Abstract
The prevalence and perceived safety of marijuana use in pregnancy are increasing with expanding legalization. Marijuana crosses the placenta and passes into breastmilk, resulting in fetal and neonatal exposure. Many women cite medical reasons for prenatal marijuana use such as nausea and vomiting of pregnancy, anxiety, and chronic pain. The scientific literature regarding marijuana in pregnancy is mixed resulting in confusion among practitioners as to how to counsel women about risks of use. In addition, there is a paucity of literature related to marijuana use and breastfeeding. Existing pregnancy studies are predominantly retrospective cohorts with a reliance on self-report for ascertainment of exposure, which underestimates use. Many studies fail to adjust for important confounding factors such as tobacco use and sociodemographic differences. Despite the limitations of the existing evidence, there are animal and human data suggesting potential harms of cannabis use. The harms are biologically plausible given the role of the endocannabinoid system in pregnancy implantation, placentation, and fetal neurological development. Two recent systematic reviews and meta-analyses found an association between marijuana use and adverse perinatal outcomes, especially with heavy marijuana use. In addition, three longitudinal cohort studies demonstrate a possible effect of prenatal marijuana exposure on long-term neurobehavioral outcomes. Marijuana use may be associated with growth restriction, stillbirth, spontaneous preterm birth and neonatal intensive care unit admission. Therefore, women should be advised to refrain from using marijuana during pregnancy and lactation.
Introduction
Cannabis (or marijuana) contains over 400 chemical entities, and is consumed via different modalities including vaping, dabbing, smoking and eating. Marijuana is now legalized for medicinal use in 29 states plus Washington DC and for recreational use in 8 states plus Washington DC. In addition to the psychoactive component, delta-9-tetrahydrocannabinol (THC), there are other components of cannabis that have generated interest for potential therapeutic properties. Women report using marijuana in pregnancy for treatment of nausea, anxiety and pain2; however, marijuana crosses the placenta and may have adverse effects on the developing fetus.1,3
As legalization expands, there is renewed interest in the health effects of marijuana, yet there remains uncertainty regarding maternal and neonatal outcomes with prenatal marijuana use. The confusion surrounding the effect of marijuana on perinatal outcomes does not stem from a lack of available literature. Instead the lack of clarity regarding anticipated outcomes is a result of the heterogeneity of findings for the association between marijuana use and adverse pregnancy outcomes. There is, however, an almost complete lack of data regarding marijuana use and breastfeeding.
Two recent systematic reviews and meta-analyses provide a comprehensive review of the human literature related to marijuana and pregnancy outcomes.4,5 Given the recent publication of these articles, we did not pursue further meta-analysis. Rather we hope to provide evidence-based information regarding the biologic plausibility of existing findings and expand on outcomes not addressed in the meta-analyses in order to provide a practical review of the available literature.
Prevalence of Marijuana Use in Pregnancy and Perceived Safety
The proportion of women using marijuana during pregnancy increased from 2.37% (95% CI 1.85–3.04) in 2002 to 3.85% (95% CI 2.87–5.18) in 2014 based on self-reported data from the National Surveys of Drug Use and Health.6 However, self-report likely underestimated the prevalence of use. In a Kaiser population with universal self-report and urine toxicology screening, the rate of use was 7.1% (95% CI 6.7–7.5%) in 2016, and over half of the women using marijuana were identified only by toxicology testing.
There is also an increasing perception of safety. Jarlenski and colleagues published a research letter utilizing data from the National Surveys of Drug Use and Health from 2005–12.7 Survey respondents were asked, “How much do people risk harming themselves physically and in other ways when they smoke marijuana once or twice per week?” The proportion of pregnant women without use in the past 30 days who reported “no risk” of harm increased from 3.5% to 16.5% over the study time period. The proportion of pregnant women with recent use who reported “no risk” of harm was even higher increasing from 25.8% to 65.4%.7
Anecdotally women report ongoing use in pregnancy to relieve nausea, decrease pain, and for psychiatric disorders such as anxiety and depression. In a cross-sectional survey (N=1,749), the majority of women reported use to help with depression and anxiety (63%) followed by help with pain (60%).2 Only 39% of the women with current use reported using marijuana for fun or recreation. Given that women perceive medical benefits of marijuana use, there is an opportunity for providers to query women as to the reasons for use and discuss alternative therapies.
Pharmacology of Cannabis
Cannabis plants produce over 400 chemical entities and over 60 cannabinoids, which can have both physical and mental effects when consumed.8 The main psychoactive component of cannabis, delta-9-THC, acts upon type 1 (CB1) and type 2 (CB2) cannabinoid receptors that are expressed throughout the central nervous system and peripheral tissues.9
Our contemporary understanding of the effects of exogenous cannabinoids (eg delta-9-THC) on humans stems predominantly from studying endogenous CB1 receptor agonists such as anandamide and 2-arachidonoyl glycerol. Unlike typical transmitters, anandamide and 2-arachidonoyl glycerol are produced only when and where they are needed. Their action is presynaptic (in a retrograde manner) rather than postsynaptic which makes inhibition of various excitatory or inhibitory neurotransmitter systems possible.8
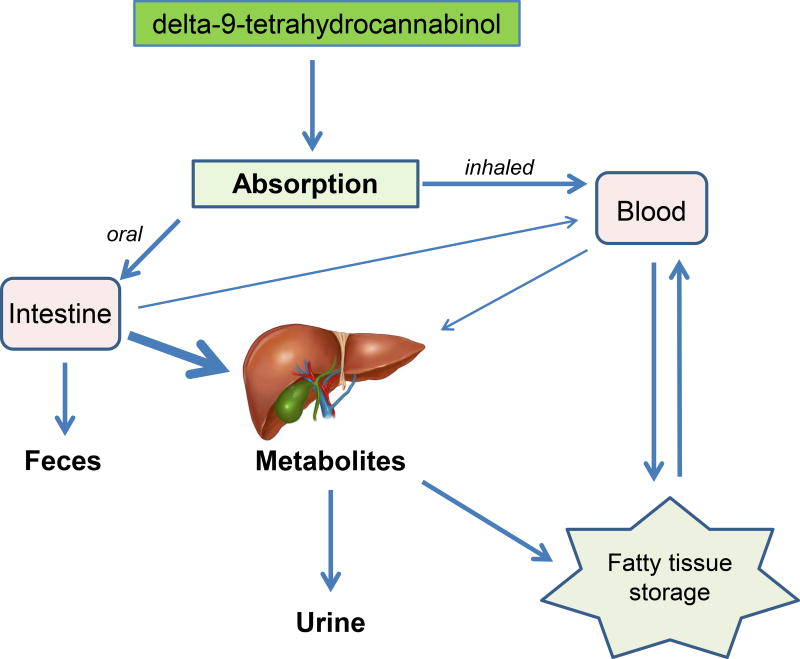
The effects of endocannabinoids are dependent upon rate of synthesis, cellular uptake, and degradation. Endocannabinoids are rapidly removed by a membrane transport process whereas exogenous cannabinoids such as delta-9-THC are metabolized by the liver, and stored in peripheral tissues as stable metabolites (Figure 1).8 The metabolites are then excreted by the kidney over time.
Figure 1.
After consumption, the primary psychoactive component of marijuana, delta-9-tetrahydrocannabinol, undergoes various absorption, metabolism, and excretion pathways based on method of administration. Illustration of the human liver anatomy © Erhan Akin, Dreamstime.com. Used with permission.
Potential Therapeutic Uses for Cannabis
There is growing evidence that the endocannabinoid system plays a role in a variety of medical conditions. Exogenous cannabinoids such as delta-9-THC and cannabidiol may function at cannabinoid and other receptors to improve symptoms of conditions with a relative endocannabinoid deficiency such as migraines, fibromyalgia, and irritable bowel syndrome.11
Cannabinoid receptors represent potential treatment targets for several neurological disorders. Seizures trigger homeostatic changes in CB1 receptors found in the hippocampus.12 Animal models demonstrate that activation of CB1 receptors reduces seizure severity. In addition, patients with temporal lobe epilepsy have decreased levels of anandamide in cerebrospinal fluid samples compared with healthy patients.13 However, efficacy of exogenous cannabinoids for treatment of epilepsy in children has been inconsistent across randomized clinical trials and notably ineffective in adults.14
Cannabis has been found to relieve some symptoms related to cancer or cancer treatment including antiemetic effects, appetite stimulation, pain relief, and improved sleep.14 Pre-clinical studies utilizing delta-9-THC and cannabidiol have demonstrated anti-tumor effects in glioma, melanoma, pancreatic, and hepatic cancer cells by inducing cancer cell death, and inhibiting angiogenesis and metastasis while protecting healthy tissue from cell death.15
CB1 receptors in the basal ganglia affect mechanisms of muscle spasticity.16 Several randomized controlled trials demonstrate that oral cannabis extracts significantly improve mobility and perceptions of muscle spasticity and pain in patients with multiple sclerosis.17 A cannabinoid-based product is now available in 30 countries for treatment of spasticity related to multiple sclerosis.
Severe or intractable nausea is a qualifying condition in most states where medical cannabis laws are enacted. Cannabinoids can block both acute, delayed, and potentially anticipatory nausea and vomiting.18 Cannabis-based treatments had superior efficacy to prochlorperazine and similar efficacy to ondansetron in randomized controlled trials of cancer patients.19,20 However, there are no data to support the efficacy of marijuana for nausea and vomiting of pregnancy, and given the potential harmful effects for the fetus, cannabis should not be recommended as a treatment for pregnant women.
Cannabis is being investigated as a therapy for post-traumatic stress disorder. Patients with post-traumatic stress disorder have lower peripheral anandamide levels and increased CB1 receptors in the brain compared to those without post-traumatic stress disorder.21 Studies have demonstrated significant decreases in nightmare occurrence and severity, as well as subjective improvement in sleep time, quality, flashbacks, and night sweats in patients with post-traumatic stress disorder treated with cannabis.22
In summary, exogenous cannabinoids (eg delta-9-THC and cannabidiol) have potential therapeutic effectiveness for conditions in which there is a relative endocannabinoid deficiency or hypofunction. However, more research is needed to provide adequate support for expanded medical cannabis use as the available body of evidence is insufficient and often conflicting.
Importance of the Endocannabinoid System in Pregnancy
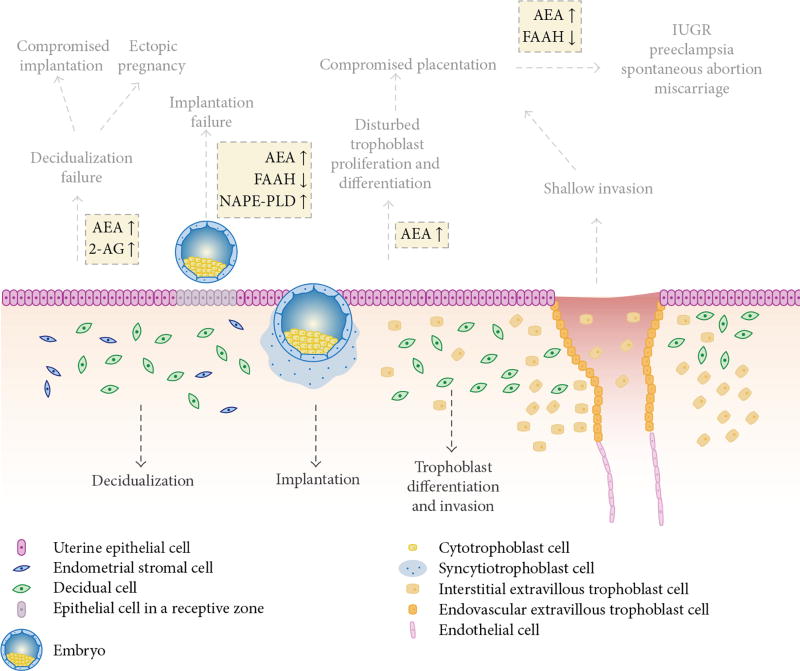
The endocannabinoid system plays an important role in implantation and pregnancy maintenance. The pregnancy implantation site expresses low levels of anandamide while adjacent sites express higher levels of anandamide to assure highly synchronized communication between the embryo and the endometrium.9 Maintaining a balance of anandamide synthesis and degradation is required for successful embryonic passage through the oviduct and implantation (Figure 2).9
Figure 2.
This is a schematic representation of endocannabinoid signaling at the site of implantation and potential adverse effects. Physiologic and molecular processes involving anandamide (AEA) are normally tightly regulated by N-acylphosphatidylethanolamine-specific phospholipase D (NAPE-PLD) and fatty acid amide hydrolase (FAAH) for synthesis and degradation, respectively. Disruption of endocannabinoid signaling (shown in yellow boxes) can result in reprogramming of cellular function at the implantation site. Reprinted under the Creative Commons Attribution License from Fonseca BM, Correia-da-Silva G, Almada M, Costa MA, and Teixeria NA. The endocannabinoid system in the postimplantation period: a role during decidualization and placentation. Int J Endocrinol 2013;2013:510540. http://dx.doi.org/10.1155/2013/510540.
Upon implantation, activated blastocysts have higher expression of CB1 receptors than dormant blastocysts and anandamide levels remain tightly regulated without variation in the first and second trimesters of pregnancy.9 Plasma anandamide levels are elevated in women with nonviable first trimester pregnancies compared to those with viable pregnancies.9 Furthermore, higher anandamide levels are associated with miscarriage and low levels of progesterone in some studies.9 However, one prospective study found no difference between plasma levels of anandamide in asymptomatic women sampled at 6–10 weeks gestation who miscarried compared to those who did not.23 Several studies in humans and mice have provided evidence that CB1 and CB2 receptors are expressed in the decidualization process of differentiation and remodeling.9
During fetal life, the CB1 receptor plays a major role in brain development by regulating neural progenitor differentiation into neurons and glia and guiding axonal migration and synaptogenesis. By two weeks’ gestation in mice and 19 weeks’ gestation in humans, the fetus has the complete array of cannabinoid receptors.9,24 However, in both rats and humans, the number of CB1 receptors is substantially higher in fetal brains compared to adult brains.24,25 The increased concentration of CB1 receptors in the fetus has been attributed to key developmental events including cell proliferation and migration, and axonal elongation with eventual synaptogenesis and myelogenesis.
Rats exposed to cannabinoids during pregnancy or lactation demonstrate motor hyperactivity in infancy and adolescence, but not adulthood.26 When rats were exposed prenatally to low or moderate levels of delta-9-THC, cognitive impairments were induced with long-term memory impairment and short-term olfactory memory.27 These impairments were associated with long-lasting changes in the expression of genes related to glutamatergic neurotransmission. In addition, long-lasting changes in emotional reactivity of offspring have been observed with less social interaction and social play at adolescence.28
Additional insights regarding the impact of cannabis on the developing fetal human brain suggest critical interruptions in the endocannabinoid system are possible. For example, repeated delta-9-THC exposure disrupts endocannabinoid signaling, particularly with the CB1 cannabinoid receptor, resulting in a “rewiring” of the fetal cortical circuitry.29
In summary, both animal and human studies demonstrate the importance of appropriate endocannabinoid signaling for normal fetal development. The endocannabinoid system also plays an active role in pregnancy implantation and placental development. With a clear understanding of the role of the endocannabinoid system, one can see how manipulating it with prenatal administration of exogenous cannabis could have subtle, but significant effects on human offspring.
Marijuana and Adverse Pregnancy Outcomes
Human evidence regarding the association between prenatal marijuana use and adverse pregnancy outcomes is mixed. Practitioners should be aware that there are significant limitations to the existing literature. Marijuana use is often not quantified and studies are limited by ascertainment of marijuana exposure through self-report which underestimates the prevalence of use.1 Biological sampling should be used to accurately determine the effects of prenatal marijuana use on maternal and neonatal outcomes. Important confounders, such as education level and concurrent tobacco use, will need to be measured thoughtfully and prospectively in order to evaluate the independent role of marijuana use in pregnancy outcomes.
The National Academy of Sciences published a consensus document in 2017 detailing the evidence related to the health effects of cannabis and cannabinoids.14 The committee reported that smoking cannabis during pregnancy was linked to lower birth weight in offspring. They also concluded that there is limited evidence of a statistical association between prenatal marijuana use and both maternal pregnancy complications and neonatal intensive care unit (NICU) admission. The committee found insufficient evidence to support or refute associations between marijuana and later outcomes in the offspring such as cognition and academic achievement. The lack of conclusive evidence regarding later childhood outcomes is predominantly a result of mixed findings as well as difficulty in attributing any observed differences to prenatal exposure rather than subtle differences in environment throughout childhood and adolescence that could not be measured.
Two recent systematic reviews and meta-analyses provide a comprehensive review of the literature which can be translated into guidance for practitioners to counsel women regarding marijuana use in pregnancy. One of these (Gunn et al) was published prior to the National Academy of Sciences report, and was drawn upon heavily in the committee’s evaluation of the existing literature. Gunn et al identified 6,854 articles, fully screened 881 articles, and included 24 articles (1 cross-sectional, 1 case-control and 22 cohort studies) in a systematic review.5 The authors created a comprehensive list of maternal and neonatal outcomes of interest, and completed a meta-analysis for any outcomes assessed in three or more studies.
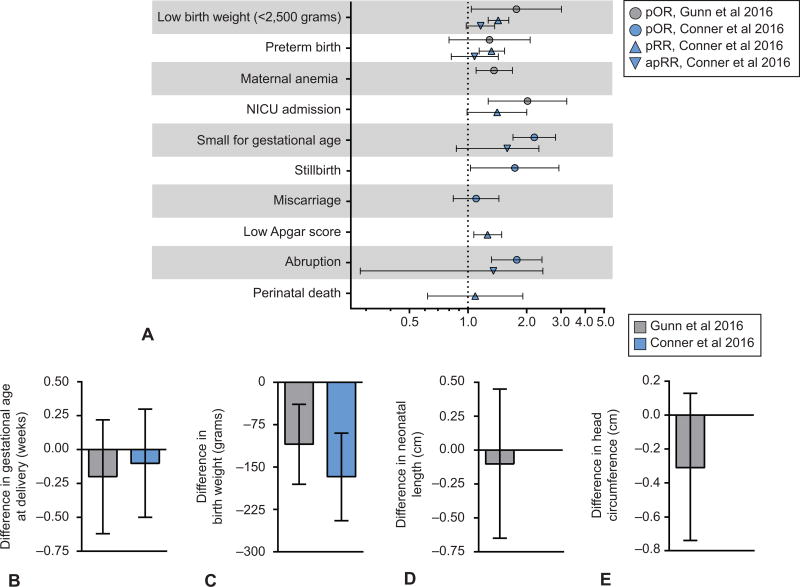
They found an association between prenatal marijuana use and anemia (pooled odds ratio from 6 studies: 1.36, 95% CI 1.10–1.69), low birth weight <2500gms (pooled odds ratio from 7 studies: 1.77, 95% CI 1.04–3.01 with a mean pooled birthweight difference of 109 gms.), and higher odds of neonatal intensive care unit admission (NICU) (pooled odds ratio from 4 studies: 2.02, 95% CI 1.27–3.21). They did not detect an association with preterm birth (pooled odds ratio from 9 studies: 1.29, 95% CI 0.80–2.08). These authors concluded that further study is needed, especially for maternal outcomes and were concerned about the associations between marijuana use and both low birth weight and NICU admission (Figure 3).
Figure 3.
Graphical representation of results from two recent meta-analyses evaluating the effect of prenatal marijuana use on maternal and neonatal outcomes. Pooled adjusted estimates are based on a pooling of adjusted estimates from individual studies which all adjusted for tobacco, some also adjusted for other illicit drugs and other sociodemographic factors. A. Pooled odds ratios and relative risks for adverse perinatal outcomes with prenatal marijuana exposure. B. Pooled difference in gestational age at delivery in weeks associated with marijuana exposure. C. Pooled difference in birthweight (grams) of newborns associated with marijuana exposure. D. Pooled difference in neonatal length (cm) associated with marijuana exposure. E. Pooled difference in head circumference (cm) associated with marijuana exposure. pRR, pooled relative risk; pOR, pooled odds ratio; apRR; adjusted pooled relative risk; NICU, neonatal intensive care unit.
In a second meta-analysis, Conner and colleagues evaluated the association between marijuana use and low birth weight or preterm birth less than 37 weeks gestation.4 The authors identified 4,875 studies and ultimately included 31 studies in the meta-analysis. In initial pooled estimates, there was an association with low birth weight and preterm birth. However, after adjustment for confounding factors such as tobacco use, there was no association between any marijuana use and low birth weight (adjusted pooled relative risk: 1.16, 95% CI 0.98–1.37) or preterm birth (adjusted pooled relative risk: 1.08, 95% CI 0.82–1.43) (Figure 3). In a planned subanalysis of women reporting moderate to heavy marijuana use (at least once per week), there was an association with both low birth weight (RR 1.90, 95% CI 1.44–2.45) and preterm birth (RR 2.04, 95% CI 1.32–3.17).
There are some studies included in each meta-analysis that are not included in the other. This discrepancy is in part a result of a focus on neonatal outcomes in the Conner et al meta-analysis, and both maternal and neonatal outcomes in the Gunn et al meta-analysis. Differences in results may also be due to adjustment for confounding factors in the Conner et al analysis. A summary of the odds ratios and relative risks for adverse outcomes in these meta-analyses are depicted in Figure 3.
Fetal Growth
Recent meta-analyses demonstrate an association between marijuana use and low birth weight as noted previously.4,5 Importantly, Conner and colleagues only observed this association with heavy marijuana use.4 A relationship between aberrant fetal growth and prenatal marijuana use was also recognized in the National Academy of Sciences report.14 This association is biologically plausible given the importance of the endocannabinoid system in pregnancy implantation and placental formation.
Essentially all of the studies that evaluate the relationship between marijuana use and fetal growth utilize a primary endpoint of either birth weight, low birth weight (<2500 gms), or small for gestational age (<10% birthweight for gestational age and sex). Only the Generation R study evaluated fetal growth prospectively in a population of women with marijuana use.30 In this study, the investigators demonstrated a relative “dose response” effect of marijuana on fetal growth with greater growth decrements demonstrated with increasing use. Fetuses exposed to marijuana in early pregnancy (n=214) grew 11.2 grams (−15.3 to −7.1 gms) per week less than non-users whereas those with ongoing use grew 14.4 grams (−22.9 to −5.9 gms) per week less than non-users. However, given the limited evidence for antenatally detected abnormal growth, Doppler studies and serial growth ultrasounds are not recommended strictly for the indication of marijuana use in the absence of clinical concern for growth restriction.
Preterm Birth
Preterm birth less than 37 weeks is commonly evaluated in the marijuana and pregnancy literature. However, data are inconsistent for this outcome.1 Odds ratios from meta-analyses for the association between marijuana use and preterm birth are presented in Figure 3. Although Conner and colleagues found no association between any marijuana use and preterm birth, there was an association between heavy marijuana use and preterm birth.4 Of note, the majority of the studies included in the meta-analyses did not classify preterm birth into spontaneous or iatrogenic.
There are three recent studies demonstrating an association between marijuana use and spontaneous preterm birth. The first is an observational study of nulliparous women (N=3,184) by Dekker and colleagues in which women who used marijuana pre-pregnancy had increased odds of spontaneous preterm birth with intact membranes (aOR 2.34, 95% CI 1.22–4.52).31 Similarly in a secondary analysis of a prospectively collected cohort, Saurel-Cubizolles et al found an increased risk of spontaneous preterm birth among women with marijuana use compared to non-users (OR 2.15, 95% CI 1.10–4.18).32 However, this association was no longer significant when evaluating women with only marijuana use and no tobacco use. Finally, Leemaqz and colleagues found an increased risk of spontaneous preterm birth after adjustment for tobacco exposure (aOR 2.28, 95% CI 1.45–3.59).33 Future research efforts need to classify women as having either iatrogenic or spontaneous preterm birth to better elucidate the role of marijuana use (if any) in this important perinatal outcome.
Stillbirth
There is a relative paucity of evidence related to stillbirth and prenatal marijuana use. This is predominantly a result of stillbirth being excluded from many existing studies. Stillbirth and perinatal death were included as secondary outcomes in the Conner et al meta-analysis with only two available studies for stillbirth and three for perinatal death.4 Marijuana use was associated with stillbirth (pooled OR 1.74, 95%CI 1.03–2.93) but not perinatal death (pooled RR 1.09, 95% CI 0.62–1.91). Gunn and colleagues also found no association with perinatal mortality.5
Varner and colleagues completed a secondary analysis of National Institutes of Child Health and Human Development Stillbirth Collaborative Research Network data including 1,468 women with umbilical cord specimens available.34 Of these, 3.9% of stillbirths and 1.7% of live births had cord homogenate positive for THC metabolites. Marijuana use as measured by cord homogenate assays was associated with stillbirth (OR 2.34, 95% CI 1.13–4.81), and this association persisted after adjustment for tobacco use with serum cotinine.
Congenital Anomalies
There is insufficient evidence to support an association between marijuana use and any specific congenital abnormality.1 Many studies evaluating the effect of marijuana on embryogenesis do not specify whether use was during the critical period of development. In addition the majority are subject to recall bias with marijuana use ascertained by self-report in the postpartum period. The Gunn et al meta-analysis found no association between marijuana use and congenital birth defects.5
In a review of the available scientific evidence the Retail Marijuana Public Health Advisory Committee for the Colorado Department of Public Health and Environment found limited evidence for an association between marijuana use and isolated ventricular septal defects based on a single study of 122 cases of isolated simple ventricular septal defect and 3,029 controls.35 Following adjustment for maternal age, race, overt diabetes, and multivitamin use, periconceptional marijuana use as ascertained by maternal self-report was associated with ventricular septal defect (OR 1.90, 95% CI 1.29–2.81). This work has not been replicated. At this time, women should be counseled that there is no consistent association between marijuana and congenital birth defects; however, there is also insufficient evidence to demonstrate safety.
Neonatal Morbidity and Neonatal Intensive Care Unit Admission
Marijuana use was associated with neonatal intensive care unit (NICU) admission in the Gunn et al meta-analysis (pooled OR 2.02, 95% CI 1.27–3.21) but not in the Conner et al meta-analysis (pooled RR 1.41, 95% CI 0.99–2.0).4,5 Despite these differential findings, there is an increasing body of evidence indicating that marijuana use may be associated with neonatal morbidity.
Warshak and colleagues demonstrated an increased risk of NICU admission among marijuana users compared to non-users in a single center retrospective cohort study (17.2% versus 12.5%, aOR 1.54, 95% CI 1.14–2.07).36 Metz and colleagues found similar rates of NICU admission between marijuana-exposed (16.9%) and marijuana non-exposed (9.5%) groups among the live births in the SCRN database.37 However, this difference was not significant in the setting of a smaller sample size. There was, however, an increased risk of composite neonatal morbidity consisting of respiratory, neurologic, infection and hematologic morbidity or death prior to hospital discharge (aOR 3.11, 95% CI 1.40–6.91).
Neonatal withdrawal syndrome from marijuana is not well described. A few articles report increased tremor, irritability, hand-to-mouth activity, and startle response among exposed neonates.5 However, others demonstrate no difference on neonatal behavioral assessment scales.5 It is unclear what factors and neonatal diagnoses drive the observed increase in NICU admission.
Long-Term Adverse Neurological Events
None of the existing meta-analyses address the effects of prenatal marijuana use on neurobehavioral outcomes in the offspring. Evidence related to neurodevelopmental outcomes comes predominantly from three longitudinal human studies: Ottawa Prenatal Prospective Study, Maternal Health Practices and Child Development, and Generation R (Table 1). For all of these studies, marijuana use was ascertained by maternal self-report.
Table 1.
Summary of Longitudinal Human Studies Evaluating Effect of Prenatal Marijuana Use on Neurobehavioral Outcomes
| Study Setting | Population | Major Findings1,50 |
|---|---|---|
| Ottawa Prenatal Prospective Study (N=698)51 Ottawa, Canada 1978 | Middle-income, predominantly Caucasian |
|
| Maternal Health Practices and Child Development Study (N=564)52 Pittsburgh, Pennsylvania 1982 | Low-income, predominantly African-American |
|
| Generation R Study (N=9,778)53 Rotterdam, Netherlands 2001 | Higher socioeconomic status, multi-ethnic |
|
The National Academy of Sciences report found insufficient evidence to support or refute an association between maternal marijuana use and later childhood outcomes such as cognition and academic achievement.14 Considering the findings from the longitudinal human studies, the animal data related to neurodevelopment, and one human study demonstrating decreased dopamine receptors in 18–22 week fetuses following pregnancy termination who were exposed to cannabis in utero compared to those who were not38, we conclude that there remain concerns related to neurologic development with prenatal marijuana use. The authors recognize that it is difficult to complete longitudinal studies which adequately control for the childhood environment to evaluate the independent effects of in utero exposure. It should also be noted that these longitudinal studies may not reflect the effects of contemporary products with higher concentrations of delta-9-THC.
Breastfeeding
A survey of lactation consultants in New England demonstrates the spectrum of opinions of lactation professionals regarding breastfeeding in the setting of marijuana use. Of the 74 lactation professionals surveyed 41% reported their recommendation would depend on the amount of marijuana use, 44% would recommend breastfeeding despite marijuana use given the other known benefits of breastfeeding, and 15% would recommend not breastfeeding with marijuana use.39
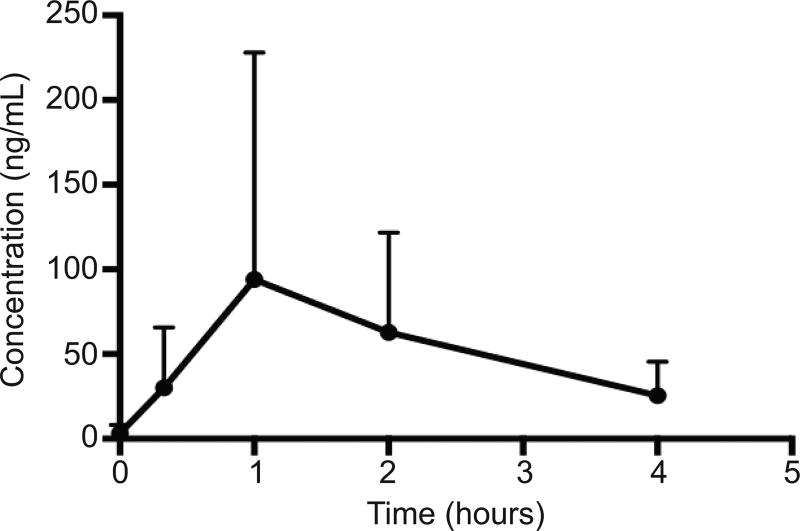
Difference in opinion regarding breastfeeding and marijuana use likely stems from a paucity of data. Using samples from two patients, Reyes-Perez et al demonstrated excretion of THC into the breastmilk with a relative infant dose of 0.8% which means approximate consumption by the infant is 0.8% of its mother’s dose per kilogram.40 Baker and colleagues studied transfer of delta-9-THC into the breastmilk of eight women after consumption of a cannabis product with known concentration of delta-9-THC. Delta-9-THC was detected in pumped breastmilk at an estimated mean of 2.5% (range 0.4%-8.7%) of the maternal dose, and the average absolute infant dose was estimated at 8 micrograms per kilogram per day. The mean concentrations of delta-9-THC in the breastmilk from 20 minutes to 4 hours post-inhalation are depicted in Figure 4. While this study has significant limitations as a result of sampling of breastmilk in an uncontrolled environment, and small sample size, it provides preliminary data supporting transfer of delta-9-THC into breastmilk.41
Figure 4.
Mean concentration-time profile of delta-9-tetrahydrocannabinol in human milk (mean±standard deviation, n=8). Reprinted from Baker T, Datta P, Rewers-Felkins, K, Thompson, H, Kallem RR, Hale TW. Transfer of inhaled cannabis into human breast milk. Obstet Gynecol 2018;131:783–788.
Cannabis concentration in the breastmilk is likely related to maternal dose, frequency of dosing, simple diffusion, and trapping within the breastmilk due to lipophilicity. The bioavailability of marijuana metabolites ingested by neonates in the breastmilk is largely unknown. In one chronic, heavy user, the milk:plasma ratio was noted at 8:1 and detectable metabolites were found in the neonate’s feces.42 Baker and colleagues did not measure a milk:plasma ratio, nor obtain neonatal samples.
There are conflicting data regarding outcomes of infants exposed to cannabis during breastfeeding. In one study, 136 breast-fed infants were assessed at one year for motor and mental development.43 The 68 infants exposed to cannabis during the first month postpartum showed an association of decreased motor development at one year compared to matched controls. Specifically, there was a 14 ± 5 point decrease in the Bayley index of infant motor development. However, the authors believed that marijuana use during pregnancy confounded the association.
Another study compared 27 breast-fed infants exposed to cannabis to 35 unexposed breast-fed infants.44 At one year, no differences were noted for motor and mental skills using the Bayley Scales of Infant Development. The authors noted that statistical analyses were limited due to a small sample size and lack of comparability regarding dose and duration of exposure.
The paucity of clinical evidence has made it difficult for organizations to make definitive recommendations regarding cannabis use during lactation. Both ACOG and the American Academy of Pediatrics recommend that women refrain from using cannabis while lactating.3,45 The Academy of Breastfeeding Medicine states breastfeeding mothers “should be counseled to reduce or eliminate their use of cannabis to avoid exposing their infants and advised of the possible long-term neurobehavioral effects from continued use”.46 They ask clinicians to consider the wide range of occasional, regular medical, and heavy exposure to cannabis and urge caution when breastfeeding occurs with cannabis use.
The authors believe that discontinuation of cannabis provides the least risk and highest safety profile for mother and baby. If discontinuation is not possible, women should be encouraged to limit use as much as they can. For women who use cannabis for medical indications, alternative therapies with more safety data during breastfeeding should be considered. If women continue to use cannabis while breastfeeding after counseling, it is reasonable to provide lactation support per standard of care at the birthing facility given the limited data regarding passage of cannabis into the breastmilk, and the many known benefits of breastfeeding for both the mother and neonate. Recommendations regarding breastfeeding will evolve as more evidence becomes available. In the meantime, the lack of data should not be interpreted as an endorsement of safety.
Counseling Patients Regarding Marijuana Use in Pregnancy and When Breasfeeding
All women should be verbally screened for drug use during the course of standard prenatal care. Screening for drug use should be completed with the goal of providing counseling regarding potential adverse effects and referral to resources to assist with cessation when needed. Some practitioners use biologic sampling to confirm self-report. The utility of this is unknown as women who self-report are likely using marijuana and would be candidates for intervention with or without a positive urine test.
Holland et al recorded 468 patient interactions during which 90 pregnant patients disclosed marijuana use to 47 different healthcare providers.48 The providers responded to the disclosure only 48% of the time. When providers did respond to the disclosure, they discussed implications of exposure such as referral to social services rather than investigating why patients were using, and educating them about possible risks. There are clearly opportunities for improvement in how healthcare providers counsel women regarding use.
Women also seek information regarding perinatal marijuana use from other sources. In qualitative work by Jarlenski and colleagues, women were unlikely to obtain information about marijuana use from their healthcare providers. Instead women relied on anecdotal experiences, advice from friends and family, and Internet searching.49 It is unclear from this study if women intentionally do not seek information from healthcare workers, or whether the information that we provide is not perceived as valuable or helpful. In addition women may seek advice directly from dispensary employees. In a cross-sectional study of randomly selected dispensaries in Colorado, 69% recommended cannabis products to a woman posing as pregnant with nausea in the first trimester.54 Many endorsed safety and few recommended consultation with a healthcare provider without prompting.54
With the time pressures of obstetric practice, clinicians need resources to provide information regarding prenatal marijuana use to patients in an efficient way. Following the legalization of marijuana in Colorado, the Colorado Department of Public Health and Environment assembled a task force to review the scientific literature regarding the health effects of marijuana on mothers and neonates (Figure 5). Following this review, the task force translated their findings into documents that can be used by clinicians to assist with responding to patient concerns and questions regarding marijuana use in pregnancy. This guidance and resources for distribution to patients can be found at the following web address: https://www.colorado.gov/pacific/cdphe/marijuana-clinical-guidelines and as Appendix 1, available online at http://links.lww.com/xxx. Additionally, ACOG recently published "Marijuana and Pregnancy: Frequently Asked Questions" with patient information that may help providers in answering questions and having conversations about marijuana use. The ACOG resource is available on-line at https://www.acog.org/Patients/FAQs/Marijuana-and-Pregnancy. Women may or may not opt to cease using marijuana, but healthcare providers should be informing pregnant women of possible risks.
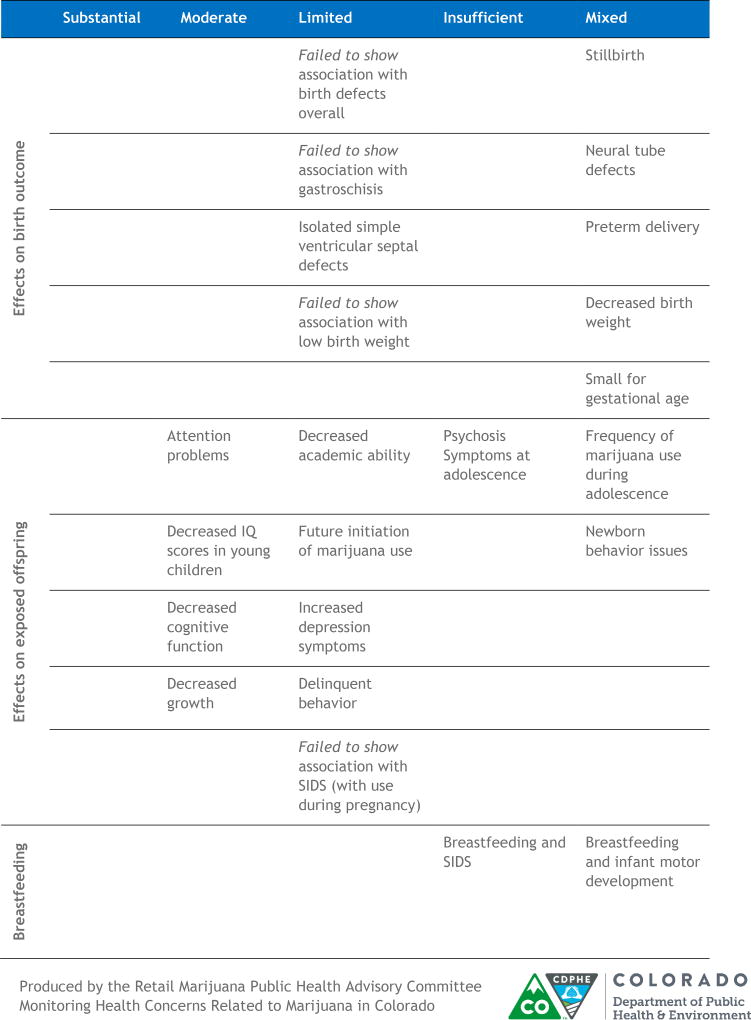
Figure 5.
Tabular representation of the Colorado Department of Public Health and Environment Retail Marijuana Public Health Advisory Committee Summary of Available Scientific Evidence. Substantial evidence was robust findings that support an association. Moderate evidence was findings support an association but with some limitations. Limited evidence was modest findings support an association but with substantial limitations. Insufficient evidence was not enough studies to conclude whether or not there is an association. Mixed evidence was defined as both supporting and non-supporting findings for an association with neither direction dominating. Reprinted with permission from the Colorado Department of Public Health and Environment. Environment’s Monitoring Health Concerns Related to Marijuana in Colorado: 2016 Report, Pregnancy and Breastfeeding evidence summary table.
Summary of Clinical Recommendations
The heterogeneity of findings in the scientific literature leads to uncertainty in counseling women regarding marijuana use in pregnancy. While more evidence is needed for informed decision-making, it seems reasonable to follow ACOG guidelines recommending that women be discouraged from using marijuana during pregnancy and lactation.3 The rationale to follow these recommendations stems from a growing body of studies showing potential harm to fetuses with evidence of decreased growth (in particular with heavy use), and concern from longitudinal studies for long-term neurologic effects. Marijuana use may be associated with spontaneous preterm birth, stillbirth, and neonatal intensive care unit admission. The health effects on the mother remain largely unknown.14
We recognize that there is still uncertainty regarding the effects of prenatal marijuana use, and even more so for marijuana use while breastfeeding. As practitioners, we can be honest with women regarding the uncertain effects of marijuana, but still express concern for fetal harm based on the available evidence. A better understanding of why women are using marijuana during pregnancy may enable a conversation of alternative therapies for which we have extensive safety and efficacy data. Further evidence to guide counseling of women as to the anticipated effects of prenatal marijuana use will allow for informed decision-making and help promote appropriate public health policies as legalization expands.
Supplementary Material
Acknowledgments
Dr. Metz is supported by the National Institute on Child Health and Human Development under award number 5K12HD001271-18. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors thank Amanda Allshouse for creating Figure 3. Ms. Allshouse is supported by the Department of Obstetrics and Gynecology at Denver Health and Hospital Authority.
Financial Disclosure: Dr. Borgelt received grant funding from the Colorado Department of Public Health and Environment (CDPHE) for a study evaluating the use of cannabidiol for the treatment of refractory pediatric epilepsy. Additionally she has provided continuing education for pharmacists through PharmCon, Inc. and served on 7 different working groups for the Colorado Department of Revenue and CDPHE regarding use of cannabis and patient safety.
Footnotes
Dr. Metz did not report any potential conflicts of interest.
References
- 1.Metz TD, Stickrath EH. Marijuana Use in Pregnancy and Lactation: A Review of the Evidence. Am J Obstet Gynecol. 2015;213(6):761–78. doi: 10.1016/j.ajog.2015.05.025. [DOI] [PubMed] [Google Scholar]
- 2.Retail Marijuana Public Health Advisory Committee. Monitoring Health Concerns Related to Marijuana in Colorado: 2016. Changes in Marijuana Use Patterns, Systematic Literature Review, and Possible Marijuana-Related Health Effects. Colorado Department of Public Health and Environment; 2016. https://drive.google.com/file/d/0B0tmPQ67k3NVQlFnY3VzZGVmdFk/view. [Google Scholar]
- 3.Marijuana use during pregnancy and lactation. Committee Opinion No. 722. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2017;130:e205–e9. doi: 10.1097/AOG.0000000000002354. [DOI] [PubMed] [Google Scholar]
- 4.Conner SN, Bedell V, Lipsey K, Macones GA, Cahill AG, Tuuli MG. Maternal Marijuana Use and Adverse Neonatal Outcomes: A Systematic Review and Meta-analysis. Obstet Gynecol. 2016;128:713–23. doi: 10.1097/AOG.0000000000001649. [DOI] [PubMed] [Google Scholar]
- 5.Gunn JK, Rosales CB, Center KE, et al. Prenatal exposure to cannabis and maternal and child health outcomes: a systematic review and meta-analysis. BMJ Open. 2016;6(4):e009986. doi: 10.1136/bmjopen-2015-009986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown QL, Sarvet AL, Shmulewitz D, Martins SS, Wall MM, Hasin DS. Trends in Marijuana Use Among Pregnant and Nonpregnant Reproductive-Aged Women, 2002–2014. JAMA. 2017;317(2):207–9. doi: 10.1001/jama.2016.17383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jarlenski M, Zank J, Bodnar LM, Koma JW, Chang JC, Bogen DL. Trends in perception of risk of regular marijuana use among US pregnant and nonpregnant reproductive-aged women. Am J Obstet Gynecol. 2017;217(6):705–7. doi: 10.1016/j.ajog.2017.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mechoulam R, Parker LA. The endocannabinoid system and the brain. Annu Rev Psychol. 2013;64:21–47. doi: 10.1146/annurev-psych-113011-143739. [DOI] [PubMed] [Google Scholar]
- 9.Correa F, Wolfson ML, Valchi P, Aisemberg J, Franchi AM. Endocannabinoid system and pregnancy. Reproduction. 2016;152:R191–R200. doi: 10.1530/REP-16-0167. [DOI] [PubMed] [Google Scholar]
- 10.Campos AC, Fogaca MV, Sonego AB, Guimaraes FS. Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacol Res. 2016;112:119–27. doi: 10.1016/j.phrs.2016.01.033. [DOI] [PubMed] [Google Scholar]
- 11.Russo EB. Clinical Endocannabinoid Deficiency Reconsidered: Current Research Supports the Theory in Migraine, Fibromyalgia, Irritable Bowel, and Other Treatment-Resistant Syndromes. Cannabis Cannabinoid Res. 2016;1:154–65. doi: 10.1089/can.2016.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alger BE. Seizing an opportunity for the endocannabinoid system. Epilepsy Curr. 2014;14:272–6. doi: 10.5698/1535-7597-14.5.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romigi A, Bari M, Placidi F, et al. Cerebrospinal fluid levels of the endocannabinoid anandamide are reduced in patients with untreated newly diagnosed temporal lobe epilepsy. Epilepsia. 2010;51:768–72. doi: 10.1111/j.1528-1167.2009.02334.x. [DOI] [PubMed] [Google Scholar]
- 14.Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda; Board on Population Health and Public Health Practice; Health and Medicine Division; National Academies of Sciences. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. http:www.nap.edu/246252017. [PubMed]
- 15.Davis MP. Cannabinoids for Symptom Management and Cancer Therapy: The Evidence. J Natl Compr Canc Netw. 2016;14:915–22. doi: 10.6004/jnccn.2016.0094. [DOI] [PubMed] [Google Scholar]
- 16.Arjmand S, Vaziri Z, Behzadi M, Abbassian H, Stephens GJ, Shabani M. Cannabinoids and Tremor Induced by Motor-related Disorders: Friend or Foe? Neurotherapeutics. 2015;12:778–87. doi: 10.1007/s13311-015-0367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Syed YY, McKeage K, Scott LJ. Delta-9-tetrahydrocannabinol/cannabidiol (Sativex(R)): a review of its use in patients with moderate to severe spasticity due to multiple sclerosis. Drugs. 2014;74:563–78. doi: 10.1007/s40265-014-0197-5. [DOI] [PubMed] [Google Scholar]
- 18.Sharkey KA, Darmani NA, Parker LA. Regulation of nausea and vomiting by cannabinoids and the endocannabinoid system. Eur J Pharmacol. 2014;722:134–46. doi: 10.1016/j.ejphar.2013.09.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ungerleider JT, Andrysiak TA, Fairbanks LA, Tesler AS, Parker RG. Tetrahydrocannabinol vs. prochlorperazine. The effects of two antiemetics on patients undergoing radiotherapy. Radiology. 1984;150:598–9. doi: 10.1148/radiology.150.2.6318262. [DOI] [PubMed] [Google Scholar]
- 20.Meiri E, Jhangiani H, Vredenburgh JJ, et al. Efficacy of dronabinol alone and in combination with ondansetron versus ondansetron alone for delayed chemotherapy-induced nausea and vomiting. Curr Med Res Opin. 2007;23:533–43. doi: 10.1185/030079907x167525. [DOI] [PubMed] [Google Scholar]
- 21.Neumeister A, Normandin MD, Pietrzak RH, et al. Elevated brain cannabinoid CB1 receptor availability in post-traumatic stress disorder: a positron emission tomography study. Mol Psychiatry. 2013;18:1034–40. doi: 10.1038/mp.2013.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roitman P, Mechoulam R, Cooper-Kazaz R, Shalev A. Preliminary, open-label, pilot study of add-on oral Delta9-tetrahydrocannabinol in chronic post-traumatic stress disorder. Clin Drug Investig. 2014;34:587–91. doi: 10.1007/s40261-014-0212-3. [DOI] [PubMed] [Google Scholar]
- 23.Tong S, Ngian GL, Onwude JL, et al. Diagnostic accuracy of maternal serum macrophage inhibitory cytokine-1 and pregnancy-associated plasma protein-A at 6–10 weeks of gestation to predict miscarriage. Obstet Gynecol. 2012;119:1000–8. doi: 10.1097/AOG.0b013e3182518fd3. [DOI] [PubMed] [Google Scholar]
- 24.Mato S, Del Olmo E, Pazos A. Ontogenetic development of cannabinoid receptor expression and signal transduction functionality in the human brain. Eur J Neurosci. 2003;17:1747–54. doi: 10.1046/j.1460-9568.2003.02599.x. [DOI] [PubMed] [Google Scholar]
- 25.Berrendero F, Sepe N, Ramos JA, Di Marzo V, Fernandez-Ruiz JJ. Analysis of cannabinoid receptor binding and mRNA expression and endogenous cannabinoid contents in the developing rat brain during late gestation and early postnatal period. Synapse. 1999;33:181–91. doi: 10.1002/(SICI)1098-2396(19990901)33:3<181::AID-SYN3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 26.Mereu G, Fa M, Ferraro L, et al. Prenatal exposure to a cannabinoid agonist produces memory deficits linked to dysfunction in hippocampal long-term potentiation and glutamate release. Proc Natl Acad Sci U S A. 2003;100:4915–20. doi: 10.1073/pnas.0537849100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campolongo P, Trezza V, Cassano T, et al. Perinatal exposure to delta-9-tetrahydrocannabinol causes enduring cognitive deficits associated with alteration of cortical gene expression and neurotransmission in rats. Addict Biol. 2007;12:485–95. doi: 10.1111/j.1369-1600.2007.00074.x. [DOI] [PubMed] [Google Scholar]
- 28.Trezza V, Campolongo P, Cassano T, et al. Effects of perinatal exposure to delta-9-tetrahydrocannabinol on the emotional reactivity of the offspring: a longitudinal behavioral study in Wistar rats. Psychopharmacology (Berl) 2008;198:529–37. doi: 10.1007/s00213-008-1162-3. [DOI] [PubMed] [Google Scholar]
- 29.Tortoriello G, Morris CV, Alpar A, et al. Miswiring the brain: Delta9-tetrahydrocannabinol disrupts cortical development by inducing an SCG10/stathmin-2 degradation pathway. EMBO J. 2014;33:668–85. doi: 10.1002/embj.201386035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El Marroun H, Tiemeier H, Steegers EA, et al. Intrauterine cannabis exposure affects fetal growth trajectories: the Generation R Study. J Am Acad Child Adolesc Psychiatry. 2009;48:1173–81. doi: 10.1097/CHI.0b013e3181bfa8ee. [DOI] [PubMed] [Google Scholar]
- 31.Dekker GA, Lee SY, North RA, McCowan LM, Simpson NA, Roberts CT. Risk factors for preterm birth in an international prospective cohort of nulliparous women. PloS one. 2012;7:e39154. doi: 10.1371/journal.pone.0039154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saurel-Cubizolles MJ, Prunet C, Blondel B. Cannabis use during pregnancy in France in 2010. BJOG. 2014;121:971–7. doi: 10.1111/1471-0528.12626. [DOI] [PubMed] [Google Scholar]
- 33.Leemaqz SY, Dekker GA, McCowan LM, et al. Maternal marijuana use has independent effects on risk for spontaneous preterm birth but not other common late pregnancy complications. Reprod Toxicol. 2016;62:77–86. doi: 10.1016/j.reprotox.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 34.Varner MW, Silver RM, Rowland Hogue CJ, et al. Association between stillbirth and illicit drug use and smoking during pregnancy. Obstet Gynecol. 2014;123:113–25. doi: 10.1097/AOG.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams LJ, Correa A, Rasmussen S. Maternal lifestyle factors and risk for ventricular septal defects. Birth Defects Res A Clin Mol Teratol. 2004;70:59–64. doi: 10.1002/bdra.10145. [DOI] [PubMed] [Google Scholar]
- 36.Warshak CR, Regan J, Moore B, Magner K, Kritzer S, Van Hook J. Association between marijuana use and adverse obstetrical and neonatal outcomes. J Perinatol. 2015;35:991–5. doi: 10.1038/jp.2015.120. [DOI] [PubMed] [Google Scholar]
- 37.Metz TD, Allshouse AA, Hogue CJ, et al. Maternal marijuana use, adverse pregnancy outcomes, and neonatal morbidity. Am J Obstet Gynecol. 2017;217(4):478.e1–478.e8. doi: 10.1016/j.ajog.2017.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hurd YL, Wang X, Anderson V, Beck O, Minkoff H, Dow-Edwards D. Marijuana impairs growth in mid-gestation fetuses. Neurotoxicol Teratol. 2005;27:221–9. doi: 10.1016/j.ntt.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 39.Bergeria CL, Heil SH. Surveying Lactation Professionals Regarding Marijuana Use and Breastfeeding. Breastfeed Med. 2015;10:377–80. doi: 10.1089/bfm.2015.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Djulus J, Moretti M, Koren G. Marijuana use and breastfeeding. Can Fam Physician. 2005;51:349–50. [PMC free article] [PubMed] [Google Scholar]
- 41.Baker T, Datta P, Rewers-Falkins K, et al. Transfer of Inhaled Cannabis Into Human Breastmilk. Obstet Gynecol. 2018;131(5):783–8. doi: 10.1097/AOG.0000000000002575. [DOI] [PubMed] [Google Scholar]
- 42.Perez-Reyes M, Wall ME. Presence of delta9-tetrahydrocannabinol in human milk. N Engl J Med. 1982;307:819–20. doi: 10.1056/NEJM198209233071311. [DOI] [PubMed] [Google Scholar]
- 43.Astley SJ, Little RE. Maternal marijuana use during lactation and infant development at one year. Neurotoxicol Teratol. 1990;12:161–8. doi: 10.1016/0892-0362(90)90129-z. [DOI] [PubMed] [Google Scholar]
- 44.Tennes K, Avitable N, Blackard C, et al. Marijuana: prenatal and postnatal exposure in the human. NIDA Res Monogr. 1985;59:48–60. [PubMed] [Google Scholar]
- 45.American Academy of Pediatrics. Breastfeeding and the use of human milk. Pediatrics. 2012;129:e827–41. doi: 10.1542/peds.2011-3552. [DOI] [PubMed] [Google Scholar]
- 46.Reece-Stremtan S, Marinelli KA. Academy for Breastfeeding Medicine clinical protocol #21: guidelines for breastfeeding and substance use or substance use disorder, revised 2015. Breastfeed Med. 2015;10:135–41. doi: 10.1089/bfm.2015.9992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Colby JM. Comparison of umbilical cord tissue and meconium for the confirmation of in utero drug exposure. Clin Biochem. 2017;50:784–90. doi: 10.1016/j.clinbiochem.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 48.Holland CL, Rubio D, Rodriguez KL, et al. Obstetric Health Care Providers' Counseling Responses to Pregnant Patient Disclosures of Marijuana Use. Obstet Gynecol. 2016;127:681–7. doi: 10.1097/AOG.0000000000001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jarlenski M, Tarr JA, Holland CL, Farrell D, Chang JC. Pregnant Women's Access to Information About Perinatal Marijuana Use: A Qualitative Study. Womens Health Issues. 2016;26:452–9. doi: 10.1016/j.whi.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.El Marroun H, Tiemeier H, Steegers EA, et al. A prospective study on intrauterine cannabis exposure and fetal blood flow. Early Hum Dev. 2010;86:231–6. doi: 10.1016/j.earlhumdev.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 51.Fried PA, Watkinson B, Gray R. Differential effects on cognitive functioning in 9- to 12-year olds prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol. 1998;20:293–306. doi: 10.1016/s0892-0362(97)00091-3. [DOI] [PubMed] [Google Scholar]
- 52.Huizink AC. Prenatal cannabis exposure and infant outcomes: overview of studies. Prog Neuropsychopharmacol Biol Psychiatry. 2014;52:45–52. doi: 10.1016/j.pnpbp.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 53.Jaddoe VW, van Duijn CM, Franco OH, et al. The Generation R Study: design and cohort update 2012. Eur J Epidemiol. 2012;27:739–56. doi: 10.1007/s10654-012-9735-1. [DOI] [PubMed] [Google Scholar]
- 54.Dickson B, Mansfield C, Guiahi M, et al. Recommendations from cannabis dispensaries about first-trimester cannabis use. Obstet Gynecol. 2018;131(6) doi: 10.1097/AOG.0000000000002619. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.