The development of new drugs for tuberculosis (TB) is not for the faint of heart, in addition to all the typical hurdles to developing a new anti-infective there are additional complications resulting from the pathology induced by the disease. TB is a disease of the lungs: it is transmitted by inhalation of an aerosol droplet containing the bacterium, which implants in small alveoli, and then invades and multiplies in the interstitial spaces in the lung forming granulomas (nodules of immune cells recruited as the body attempts to wall off the invading bacteria). Eventually these granulomas undergo necrosis to form cavities and leak out into the airways, spilling bacteria into the lungs and inducing coughing to enable transmission to a new host. A single patient with TB can have dozens of individual lesions, all at different stages of maturation. Endpoints in Phase 3 clinical trials of new TB drugs focus on achieving disease-free status in patients one year after completion of therapy (which is often very protracted as the standard of care for pulmonary TB patients globally involves six months of treatment). In contrast endpoints in Phase 2 clinical trials focus on short-term killing bacteria in expectorated sputum over the first few months of treatment. Unfortunately the endpoints in these two different stages of clinical evaluation are not well correlated as has been re-emphasized recently by the failure of three large Phase 3 studies attempting to shorten the standard six month regimen to four months by including a fluoroquinolone as part of a four-drug regimen 1–3. Despite achieving earlier sterilization of sputum in preceding Phase 2 studies and within the Phase 3 trials themselves, more patients in the four-month fluoroquinolone arms suffered relapse with disease after treatment was discontinued. These patients obviously were not cured, thus bacteria in the sputum do not tell a complete story, nor would one expect them to since only certain lesions produce bacteria that are represented in the airways.
Curing all patients with a shortened TB treatment regimen remains a challenging task and requires developing an understanding of what leads to a relapse (defined as recurrent disease with the same bacterial strain after apparent initial cure) in a subset of treated patients. Broadly put this scientific debate revolves around two different hypotheses, one that argues that relapse is due to a subset of bacteria with unique physiology (imposed by drugs, the immune system, physical properties of the local environment, or subpopulations that arise stochastically)4, and another that maintains that relapse is due to bacteria that exist within an area of unique pathology where their exposure to antibiotics is limited5. There are elements of truth and experimental data in support of both theories and it would seem likely that each has some validity. What has been missing until recently has been translational studies that move beyond simply measurements of bacteria in sputum to really understand the basis of relapse.
One area where this is changing involves advances in radiologic imaging and computational image analysis that appear to allow for precise quantitation of all types of lesion pathology present in the lungs of TB patients. Computed tomography (CT) is a type of exam that can generate 3D maps representing every voxel of the lung at mm resolution and report its density to X-rays.
Computationally it is possible to distinguish features that are not normally present in healthy lungs, and, more importantly, quantitate the change in such features over serial exams. In a recent study, changes in abnormal CT lung density after two months of treatment were shown to accurately predict how patients would fare two years later, after they had completed treatment6. Positron-emission tomography (PET) scanning involves administering a radioactive probe (the most widely used is [18F]-2-fluoro-2-deoxyglucose, which is taken up by immunologically active cells that rapidly take up glucose due to inflammation) and collecting information about the location of positron emission and hence location of the probe. TB lesions, and likely lesions from many pathogens, are sites of intense inflammation and quantification of changes in the extent of inflammation during the first two months of treatment predicted ultimate treatment outcome just as well as CT changes6. Importantly both these radiologic exams have also been applied to non-human primate models offering a smooth pathway between a realistic animal model and clinical trials7. Although the initial studies in humans were done two months after treatment started, results in rabbits suggest that radiographic changes due to chemotherapy are quantifiable as early as 1 week after initiation. In addition, many groups are actively pursuing promising PET probes that report on processes specific to TB that might offer a quantitative means of assessing bacterial numbers in specific lesions8,9.
The search for markers to determine when treatment is complete has also been advanced by looking at changes in circulating levels of various immunologically active molecules in blood from patients on treatment10 as well as by analysis of changes in metabolites in urine during the first month of treatment11 and both appear highly promising. Larger cohorts of patient samples, preferably from trials designed to attempt treatment shortening, are urgently needed for all these techniques to assess the predictive validity of such markers.
Imaging mass spectrometry (IMS) is another technique that advances the understanding of persisting bacterial populations. IMS creates two dimensional ion maps from excised tissue samples allowing the evaluation of drug distribution across relatively large areas. The lung has long been thought to be a “blood organ,” suggesting that the circulating concentration of drug in the bloodstream approximates that in TB lesions. Recent work using IMS in animal models of disease clearly shows this assumption to be false in most cases5,12 with some drugs penetrating lesions poorly or not at all while others freely diffuse into, and out of, affected areas. A clinical trial to validate these findings in human TB patients has recently concluded and the results will soon be reported (ClinicalTrials.gov identifier: NCT00816426). At the moment we have only superficial ideas of what physicochemical properties might facilitate lesion penetration, and little understanding of the potential role of human efflux systems that might affect pulmonary drug concentrations but there has been a quantum leap forward in our appreciation of the importance of this knowledge if we are to achieve success with shorter TB therapies.
All of these exciting new techniques have tremendous potential to inform TB drug development and affect millions of patients. The first randomized, placebo-controlled clinical trial of any medical intervention was performed in TB patients treated with streptomycin and up until now we’ve used the same methodologies as were used in that trial more than half a century ago. Today, science has advanced; it’s time for our clinical methodology to catch up.
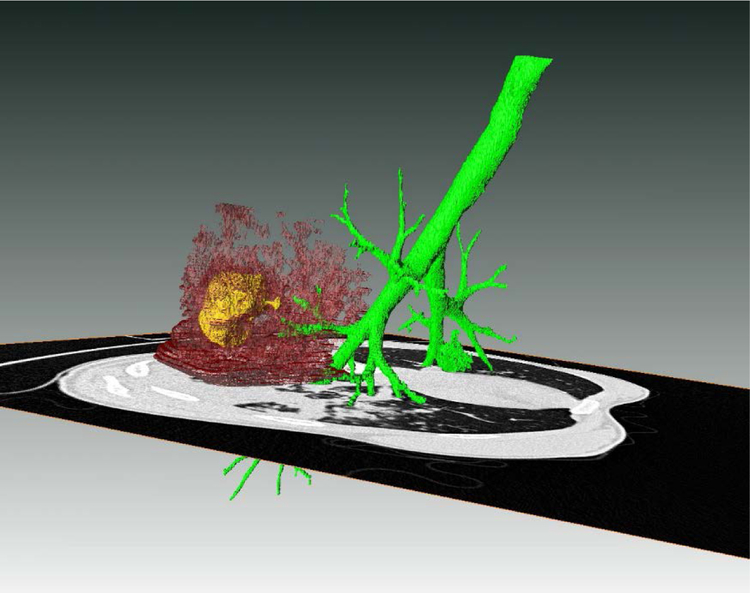
Figure 1.
Rendering of abnormal CT density in the lungs of a patient with pulmonary TB. The green structure reflects the airways; the maroon is density associated with TB lesions; and the yellow represents air inside of a cavitary lesion. Also shown is a 2D axial slice of the CT scan from which the rendering was derived. Produced in Amira 5.6.0 (FEI Visualization Sciences Group).
REFERENCES
- 1.Jindani A, Harrison TS, Nunn AJ, et al. High-dose rifapentine with moxifloxacin for pulmonary tuberculosis. The New England journal of medicine 2014;371:1599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merle CS, Fielding K, Sow OB, et al. A four-month gatifloxacin-containing regimen for treating tuberculosis. The New England journal of medicine 2014;371:1588–98. [DOI] [PubMed] [Google Scholar]
- 3.Gillespie SH, Crook AM, McHugh TD, et al. Four-month moxifloxacin-based regimens for drug-sensitive tuberculosis. The New England journal of medicine 2014;371:1577–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKinney JD. In vivo veritas: the search for TB drug targets goes live. Nature medicine 2000;6:1330–3. [DOI] [PubMed] [Google Scholar]
- 5.Dartois V The path of anti-tuberculosis drugs: from blood to lesions to mycobacterial cells. Nature reviews Microbiology 2014;12:159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen RY, Dodd LE, Lee M, et al. PET/CT imaging correlates with treatment outcome in patients with multidrug-resistant tuberculosis. Science translational medicine 2014;6:265ra166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coleman MT, Chen RY, Lee M, et al. PET/CT imaging reveals a therapeutic response to oxazolidinones in macaques and humans with tuberculosis. Science translational medicine 2014;6:265ra167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Urbanek BL, Wing DC, Haislop KS, et al. Chemoenzymatic synthesis of trehalose analogues: rapid access to chemical probes for investigating mycobacteria. Chembiochem : a European journal of chemical biology 2014;15:2066–70. [DOI] [PubMed] [Google Scholar]
- 9.Backus KM, Boshoff HI, Barry CS, et al. Uptake of unnatural trehalose analogs as a reporter for Mycobacterium tuberculosis. Nature chemical biology 2011;7:228–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walzl G, Ronacher K, Hanekom W, Scriba TJ, Zumla A. Immunological biomarkers of tuberculosis. Nature reviews Immunology 2011;11:343–54. [DOI] [PubMed] [Google Scholar]
- 11.Mahapatra S, Hess AM, Johnson JL, et al. A metabolic biosignature of early response to anti-tuberculosis treatment. BMC infectious diseases 2014;14:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kjellsson MC, Via LE, Goh A, et al. Pharmacokinetic evaluation of the penetration of antituberculosis agents in rabbit pulmonary lesions. Antimicrobial agents and chemotherapy 2012;56:446–57. [DOI] [PMC free article] [PubMed] [Google Scholar]