed] [Google Scholar]
- 42. Cameron N. The human growth curve, canalization and catch-up growth. In: Cameron N Bogin B. Human Growth and Development. 2nd ed.Boston, MA: Academic Press; 2012. pp. 1–22. [Google Scholar]
- 43. Foote JM. Optimizing linear growth measurement in children. J Pediatr Health Care: Official Publication of National Association of Pediatric Nurse Associates & Practitioners. 2014;28(5):413–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.