ABSTRACT
Diet is proposed to have a stimulatory or preventative influence on mental health and the risk of depressive symptoms, given that the diet can have either a pro- or an anti-inflammatory effect. This study aimed to collate the relation between dietary inflammatory potential and the risk of depression. PubMed, Google Scholar, ScienceDirect, and Scopus databases, as well as Google were searched for articles published at any date until May 2018. Original English-language articles involving human participants and studies that investigated the association between dietary inflammatory potential and the risk of developing depression were included. Duplicated and irrelevant reports were screened out and data were extracted during critical analysis. Our search method initially identified 173 articles, of which 48 remained after duplicates had been removed. Thirteen articles were screened and identified as being relevant to the study topic. After critical analysis, 12 articles were included in the final analysis. All of the articles but 1 reported that higher dietary inflammatory index (DII) is associated with a higher risk of developing depression. Three studies indicated that DII was positively correlated with circulating inflammatory markers; however, in these studies increased concentrations of circulating inflammatory markers did not predict the diet–depression relation. Low literacy, unhealthy lifestyle, nutritional status, marital status, and age were potent contributory factors to whether or not a diet with inflammatory potential was consumed. These findings support the hypothesis that the DII is an appropriate tool for measuring dietary inflammatory potential, and reinforce the role of diets with inflammatory potential in the pathophysiology of depression.
Keywords: depression, dietary inflammatory index, stress, anxiety, mental health
Introduction
Depression is a prevalent and serious medical disease that adversely influences a person's feelings, thoughts, and actions and is related to increased levels of disability, morbidity, and mortality. Depression has been projected to affect 1 in 15 adults (6.7%) annually (1); it has been ranked 11th out of 291 diseases and injuries in regards to disability-adjusted life years, according to the most recent Global Burden of Disease study (2), and contributes to 4.3% of the total worldwide burden of disease, according to WHO estimations (3). Several factors, including biochemistry (alterations in certain chemicals in the brain) (4, 5), genetics (6), personality (individuals with low self-esteem or with high stress) (7, 8), and environmental factors, including persistent exposure to violence, neglect, abuse, or poverty, can play a role in the development of depression (9, 10). Recently, diet and circulating inflammation markers have been reported to be major contributors to the incidence of depression (11, 12).
The diet contains several bioactive parameters with pro- or anti-inflammatory features (13) and is proposed to have a stimulatory or preventive influence on depressive symptoms, in part due to the potency of certain food elements in affecting inflammatory pathways (14). For example, increased intake of refined carbohydrates and refined vegetable oils rich in omega-6 fatty acids such as arachidonic acid (a precursor to proinflammatory eicosanoids) and reduced intake of long-chain ω-3 fatty acids such as EPA (which produces anti-inflammatory eicosanoids) led to the production of arachidonic acid, thereby leading to elevated inflammation in various organs (15). The dietary inflammatory index (DII) was designed to evaluate whole-diet inflammatory capacity according to the pro- and anti-inflammatory efficacy of different dietary components on various circulating inflammatory biomarkers (13). The details on calculating and scoring the DII have been presented elsewhere (13). Briefly, the DII score is based on a review of 1943 qualified studies that investigated the influence of dietary parameters on 6 inflammatory markers: IL-1β, IL-4, IL-6, IL-10, TNF-α, and C-reactive protein (CRP). Forty-five food parameters from 11 food consumption data sets from countries around the world were identified as being related to the inflammatory markers. Each article was given 1 of 3 possible values, i.e., +1 (significantly increased inflammation), −1 (significantly decreased inflammation), or 0 (no change in inflammation), based on the food parameter. The articles were then weighed using the study characteristics, and the pro- and anti-inflammatory fractions for each food parameter were calculated using the weighted values of the articles. The anti-inflammatory fraction was subtracted from the proinflammatory fraction, and each food component was allocated an inflammatory effect score. For each food parameter, z scores were calculated and converted to a centered percentile, based on individual dietary intake data, the world mean, and the SD, and the gained scores were then multiplied by the corresponding inflammatory effect score to obtain a food parameter–specific DII score. The DII scores of entire food components were then summed to derive the overall DII score for each individual in the study. A higher DII score indicates greater proinflammatory potential of the diet, and vice versa (Supplemental Table 1).
The effects of a diet with a higher DII score (indicating a proinflammatory diet) on mental health and the development of depression have been assessed (16–18). However, there has been, to our knowledge, no comprehensive report summarizing these investigations. Therefore, this systematic review was designed to collate the relations between diets with inflammatory potential and the risk of depression.
Methods
The search strategy, screening, and selection criteria were as follows. This review was conducted according to the guidelines indicated in the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) protocols, 2015 statement. The PubMed, Google Scholar, ScienceDirect, and Scopus databases were searched, as was Google, for any articles published until May 2018. Original full-text English-language articles with human participants were included. Observational studies and journal articles were included whereas review articles were excluded. Articles that investigated the association between dietary inflammatory potential and the risk of developing depression were included. The following keywords were used: “dietary inflammatory index” or “dietary inflammatory potential” or “inflammatory dietary pattern” or “inflammatory diet” in the title and “depression” or “depressive symptoms” or “mental health” or “stress” or “anxiety” in the title or abstract. We manually reviewed the reference lists of articles that were included to identify further studies, and emailed authors when the full text of an article was not available. The research question was: Is the DII associated with the incidence of depressive symptoms in humans?
The articles identified in the search were saved in an EndNote software (Thomson Reuters, philadelphia, PA, USA) file and sorted to remove duplicate reports. The remaining titles and abstracts were reviewed to screen for articles with the correct scope for this review. The full texts of the screened articles were then critically analyzed separately for eligibility and data extraction. We excluded studies that assessed the relation between the DII and other brain disorders, e.g., neurodegenerative diseases including Alzheimer's or Parkinson's disease.
Results
Study characteristics and selection
As presented in Figure 1, we initially identified 173 titles and abstracts using the aforementioned search method. Forty-eight studies remained after duplicate studies had been removed. During the screening phase, 13 articles were identified as being relevant to the study topic. During critical analysis of the screened articles, we excluded 1 article because the full text was unavailable (19). Eventually, 12 articles that matched the study scope were included in the final review and analysis (Figure 1).
FIGURE 1.
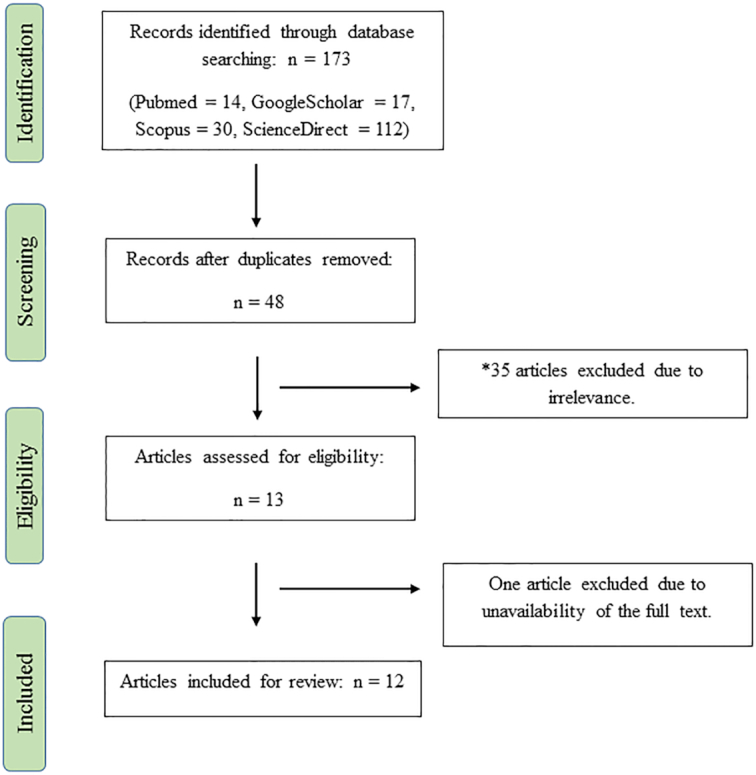
Flow diagram of the study. *Articles that investigated the association between dietary inflammatory potential and the risk of developing depression were included in this review.
Table 1 shows that all of the studies included were published or accepted in 2013–2018. Out of 45 food parameters (Supplemental Table 1), 24–37 were used for evaluation of the DII. All of the studies were conducted on the general adult population and used a cohort or cross-sectional design, and there were no clinical studies investigating the association of the DII with depression-related neuropsychological function or chemical biomarkers.
TABLE 1.
Characteristics and extracted data of the included studies1
| Authors | Country | Type of study | Target population | Population, n | Method of DII evaluation | Age, y | Primary outcome | Number of food parameters of 45 | Duration of follow-up, y | Findings |
|---|---|---|---|---|---|---|---|---|---|---|
| Adjibade et al. (20) | France | Cohort | General population | 3523 | Repeated 24-h dietary records | 35–60 | Depression | 36 | 12.6 | In the full sample, DII was not associated with incident depressive symptoms (OR: 1.07; 95% CI: 0.66, 0.72). Men (OR: 2.32; 95% CI: 1.01, 5.35), smokers (OR: 2.21; 95% CI: 1.08, 4.52), and less physically active participants (OR: 2.07; 95% CI: 1.05, 4.07) with a higher DII had a higher risk of incident depressive symptoms.2 Those with high DII were mostly women (P < 0.0001), younger (P < 0.0001), less educated (P < 0.0001), less physically active (P < 0.0001), and with normal BMI (67%).2 |
| Akbaraly et al. (21) | United Kingdom | Cohort | Middle-aged men and men from Whitehall II sdy | 4246 | FFQ | 35–55 | Depression | 27 | 5 | DII was correlated with recurrent depressive symptoms only in women (OR: 2.83; 95% CI: 1.48, 5.42). Those with higher DII score were mostly current smokers (P < 0.001), more physically inactive (P < 0.001), living alone (P = 0.01), had less energy intake (P < 0.001), a low socioeconomic position (P < 0.001), and higher concentrations of IL-6 (P = 0.006) and CRP (P = 0.002).2 Inflammatory markers did not predict the association between DII and recurrent depressive symptoms. |
| Bergmans and Malecki (22) | United States | Cross-sectional | Adults | 11,592 | 24-h dietary recalls | ≥20 | Depression and distress | 27 | — | DII↑→ Depression↑ (OR: 2.26; 95% CI: 1.60, 3.20); frequent distress↑ (OR: 1.81; 95% CI: 1.20, 2.71). Those with high DII were mostly females, more current smokers, obese, were worse regarding education, employment, smoking status, health insurance status, and poverty:income ratio, and had lower supplement use and total energy intake (all, P < 0.0001).2 |
| Jorgensen et al. (16) | United States | Cross-sectional | Adults without CVD | 11,624 | 24-h dietary recall | ≥18 | Depression | 28 | — | DII↑→ Depressive risk↑ (OR: 1.78; 95% CI: 1.32, 2.40). CRP was significantly correlated with DII score (P < 0.001). CRP concentration was higher in those with current depression (P < 0.0001). |
| Lucas et al. (23) | United States | Cohort | Women without depression | 43,685 | FFQ | 50–77 | Depression | — | 12 | DII↑→ Depressive risk↑ (OR: 1.41; 95% CI: 1.22, 1.63). Those with high IDP were more likely to be a never smoker, with higher BMI, be less physically active, with a high total energy intake, be lesser users of multivitamins, have lower intake of caffeine and alcohol, and high prevalence of cardiometabolic diseases (all, P < 0.0001).2 |
| Phillips et al. (18) | Ireland | Cross-sectional | Adults | 2047 | FFQ | 50–69 | Depression and anxiety | 26 | — | DII↑→ Depressive risk↑ (OR: 2.23; 95% CI: 1.15, 4.36). In gender-stratified analyses correlations were noted in women only. Those with higher DII were more male (P < 0.0001), sedentary (P < 0.0001), and more current smokers (P = 0.001).2 The relation between DII and mental health is not regulated by circulating inflammatory status. |
| Shivappa et al. (24) | United States | Cohort | Adults at risk of arthritis | 3648 | FFQ | 45–79 | Depression | 24 | 8 | DII↑→ Depressive risk↑ (HR: 1.24; 95% CI: 1.01, 1.53). Those with high DII were younger (P < 0.0001), more males (P < 0.0001), smokers (P = 0.001), less educated (P = 0.002), obese (P < 0.0001), and used statins less frequently (P < 0.0001).2 |
| Shivappa et al. (25) | Iran | Cross-sectional | Adolescent girls | 299 | FFQ | 15–18 | Stress | 31 | — | Girls with higher DII had higher BMI and DASS stress scores (β: 2.75; 95% CI: 1.05, 4.46) and had a 3.48-fold risk of having at least a moderate level of stress. |
| Shivappa et al. (26) | Australia | Cohort | Middle-aged women | 6438 | FFQ | 45–50 | Depression | 26 | 12 | DII↓→ Depression risk↓ (RR: 0.81; 95% CI: 0.69, 0.96) Women with higher DII more often had lower education (P = 0.02), were separated, divorced, or widowed (P < 0.0001), had low physical activity (P < 0.0001), and were more current smokers (P < 0.0001).2 |
| Sánchez-Villegas et al. (17) | Spain | Cohort | University graduates | 15,093 | FFQ | Mean ± SD: 38.28 ± 11.96 | Depression | 28 | 10 | DII↑→ Depression risk↑ (HR: 1.47; 95 % CI: 1.17, 1.85). Association of DII with depression was stronger among older individuals (HR: 2.70; 95% CI: 1.22, 5.97) and among those with cardiometabolic comorbidities (HR: 1.80; 95% CI: 1.70, 2.57). Those with higher DII were more men, single, younger, had lower daily energy intake, and were more current smokers (all, P < 0.001).2 |
| Vermeulen et al. (28) | Italy | Cohort | Older adults InCHIANTI study | 827 | FFQ | ≥65 | Depression | — | 3, 6, 9 | No longitudinal association was found of high inflammatory dietary pattern I with depression (OR: 0.90; 95% CI: 0.55, 1.45) or depressive symptoms (B = 0.04; 95% CI: –0.06, 0.13). Dietary pattern was related to circulating inflammatory markers. Participants in the highest quartile of inflammatory dietary pattern I were older (P = 0.004), less educated (P = 0.01), more inactive (P = 0.03), more a never smoker (P = 0.01), had lower energy intakes (P < 0.001), and higher concentrations of TNF-α (P < 0.001) and CRP (P < 0.001).2 Participants in the highest quartile of inflammatory dietary pattern II were more often female (P = 0.007), less educated (P = 0.007), had higher energy intakes (P = 0.02), used more anti-inflammatory drugs (P = 0.02), and had higher concentrations of IL-18 (P = 0.001).2 |
| Wirth et al. (27) | United States | — | US NHANES | — | 24-h dietary recalls | — | Depression | — | — | DII scores were correlated with depressive symptoms among women (OR: 1.30; 95% CI: 1.00, 1.68). Risk of depressive symptoms was 30% greater among women with higher DII. |
CRP, C-reactive protein; DASS, Depression Anxiety Stress Scale; DII, dietary inflammatory index; IDP, inflammatory dietary pattern.
Compared with those who had lower DII.
Age, sex, race/ethnicity, BMI, education, energy intake, occupational status, smoking status, physical activity, comorbid diseases, and income were the common confounding factors considered in all of the studies analyzing the association between the DII and mental health status.
Association of the DII with the risk of developing depressive symptoms
In total, we included 12 studies that assessed the relation between the DII and depression. All of the articles (16–18, 20–27) that used the DII measurement tool developed by Shivappa et al. (13) reported that a higher DII was associated with a higher risk of developing depression, which may reinforce the role of diets with inflammatory potential in the pathophysiology of depression. In a cohort study of 3523 people, Adjibade et al. (20) showed that a greater DII was associated with higher occurrence of depressive symptoms in men, but not in the whole population. In a cohort study of 4246 people, Akbaraly et al. (21) indicated that the DII was correlated with recurrent depressive symptoms in women but not in men, and found that women with higher DII had greater odds of having recurrent depressive symptoms over the 5-y follow-up period. In the Seguimiento Universidad de Navarra Project, Sánchez-Villegas et al. (17) included 15,093 participants, and reported that individuals with higher DII had a 47% risk of developing depression. The Nurses’ Health Study that involved 43,685 participants indicated that the most proinflammatory diets correlated with a 30–40% increased risk of depression (23). Seven of the studies included also reported that participants with higher DII scores had greater odds of having depressive symptoms (16, 18, 22, 24–27) (Table 1). However, in the InCHIANTI study, Vermeulen et al. (28) reported that high inflammatory dietary patterns, measured using a different method to Shivappa et al. (13), were not longitudinally associated with depression and depressive symptoms.
Stress and anxiety are risk factors and major comorbidities of depression that may contribute to the pathophysiology of depression (29, 30). Two studies reported a positive correlation between higher DII scores and higher Depression Anxiety Stress Scale scores, whereas another study reported a positive correlation between higher DII scores and anxiety (18, 22, 25). In a cross-sectional study of 11,592 adults, Bergmans and Malecki (22) reported that higher DII scores were related with higher frequencies of distress. Shivappa et al. (25) performed a cross-sectional study on 299 adolescent girls and reported that girls with higher DII had higher Depression Anxiety Stress Scale-stress scores. In a cross-sectional study on 2047 adults, Phillips et al. (18) demonstrated that higher DII scores were correlated with an increased risk of anxiety; however, the association did not persist in the fully adjusted model.
Taken together, our findings support the association between the DII and depression, although some studies found that the association was gender-specific. Three studies indicated that the DII score was significantly correlated with depressive symptoms among women (18, 21, 27), whereas 1 study showed an increased correlation among men (20).
The role of circulating proinflammatory markers in the association between diet and depression
Four of the studies included demonstrated the regulatory effect of circulating inflammatory markers on the relation between the DII and mental health (16, 18, 21, 28). Three studies indicated that DII scores were positively correlated with circulating inflammatory markers; however, in these studies increased circulating inflammatory markers did not bridge the link between dietary inflammatory potential and depression. Akbaraly et al. (21) reported that women with a high DII had higher concentrations of circulating inflammatory markers, including IL-6 and CRP, and were more likely to have recurrent depressive symptoms. However, the correlation between DII and recurrent depressive symptoms was not related to plasma inflammatory marker concentrations (21). Phillips et al. (18) demonstrated that the association between DII and mental health was not related to circulating inflammatory markers. Vermeulen et al. (28) showed that participants in the highest quartile of inflammatory dietary patterns had higher TNF-α and CRP concentrations; however, they concluded that the link between dietary patterns and inflammatory markers was not involved in the increased likelihood of having depressive symptoms. Jorgensen et al. (16) indicated that individuals with current depression had higher CRP concentrations, which was positively associated with DII score.
Taken together, a positive correlation between the DII and circulating inflammatory markers may support the DII as an appropriate tool for measuring dietary inflammatory potential. However, increased inflammatory markers do not predict a link between dietary inflammatory potential and depression.
Determinants of dietary inflammatory potential
According to the articles included in this review (Table 1), the individuals with high DII scores were mostly smokers (in total, 6 articles; current smokers, 4 articles), physically less active (6 articles), less educated (7 articles), had lower energy intake (4 articles), were obese (4 articles), younger (3 articles), and single or living alone or widowed (4 articles). Other factors associated with high DII were lower income (1 article), unemployment (1 article), lower health status (1 article), low supplement use (2 articles), normal BMI (1 article), and the presence of comorbid diseases (1 article). In 4 articles, high DII scores were mostly observed in men; 3 other articles observed them in women. In all of the studies included, the effects of all of the confounder factors, including age, sex, level of literacy, smoking, physical activity, marital status, etc., were adjusted in the analysis of the relation between the DII and depression.
Discussion
An extensive body of literature has indicated that nutrition and dietary patterns in humans are linked to changes in cognition, behavior, and emotions. It has been suggested that nutritional compounds can contribute to the onset, severity, and duration of depression (31, 32). More recent reviews have mainly focused on the relation between nutrition, dietary quality, or dietary pattern and the odds of having depressive symptoms, and have indicated that nutritional inadequacies, unhealthy dietary patterns, and low-quality diets are associated with poor mental health. In a review, Rao et al. (33) reported that nutritional deficiencies, including those of vitamins (folate, vitamin B-12), minerals (iron, zinc, magnesium, selenium), and amino acids, are common in depressed patients. In addition, several reviews have indicated that vitamins (vitamins D and B), minerals (calcium, zinc, selenium), fish, and PUFAs may have a protective effect against the development of depressive symptoms (34–37). Reviews and meta-analyses have shown that unhealthy Western dietary patterns are associated with an increased risk of depression, whereas the healthy Mediterranean diet is inversely associated with the risk of depression (38, 39). In a meta-analysis of prospective studies, Molendijk et al. (40) concluded that adherence to a high-quality diet was related to a lower risk of the onset of depressive symptoms. In a systematic review, Baskin et al. (41) reported that poor-quality diets were positively associated with antenatal depressive and stress symptoms. There is also a large amount of evidence that food parameters with anti-inflammatory characteristics may be protective against depressive symptoms. For example, curcumin, fiber, and garlic are all food parameters with low DII (13) and high anti-inflammatory activity (42–45), which can decrease the development of depression. In a meta-analysis, Ng et al. (46) indicated that curcumin has significant clinical efficacy in ameliorating depressive symptoms. In a cross-sectional study, Miki et al. (47) reported that higher dietary fiber consumption from vegetables and fruits might reduce the odds of having depressive symptoms. Marschollek et al. (48) demonstrated that garlic oil dose-dependently suppresses cortical spreading depression. However, the DII is a new dietary indicator that evaluates the proinflammatory or anti-inflammatory status of the overall diet. To date, no review has assessed the relation between the DII and the risk of depressive symptoms. Here, we assessed this relation and found a positive correlation between the DII and the odds of developing depressive symptoms.
Among the studies reviewed, Vermeulen et al. (28) did not support the notion that a diet with increased inflammatory potential is associated with higher incidence of depression. In this study, dietary patterns with inflammatory potential were identified based on a set of food groups from the Italian National Food Consumption data, which showed variation in a number of inflammatory markers. This method only includes a limited number of food components when identifying the inflammatory potential of a diet (e.g., higher scores for diets with high intakes of refined grains, sweet snacks, pasta, and rice) and is likely to be unable to properly predict the inflammatory potential of the entire diet. Moreover, it does not include consumable foods from other countries, thereby studies performed in different countries may possibly not be fully comparable. The DII on the other hand, as mentioned in the Introduction to this article, is a tool that scores diets based on 45 food components from 11 food consumption data sets from countries around the world. Hence, it seems to be a more appropriate tool for determining dietary inflammatory potential.
According to the findings of the studies included in our review, a high DII score is associated with increased concentrations of circulating inflammation markers, and with an elevated risk of depression. However, the link between dietary inflammatory potential and depression did not appear to be related to increased inflammatory markers, because adjustment for inflammatory markers did not significantly attenuate the DII–depression relation. The mechanisms by which dietary inflammatory potential correlates with depressive symptoms are not fully clarified. Extensive evidence indicates that depression is associated with circulating inflammation markers, and that increased amounts of inflammation enhance the risk of developing depression (49–51). Exogenous proinflammatory cytokine infusions lead to depression-like behaviors and features. A recent meta-analysis determined that 25% of patients with hepatitis C treated with IFN developed depressive symptoms (52). Moreover, antidepressant medication, particularly serotonin inhibitors, leads to the reduced production of proinflammatory cytokines, including IL-1, IL-6, TNF-α, and IFN-γ (53, 54). These findings all indicate that an increased proinflammatory status might be involved in the pathogenesis of depression.
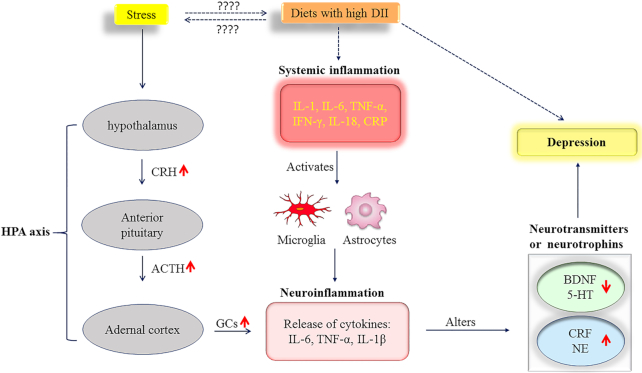
However, because concentrations of circulating inflammatory markers did not predict the diet–depression relation, according to Baron and Kenny's (55) models, it is possible that inflammation is more likely to influence the occurrence of depression by indirectly mediating the concentrations of several neurotransmitters or neuropeptides. As shown in Figure 2, it has been speculated that systemic inflammation leads to neuroinflammation through microglial and astrocyte activation (56–58). A systematic review of animal experiments noted that peripheral inflammatory stimuli lead to microglial activation and an inflammatory response in the brain (56). Hoogland et al. (57) showed that systemic stimulation with live Escherichia coli causes microglial activation and an inflammatory response in the brain. Neuroinflammation predisposes to the development of depression by increasing glutamatergic neurotransmission, reducing neurotropic factors, and altering the metabolism of neurotransmitters in the brain (59, 60). In depressed patients, changes to several neurotransmitters or neurotrophins in the central nervous system, including serotonin, norepinephrine, corticotropin-releasing factor, and brain-derived neurotrophic factor, have all been reported (61–64). In addition, inflammatory factors can modulate the concentrations of the aforementioned neurotransmitters and neuropeptides (60) and thereby may induce susceptibility to psychiatric disorders. Taken together, accounting for inflammatory factors alone may not be sufficient for identifying the diet–inflammation–depression relation without considering other interface mediators, including neurotransmitters, neurotrophins, or neuropeptides.
FIGURE 2.
A possible mechanistic model for the diet–inflammation–depression relation. Dashed arrows represent findings from the current study and solid arrows show findings from previous studies. 5-HT, serotonin; ACTH, adrenocorticotropic hormone; BDNF, brain-derived neurotrophic factor; CRF, corticotropin-releasing factor; CRH, corticotropin-releasing hormone; CRP, C-reactive protein; DII, dietary inflammatory index; GC, glucocorticoid; HPA, hypothalamic–pituitary–adrenal; NE, norepinephrine.
Another possibility is that an inflammatory diet may directly induce alterations in multiple neurobiological factors, e.g., serotonin, norepinephrine, corticotropin-releasing factor, and brain-derived neurotrophic factor, which each contribute to depression. However, the association of an inflammatory diet with the biochemical or neurochemical factors involved in the pathogenesis of depression, including neurotransmitters or neurotrophins, has not been studied, to our knowledge.
In the present study, higher DII scores were positively associated with higher stress levels. The exact mechanism for this correlation is unclear. Chronic stress is a strong risk factor for psychiatric disorders, including depression (65). Figure 2 shows that stress influences the hypothalamus and stimulates the release of corticotropin-releasing hormone, which subsequently activates the hypothalamic–pituitary–adrenal axis and increases the secretion of adrenocorticotropic hormone from the pituitary gland, contributing to an increased release of glucocorticoids from the adrenal cortex. Under normal conditions, glucocorticoids lead to the termination of the stress reaction (65, 66). However, glucocorticoid dysfunction enhances the expression and function of the inflammasome NLRP3 (NLR family pyrin domain containing 3) and promotes neuroinflammation, which might subsequently influence the concentrations of other neurochemicals and contribute to depression (66, 67).
Our findings demonstrate that low literacy and an unhealthy lifestyle (being inactive, smoking) are potent factors that negatively influence dietary behaviors. Nutritional status (obesity, lower energy intake), marital status (single or living alone or widowed), and a younger age are other factors that might affect food choices. Our findings are in line with those of previous studies. van Lenthe et al. (68) reported that higher education and higher net household income levels are connected to healthier food behaviors. Higher education was related to higher consumption of healthy foods, including fruits and vegetables. In a cohort study, Reininger et al. (69) showed that unhealthy eating behaviors were more common among younger participants. In a cross-sectional study of 7236 participants, Ruiz-Canela et al. (70) indicated a positive correlation between DII and obesity. In the Seguimiento Universidad de Navarra cohort study, Ramallal et al. (71) reported that a high-DII diet was associated with an increased risk of developing obesity during the 8.1-y follow-up.
Conclusions
Our findings support the DII as an appropriate tool for measuring dietary inflammatory potential and validate the role of diets with inflammatory potential in the pathophysiology of depression. A high DII may be correlated with increased circulating inflammatory markers. In addition, our findings suggest that low literacy, unhealthy lifestyle, poor nutritional status (obesity, lower energy intake), marital status (single or living alone or widowed), and younger age are potent factors that may contribute to having a diet with inflammatory potential. Further animal, clinical, or dietary interventional studies are needed to investigate the associations of the DII with depression-related neuropsychological function and with the neurochemical factors involved in the pathogenesis of depression, including neurotransmitters or neurotrophins, to provide more evidence of the mechanistic links between DII and depression.
Application of the findings
At whole population level, a primary prevention strategy involving the promotion of knowledge of, and attitudes towards, diets with more anti-inflammatory parameters (as listed in Supplemental Table 1) through training and advertising or marketing activities may result in healthier dietary habits. This should consequently be useful for inhibiting the occurrence of depression, particularly among smokers and people with low levels of education or sedentary lifestyles. Furthermore, the provision of dietary recommendations in psychiatric clinical care could have a place in inhibiting the development of depression.
Strengths and limitations of the study
This study's greatest strength is that all of the studies included were prospective cohort studies with large sample sizes (299–43,685 participants) and mostly had long follow-up periods (5–12.6 y). Most of the studies (10 of 12) used the same tool for assessing the DII. All of the studies included adjusted for the role of potential confounding factors (age, literacy, physical activity, marital status, etc.) in their analyses of the association between the DII and depressive symptoms. However, the studies searched had several limitations, such as varying measurement tools and cutoffs for the diagnosis of depression. No clinical study investigated the association between the DII and neurochemical factors such as neurotransmitters or neurotrophins, which are substantial contributors to the pathogenesis of depression. Further clinical studies may therefore be beneficial for determining the mechanistic pathways that regulate the relation between the DII and depression.
Supplementary Material
Acknowledgments
Both authors read and approved the final manuscript.
Notes
The authors reported no funding received for this study.
Author disclosures: SK and MA, no conflicts of interest.
Supplemental Table 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances.
Abbreviations used: CRP, C-reactive protein; DII, dietary inflammatory index.
References
- 1. American Psychiatric Association [Internet]. What is Depression?. Washington, DC: American Psychiatric Association; 2017; [cited 7 Jun, 2018]. Available from: https://www.psychiatry.org/patients-families/depression/what-is-depression. [Google Scholar]
- 2. Murray CJL, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2197–223. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization. Mental Health Action Plan 2013 - 2020. Geneva, Switzerland: WHO; 2013; [cited 7 Jun, 2018]. Available from: http://www.who.int/mental_health/publications/action_plan/en/. [Google Scholar]
- 4. Bao AM, Ruhé HG, Gao SF, Swaab DF. Neurotransmitters and neuropeptides in depression. Handb Clin Neurol. 2012;106:107–36. [DOI] [PubMed] [Google Scholar]
- 5. Werner FM, Coveñas R. Classical neurotransmitters and neuropeptides involved in major depression in a multi-neurotransmitter system: a focus on antidepressant drugs. Curr Med Chem. 2013;20(38):4853–8. [DOI] [PubMed] [Google Scholar]
- 6. Kendler KS, Ohlsson H, Lichtenstein P, Sundquist J, Sundquist K. The genetic epidemiology of treated major depression in Sweden. Am J Psychiatry. 2018;175(11):1137–44. [DOI] [PubMed] [Google Scholar]
- 7. Hilbert S, Goerigk S, Padberg F, Nadjiri A, Übleis A, Jobst A, Dewald-Kaufmann J, Falkai P, Bühner M, Naumann F et al. The role of self-esteem in depression: a longitudinal study. Behav Cogn Psychother. 2018;1–7. [DOI] [PubMed] [Google Scholar]
- 8. Fox CW, Reed DH. Inbreeding depression increases with environmental stress: an experimental study and meta-analysis. Evolution. 2011;65(1):246–58. [DOI] [PubMed] [Google Scholar]
- 9. Chan KL, Lo R, Ip P. From exposure to family violence during childhood to depression in adulthood: a path analysis on the mediating effects of intimate partner violence. J Interpers Violence. 2018;886260518790596. [DOI] [PubMed] [Google Scholar]
- 10. Behr Gomes Jardim G, Novelo M, Spanemberg L, von Gunten A, Engroff P, Nogueira EL, Cataldo Neto A. Influence of childhood abuse and neglect subtypes on late-life suicide risk beyond depression. Child Abuse Negl. 2018;80:249–56. [DOI] [PubMed] [Google Scholar]
- 11. Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctôt KL. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–57. [DOI] [PubMed] [Google Scholar]
- 12. Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. 2016;21:1696–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shivappa N, Steck SE, Hurley TG, Hussey JR, Hebert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17:1689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaplan BJ, Rucklidge JJ, Romijn A, McLeod K. The emerging field of nutritional mental health: inflammation, the microbiome, oxidative stress, and mitochondrial function. Clin Psychol Sci. 2015;3(6):964–80. [Google Scholar]
- 15. Sears B, Ricordi C. Anti-inflammatory nutrition as a pharmacological approach to treat obesity. J Obes. 2011:431985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jorgensen D, White GE, Sekikawa A, Gianaros P. Higher dietary inflammation is associated with increased odds of depression independent of Framingham Risk Score in the National Health and Nutrition Examination Survey. Nutr Res. 2018;54:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sánchez-Villegas A, Ruíz-Canela M, de la Fuente-Arrillaga C, Gea A, Shivappa N, Hébert JR, Martínez-González MA. Dietary inflammatory index, cardiometabolic conditions and depression in the Seguimiento Universidad de Navarra cohort study. Br J Nutr. 2015;114(9):1471–9. [DOI] [PubMed] [Google Scholar]
- 18. Phillips CM, Shivappa N, Hébert JR, Perry IJ. Dietary inflammatory index and mental health: a cross-sectional analysis of the relationship with depressive symptoms, anxiety and well-being in adults. Clin Nutr. 2018;37(5):1485–91. [DOI] [PubMed] [Google Scholar]
- 19. Wirth M, Shivappa N, Hurley T, Burch J, Hebert J. Association between the Dietary Inflammatory Index (DII) and depressive symptoms from the National Health and Nutrition Examination Survey. J Acad Nutr Diet. 2016;116(9):A72. [Google Scholar]
- 20. Adjibade M, Andreeva VA, Lemogne C, Touvier M, Shivappa N, Hébert JR, Wirth MD, Hercberg S, Galan P, Julia C et al. The inflammatory potential of the diet is associated with depressive symptoms in different subgroups of the general population. J Nutr. 2017;147(5):879–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Akbaraly T, Kerlau C, Wyart M, Chevallier N, Ndiaye L, Shivappa N, Hébert JR, Kivimäki M. Dietary inflammatory index and recurrence of depressive symptoms: results from the Whitehall II Study. Clin Psychol Sci. 2016;4(6):1125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bergmans RS, Malecki KM. The association of dietary inflammatory potential with depression and mental well-being among U.S. adults. Prev Med. 2017;99:313–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lucas M, Chocano-Bedoya P, Schulze MB, Mirzaei F, O'Reilly ÉJ, Okereke OI, Hu FB, Willett WC, Ascherio A. Inflammatory dietary pattern and risk of depression among women. Brain Behav Immun. 2014;36:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shivappa N, Hébert JR, Veronese N, Caruso MG, Notarnicola M, Maggi S, Stubbs B, Firth J, Fornaro M, Solmi M. The relationship between the dietary inflammatory index (DII®) and incident depressive symptoms: a longitudinal cohort study. J Affect Disord. 2018;235:39–44. [DOI] [PubMed] [Google Scholar]
- 25. Shivappa N, Hebert JR, Rashidkhani B. Association between inflammatory potential of diet and stress levels in adolescent women in Iran. Arch Iran Med. 2017;20(2):108–12. [PubMed] [Google Scholar]
- 26. Shivappa N, Schoenaker DA, Hebert JR, Mishra GD. Association between inflammatory potential of diet and risk of depression in middle-aged women: the Australian Longitudinal Study on Women's Health. Br J Nutr. 2016;116(6):1077–86. [DOI] [PubMed] [Google Scholar]
- 27. Wirth MD, Shivappa N, Burch JB, Hurley TG, Hébert JR. The Dietary Inflammatory Index, shift work, and depression: results from NHANES. Health Psychol. 2017;36(8):760–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vermeulen E, Brouwer IA, Stronks K, Bandinelli S, Ferrucci L, Visser M, Nicolaou M. Inflammatory dietary patterns and depressive symptoms in Italian older adults. Brain Behav Immun. 2018;67:290–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Plieger T, Melchers M, Montag C, Meermann R, Reuter M. Life stress as potential risk factor for depression and burnout. Burnout Res. 2015;2(1):19–24. [Google Scholar]
- 30. Xin LM, Chen L, Ji ZP, Zhang SY, Wang J, Liu YH, Yang FD, Wang G, Fang YR, Lu Z et al. Risk factors for anxiety in major depressive disorder patients. Clin Psychopharmacol Neurosci. 2015;13(3):263–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dana-Alamdari L, Kheirouri S, Noorazar SG. Serum 25-hydroxyvitamin D in patients with major depressive disorder. Iran J Public Health. 2015;44(5):690–7. [PMC free article] [PubMed] [Google Scholar]
- 32. Berridge MJ. Vitamin D and depression: cellular and regulatory mechanisms. Pharmacol Rev. 2017;69(2):80–92. [DOI] [PubMed] [Google Scholar]
- 33. Rao TS, Asha MR, Ramesh BN, Rao KS. Understanding nutrition, depression and mental illnesses. Indian J Psychiatry. 2008;50(2):77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sparling TM, Henschke N, Nesbitt RC, Gabrysch S. The role of diet and nutritional supplementation in perinatal depression: a systematic review. Matern Child Nutr. 2017;13(1):e12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gould JF, Best K, Makrides M. Perinatal nutrition interventions and post-partum depressive symptoms. J Affect Disord. 2017;224:2–9. [DOI] [PubMed] [Google Scholar]
- 36. Miller BJ, Murray L, Beckmann MM, Kent T, Macfarlane B. Dietary supplements for preventing postnatal depression. Cochrane Database Syst Rev. 2013;(10):CD009104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Spedding S. Vitamin D and depression: a systematic review and meta-analysis comparing studies with and without biological flaws. Nutrients. 2014;6(4):1501–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li Y, Lv MR, Wei YJ, Sun L, Zhang JX, Zhang HG, Li B. Dietary patterns and depression risk: a meta-analysis. Psychiatry Res. 2017;253:373–82. [DOI] [PubMed] [Google Scholar]
- 39. Martínez-González MA, Sánchez-Villegas A. Food patterns and the prevention of depression. Proc Nutr Soc. 2016;75(2):139–46. [DOI] [PubMed] [Google Scholar]
- 40. Molendijk M, Molero P, Ortuño Sánchez-Pedreño F, Van der Does W, Angel Martínez-González M. Diet quality and depression risk: a systematic review and dose-response meta-analysis of prospective studies. J Affect Disord. 2018;226:346–54. [DOI] [PubMed] [Google Scholar]
- 41. Baskin R, Hill B, Jacka FN, O'Neil A, Skouteris H. The association between diet quality and mental health during the perinatal period. A systematic review. Appetite. 2015;91:41–7. [DOI] [PubMed] [Google Scholar]
- 42. Chainani-Wu N. Safety and anti-inflammatory activity of curcumin: a component of tumeric (Curcuma longa). J Altern Complement Med. 2003;9(1):161–8. [DOI] [PubMed] [Google Scholar]
- 43. Kuo SM. The interplay between fiber and the intestinal microbiome in the inflammatory response. Adv Nutr. 2013;4(1):16–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Grooms KN, Ommerborn MJ, Pham DQ, Djoussé L, Clark CR. Dietary fiber intake and cardiometabolic risks among US adults, NHANES 1999–2010. Am J Med. 2013;126(12):1059–67..e1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Arreola R, Quintero-Fabián S, López-Roa RI, Flores-Gutiérrez EO, Reyes-Grajeda JP, Carrera-Quintanar L, Ortuño-Sahagún D. Immunomodulation and anti-inflammatory effects of garlic compounds. J Immunol Res. 2015:401630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ng QX, Koh SSH, Chan HW, Ho CYX. Clinical use of curcumin in depression: a meta-analysis. J Am Med Dir Assoc. 2017;18(6):503–8. [DOI] [PubMed] [Google Scholar]
- 47. Miki T, Eguchi M, Kurotani K, Kochi T, Kuwahara K, Ito R, Kimura Y, Tsuruoka H, Akter S, Kashino I et al. Dietary fiber intake and depressive symptoms in Japanese employees: the Furukawa Nutrition and Health Study. Nutrition. 2016;32(5):584–9. [DOI] [PubMed] [Google Scholar]
- 48. Marschollek C, Karimzadeh F, Jafarian M, Ahmadi M, Mohajeri SM, Rahimi S, Speckmann EJ, Gorji A. Effects of garlic extract on spreading depression: in vitro and in vivo investigations. Nutr Neurosci. 2017;20(2):127–34. [DOI] [PubMed] [Google Scholar]
- 49. Felger JC, Lotrich FE. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience. 2013;246:199–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Muthuramalingam A, Menon V, Rajkumar RP, Negi VS. Is depression an inflammatory disease? Findings from a cross-sectional study at a tertiary care center. Indian J Psychol Med. 2016;38(2):114–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vogelzangs N, de Jonge P, Smit JH, Bahn S, Penninx BW. Cytokine production capacity in depression and anxiety. Transl Psychiatry. 2016;6(5):e825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Udina M, Castellví P, Moreno-España J, Navinés R, Valdés M, Forns X, Langohr K, Solà R, Vieta E, Martín-Santos R. Interferon-induced depression in chronic hepatitis C: a systematic review and meta-analysis. J Clin Psychiatry. 2012;73(8):1128–38. [DOI] [PubMed] [Google Scholar]
- 53. Xia Z, DePierre JW, Nassberger L. Tricyclic antidepressants inhibit IL-6, IL-1β and TNF-α release in human blood monocytes and IL-2 and interferon-γ in T cells. Immunopharmacology. 1996;34:27–37. [DOI] [PubMed] [Google Scholar]
- 54. Lindqvist D, Dhabhar FS, James SJ, Hough CM, Jain FA, Bersani FS, Reus VI, Verhoeven JE, Epel ES, Mahan L et al. Oxidative stress, inflammation and treatment response in major depression. Psychoneuroendocrinology. 2017;76:197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Baron RM, Kenny DA. The moderator–mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–82. [DOI] [PubMed] [Google Scholar]
- 56. Hoogland IC, Houbolt C, van Westerloo DJ, van Gool WA, van de Beek D. Systemic inflammation and microglial activation: systematic review of animal experiments. J Neuroinflammation. 2015;12:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hoogland ICM, Westhoff D, Engelen-Lee JY, Melief J, Valls Serón M, Houben-Weerts JHMP, Huitinga I, van Westerloo DJ, van der Poll T, van Gool WA et al. Microglial activation after systemic stimulation with lipopolysaccharide and Escherichia coli. Front Cell Neurosci. 2018;12:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wu Z, Zhang J, Nakanishi H. Leptomeningeal cells activate microglia and astrocytes to induce IL-10 production by releasing pro-inflammatory cytokines during systemic inflammation. J Neuroimmunol. 2005;167(1–2):90–8. [DOI] [PubMed] [Google Scholar]
- 59. Sahin C, Dursun S, Cetin M, Aricioglu F. The neuroinflammation perspective of depression: reuniting the outstanding mechanisms of the pathophysiology. Klinik Psikofarmakol Bülteni. 2016;26(2):196–206. [Google Scholar]
- 60. Finnell JE, Wood SK. Neuroinflammation at the interface of depression and cardiovascular disease: evidence from rodent models of social stress. Neurobiol Stress. 2016;4:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cowen PJ, Browning M. What has serotonin to do with depression?. World Psychiatry. 2015;14(2):158–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Moret C, Briley M. The importance of norepinephrine in depression. Neuropsychiatr Dis Treat. 2011;7(Suppl 1):9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Waters RP, Rivalan M, Bangasser DA, Deussing JM, Ising M, Wood SK, Holsboer F, Summers CH. Evidence for the role of corticotropin-releasing factor in major depressive disorder. Neurosci Biobehav Rev. 2015;58:63–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kheirouri S, Noorazar SG, Alizadeh M, Dana-Alamdari L. Elevated brain-derived neurotrophic factor correlates negatively with severity and duration of major depressive episodes. Cogn Behav Neurol. 2016;29(1):24–31. [DOI] [PubMed] [Google Scholar]
- 65. Yang L, Zhao Y, Wang Y, Liu L, Zhang X, Li B, Cui R. The effects of psychological stress on depression. Curr Neuropharmacol. 2015;13(4):494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Liu YZ, Wang YX, Jiang CL. Inflammation: the common pathway of stress-related diseases. Front Hum Neurosci. 2017;11:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Busillo JM, Azzam KM, Cidlowski JA. Glucocorticoids sensitize the innate immune system through regulation of the NLRP3 inflammasome. J Biol Chem. 2011;286(44):38703–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. van Lenthe FJ, Jansen T, Kamphuis CB. Understanding socio-economic inequalities in food choice behaviour: can Maslow's pyramid help?. Br J Nutr. 2015;113(7):1139–47. [DOI] [PubMed] [Google Scholar]
- 69. Reininger B, Lee M, Jennings R, Evans A, Vidoni M. Healthy eating patterns associated with acculturation, sex and BMI among Mexican Americans. Public Health Nutr. 2017;20(7):1267–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ruiz-Canela M, Zazpe I, Shivappa N, Hébert JR, Sánchez-Tainta A, Corella D, Salas-Salvadó J, Fitó M, Lamuela-Raventós RM, Rekondo J et al. Dietary inflammatory index and anthropometric measures of obesity in a population sample at high cardiovascular risk from the PREDIMED (PREvención con DIeta MEDiterránea) trial. Br J Nutr. 2015;113(6):984–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ramallal R, Toledo E, Martínez JA, Shivappa N, Hébert JR, Martínez-González MA, Ruiz-Canela M. Inflammatory potential of diet, weight gain, and incidence of overweight/obesity: the SUN cohort. Obesity (Silver Spring). 2017;25(6):997–1005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.