Abstract
Osteogenesis imperfecta (OI) is a hereditary bone disorder most commonly caused by autosomal dominant mutations in genes encoding type I collagen. In addition to bone fragility, patients suffer from impaired longitudinal bone growth. It has been demonstrated that in OI, an accumulation of mutated type I collagen in the endoplasmic reticulum (ER) induces ER stress in osteoblasts, causing osteoblast dysfunction leading to bone fragility. We hypothesize that ER stress is also induced in the growth plate where bone growth is initiated, and examined a mouse model of dominant OI that carries a G610C mutation in the procollagen α2 chain. The results demonstrated that G610C OI mice had significantly shorter long bones with growth plate abnormalities including elongated total height and hypertrophic zone. Moreover, we found that mature hypertrophic chondrocytes expressed type I collagen and ER dilation was more pronounced compared to wild type littermates. The results from in vitro chondrocyte cultures demonstrated that the maturation of G610C OI hypertrophic chondrocytes was significantly suppressed and ER stress related genes were upregulated. Given that the alteration of hypertrophic chondrocyte activity often causes dwarfism, our findings suggest that hypertrophic chondrocyte dysfunction induced by ER stress may be an underlying cause of growth deficiency in G610C OI mice.
Keywords: Osteogenesis imperfecta, bone growth, Endoplasmic reticulum stress, hypertrophic chondrocytes
Introduction
Osteogenesis imperfecta (OI) is a genetic disorder that phenotypically causes skeletal abnormalities including bone fragility, deformity and growth impairment [1, 2]. More than 85% of OI cases are caused by autosomal dominant mutations in Col1a1 and Col1a2 encoding the α1 and α2 chains of type I procollagen, respectively, which is a main component of bone matrices [2]. The G610C mutation that changes the 610th amino acid from glycine (Gly) to cysteine (Cys) in the triple helical domain of α2 chains of type I procollagen (p.Gly777Cys) is one of the dominant forms that was originally identified in patients from the Old Order Amish kindred [3]. Mice harboring this G610C mutation exhibit skeletal phenotypes similar to OI patients with the identical mutation, such as reduced body mass, bone mineral density and bone strength [3].
The mature type I collagen is a triple helix assembled in the endoplasmic reticulum (ER) by two α1 and one α2 chains of type I procollagen. This collagen triple helix consists of a repeating Gly-X-Y sequence within each chain [4]. The dominant mutations of OI, including G610C mutation, commonly substitute Gly in this repeating sequence, which interrupts hydrogen bonding between procollagen chains and disrupts proper triple helix formation, resulting in misfolding of collagen [5]. This misfolded collagen accumulates in the ER, causing ER dilation, cell stress and dysfunction of osteoblasts in OI [5-7]. Reduction of cell stress by enhancing clearance of unfolded collagen improved dysfunction of osteoblasts isolated from G610C OI mice [5]. Therefore, osteoblast dysfunction induced by the accumulation of misfolded collagen plays a major role in pathogenesis of bone fragility in autosomal dominant OI cases.
Longitudinal bone growth occurs by endochondral ossification at the growth plate where chondrocytes proliferate, produce cartilaginous matrices and differentiate into hypertrophic chondrocytes, which are characterized by cell shape and expression of hypertrophy-related molecules such as type X collagen, matrix metalloprotease 13, alkaline phosphatase, bone sialoprotein, etc. [8-10]. Hypertrophic chondrocytes contribute to longitudinal bone growth not only by increasing their size but also playing multiple roles in endochondral ossification. Specifically, they are responsible for cartilage matrix mineralization and degradation, invasion of blood vessels, and osteo/chondro-clastogenesis and bone formation by transdifferentiating into osteoblasts [11-14]. Alteration of these activities has been shown to lead to growth deficiency [11, 15].
Contrary to bone fragility, growth deficiency in OI has not been extensively investigated and there are very few reports studying the growth plate in OI [16-18]. Analyses of the growth plate in 6 OI patients showed abnormal changes, especially in hypertrophic chondrocytes, including increased thickness of the hypertrophic zone, reduced matrix glycosaminoglycans, reduced alkaline phosphatase activity, and poor mineralization, suggesting that hypertrophic chondrocyte activities are somehow affected in OI. Therefore, to elucidate the mechanism underlying growth deficiency in OI, in this study, we examined alterations of the growth plate and function of hypertrophic chondrocytes in G610C OI mice.
Materials and Methods
Mice
Wild type C57BL/6 mice and G610C OI mice on a C57BL/6 background [3] were purchased from The Jackson Laboratory and Col1a1 3.6-GFPtpz transgenic mice [19] were kindly provided by Dr. David Rowe. These strains were maintained in the animal facility at The Research Institute at Nationwide Children’s Hospital as well as at The University of Maryland, School of Medicine. All animal protocols were approved by the Institutional Animal Care and Use Committees of both institutes.
Bone length
Both the left and right hind limbs were harvested from 3-, 6- and 12-week old G610C OI and wild type littermates and fixed in 4% paraformaldehyde for 2 days. After carefully removing soft tissues under a stereo microscope, tibia and femur lengths were measured using a digital caliper (Thermo Fisher Scientific, Waltham, MA, USA). The lengths of the right and left bones from individual mice were averaged.
Histological evaluation (Details are provided in the Supplementary Material)
Following fixation and decalcification, 6μm-thick paraffin embedded sections were stained with hematoxylin and eosin (H&E) for height measurements of the growth plate.
For GFP and type X collagen staining, 6μm-thick sections were incubated with polyclonal goat anti-GFP antibody (1:200, Novus Biologicals LLC, Littleton, CO, USA) and polyclonal rabbit anti-Collagen X antibody (1:50, Abcam, Cambridge, MA, USA) followed by incubation with Alexa Fluor 488 anti-goat IgG, Alexa Fluor 555 anti-rabbit IgG (1:200, Thermo Fisher Scientific).
For procollagen type I staining, sections were blocked with the Mouse on Mouse kit (Vector Laboratories, Burlingame, CA, USA) followed by incubation with an antibody specific for procollagen type I (1:20 dilution, SP1.D8, Developmental Studies Hybridoma Bank, Iowa, IA, USA) [20]. The antibody was visualized using ImmPACT NovaRED Peroxidase substrate (Vector Laboratories).
For 5-ethynyl-2'- deoxyuridine (EdU) staining, sections stained for EdU using the Click-iT EdU Imaging Kit (Thermo Fisher Scientific).
All images were acquired with an Axiocam HRc or MRm camera using AxioVision 4.5SP1 software (Carl Zeiss, Thornwood, NY, USA) on a Zeiss Axio Imager A1. All histological measurements were performed blindly to sample identifications.
Electron microscopy
The growth plate was dissected from distal femora of day4 neonates of G610C OI and wild type littermates under the stereo microscope. The isolated growth plates were fixed in 2.5% glutaraldehyde over night at 4°C followed by the post fixation with 1% OsO4 for 2 hours. These fixed growth plates were then embedded into epoxy resin. Sixty nm sections were collected onto CuPd grids and stained with uranyl acetate and lead citrate. Grids were viewed on a Hitachi H 7650 Transmission Electron Microscope (Hitachi High-Technologies, Chatsworth, CA, USA). ER thickness was measured at 500nm intervals in 7-12 hypertrophic chondrocytes per growth plate in 5 mice from each strain (30-170 measurements per hypertrophic chondrocyte) on acquired images [5].
Chondrocyte pellet culture
Primary chondrocytes were isolated from epiphyseal cartilage of humeri and femora harvested from day 4 neonatal G610C OI and wild type littermates as previously described [21, 22]. Freshly isolated chondrocytes were re-suspended in DMEM/F12 with L-Glutamine (Corning Life Sciences) supplemented with ascorbic acid (50μg/mL) and 10% fetal bovine serum and then 5 × 105 chondrocytes were pelleted in a 0.5mL tube by centrifugation at 400g for 5 minutes [10, 23]. These chondrocyte pellets were incubated at 37°C and the medium was changed every other day.
RNA isolation and quantitative PCR
Chondrocyte pellets were washed with cold PBS twice and disrupted in RLT buffer containing β-mercaptoethanol using a mortar and pestle (Takara BioMasher, Takara Bio Inc, Shiga, Japan) followed by homogenization using QIAshredder (QIAGEN, Gaithersburg, MD, USA). RNA was isolated from the lysate with RNeasy Micro Kit according to the manufacture’s instruction (QIAGEN). Reverse transcription was performed with High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). Quantitative PCR was performed on ABI 7900HT Fast Real-Time Systems (Thermo Fisher Scientific) using TaqMan Gene Expression Assays for glyceral-dehyde-3-phosphate dehydrogenase (Gapdh), type X collagen (Col10a1), matrix metalloprotease 13 (Mmp13), alkaline phosphatase (Alp), bone sialoprotein (Bsp), osterix (Osx), bone gamma-carboxyglutamate protein (Bglap), type I collagen (Col1a1 and Col1a2), binding- immunoglobulin protein (BiP, Hapa5), heat shock protein 47 (Serpinh1), ER degradation enhancing alpha-mannosidase like protein 1 (Edem1) and crystallin alpha b (Cryab).
Statistical Analysis
Statistical analyses were performed by unpaired two-tailed t-test for comparison of two samples and by two-way analysis of variance for samples with two variables followed by Sidak’s multiple comparisons test using Prism (Version 7, GraphPad Software Inc., La Jolla, CA, USA). A minimum of four replicates were used for statistical analyses for each experiment. All data are shown as mean ± standard error. A P-value < 0.05 was considered statistically significant.
Results
Bone growth was significantly impaired with the elongated growth plate in G610C OI mice.
We examined the bone length and growth plate histology of G610C OI mice in comparison with wild type littermates of each gender at 3, 6, and 12 weeks of age. At least 5 mice per each age and gender were evaluated. Body weight and bone length in G610C OI mice were significantly smaller for both genders at every age examined (Fig. 1A [male] and Supplemental Fig. 1A [female]). These results were consistent with the findings in OI patients harboring an identical mutation [3]. Histological analyses of the femora revealed that the height (thickness) of the growth plate (GP) and hypertrophic zone (HZ) was significantly elongated in G610C OI mice (Fig. 1B [male] and Supplemental Fig. 1B [female]).
Figure 1.
Analyses of male G610C OI mice. (A) Body weight as well as femur and tibia lengths were evaluated in G610 C OI male mice and wild type (WT) male littermates at 3, 6, 12 weeks of age (black solid line: WT, red dotted line: G610C, n≥5). (B) Lengths of the growth plate (GP) and hypertrophic zone (HZ) were measured on femoral sections stained with H&E (x 200, n≥5). All data are plotted as mean ± SEM. *P<0.05, **P<0.01 and ***P<0.001.
Type I collagen was expressed in hypertrophic chondrocytes.
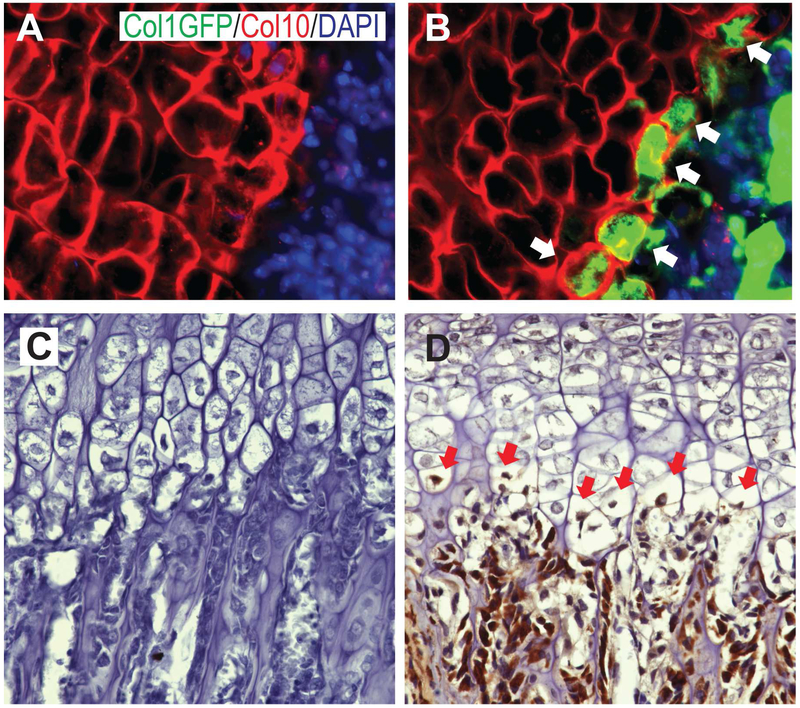
Consistent with previous reports from OI patients [16-18], the results above clearly showed that the growth plate is affected in G610C OI mice. To examine whether type I collagen is expressed in the growth plate, we analyzed Col1a1 3.6-GFPtpz transgenic mice, which express the topaz variant of GFP coincidently with endogenous Col1a1 expression [19]. Histological GFP staining of postnatal day 4 femora showed that cells adjacent to the chondro-osseous junction stained positively for both Col10 and GFP, while no GFP was detected in wild type mice (Fig. 2A and B). These results suggest that mature hypertrophic chondrocytes express type I collagen consistently with previous findings [24].
Figure 2.
Col1 expression in the growth plate. Femoral sections from day 4 neonates of wild type (A) and Col1a1 3.6-GFPtpz transgenic mice (B) were stained for GFP (green), type X collagen (Col10, red) and DAPI (blue) (x 200). (C, D) Double positive cells for GFP and type X collagen were indicated by white arrows. Femoral sections from 3-weeks old G610C OI mice were stained for type I procollagen (C: a control section without the primary antibody, D: with the primary antibody). The stained cells in the growth plate are indicated by red arrows (x 200).
To confirm that type I collagen is expressed in G610C OI hypertrophic chondrocytes, femoral sections of 3-week old G610C mice were stained with an antibody against type I procollagen, which does not recognize type I collagen in the extracellular matrix. Although the staining was not as strong as osteoblasts in the trabecular area, mature hypertrophic chondrocytes were also positively stained (Fig. 2C and D). These results indicate that mature hypertrophic chondrocytes in G610C OI mice express mutated type I collagen.
Endoplasmic reticulum was dilated in G610C OI hypertrophic chondrocytes.
In dominant forms of OI, mutated type I collagen has been demonstrated to cause ER stress in osteoblasts [1, 2]. Thus, we examined growth plate hypertrophic chondrocytes using transmission electron microscopy. Electron micrographs showed that ER dilation was more prominent in G610C OI hypertrophic chondrocytes than those from wild type littermates (Fig. 3A and B). ER thickness was increased in G610C OI hypertrophic chondrocytes (Fig. 3C) and the fraction of ER with 200nm or larger in thickness was significantly greater in G610C OI hypertrophic chondrocytes (Fig. 3D). These results suggest that ER stress is induced in hypertrophic chondrocytes in G610C OI mice.
Figure 3.
Electron microscopic analysis. Femoral sections from day 4 neonates of wild type littermates (A) and G610C OI mice (B) were scanned by electron microscopy. Swollen endoplasmic reticulum (ER) in hypertrophic chondrocytes were indicated by red asterisks. (C) ER thickness in hypertrophic chondrocytes was shown by histogram. (D) The fraction of ER with over 200nm thickness was analyzed. The data are shown as mean ± SEM.
Maturation of G610C OI hypertrophic chondrocytes was suppressed.
To examine whether ER stress cell-autonomously affects hypertrophic chondrocytes, we investigated hypertrophic differentiation of chondrocytes using a chondrocyte pellet culture which mimics hypertrophic differentiation in the growth plate [10, 23]. When chondrocytes isolated from epiphyseal cartilage were cultured as a pellet for a week, they remained immature and expressed marginal levels of hypertrophic chondrocyte related genes with no significant differences between G610C OI and wild type pellets (Fig. 4A). In contrast, chondrocytes in pellet cultures for 3 weeks became hypertrophic and increased expression levels of hypertrophic chondrocyte related genes (Fig. 4A). Consistent with the results in vivo (Fig. 2D), both Col1a1 and Col1a2 were expressed in chondrocyte pellet cultures. The expression levels of all hypertrophy-related genes tested at 3 weeks were significantly lower in G610C chondrocyte pellets than in wild type chondrocyte pellets (Fig. 4A), indicating that chondrocyte maturation is significantly suppressed in G610C OI.
Figure 4.
In vitro chondrocyte pellet culture. (A) Expression of mature hypertrophic chondrocyte related genes were examined in 1 week (1wk)- and 3 week (3wks)- pellet culture of chondrocytes (black: WT littermate chondrocyte pellet, gray: G610C OI chondrocyte pellet, n=5). (B) ER stress related genes were analyzed in the 3 week pellet culture of chondrocytes (n=5). Gene expression is shown relative to that of 1wk WT or WT. All data are shown as mean ± SEM. N.S.; not significant.
Consistent with the results shown in Fig.3, G610C chondrocyte pellets at 3 weeks expressed ER stress related genes such as Hspa5, Serpinh1, Edem1 and Cryab significantly greater than wild type chondrocytes (Fig. 4B). Contrary to G610C osteoblasts, Hspa5 which encodes binding-immunoglobulin protein (BiP) is upregulated in G610C chondrocytes.
These results demonstrate that G610C OI chondrocytes become dysfunctional when they undergo hypertrophic differentiation and increase ER stress. The expression of mutated type I collagen in pellet culture may be the source of abnormal ER stress in hypertrophic chondrocytes.
Discussion
In addition to bone fragility, growth deficiency is one of the major skeletal phenotypes in patients with OI [1]. While extensive efforts have been made to identify the mechanism of bone fragility in OI, the cause of growth deficiency in OI has been a longstanding mystery. In this study, we demonstrate the possibility that hypertrophic chondrocyte dysfunction in the growth plate induced by ER stress may cause growth deficiency in OI.
Similar to other dominant forms of OI, G610C OI mice demonstrate mutated type I collagen accumulated in the ER of osteoblasts, which induced cell stress leading to suppression of osteoblast maturation and mineral deposition [5]. Our in vitro chondrocyte pellet studies showed that G610C OI chondrocyte maturation was also suppressed, suggesting that similar mechanisms may be involved in both osteoblasts and chondrocytes to prevent differentiation. Failure in chondrocyte maturation may prevent chondrocyte clearance from the growth plate and result in elongated growth plate height.
When ER stress was induced in murine growth plate chondrocytes during the pubertal growth spurt by knocking out ERp57, a chaperone which is responsible for the correct folding of newly synthesized glycoproteins, bone growth was significantly suppressed with reduced trabecular bone formation [25]. Importantly, the reduction of bone growth was accompanied with enlarged growth plates as well as expanded hypertrophic zones. Moreover, when ER stress was introduced specifically in hypertrophic chondrocytes in the growth plate by expressing an ER stress-inducing protein under the control of Col10a1 promoter [26], the transgenic mice also exhibited shorter bones with the expanded hypertrophic zones. These results indicate that ER stress in the growth plate, especially in hypertrophic chondrocytes, impairs longitudinal bone growth. One of the chondrodysplasias, metaphyseal chondrodysplasia type Schmid (MCDS), is caused by mutations in type X collagen proteins which result in the production of abnormal proteins with improper folding leading to ER stress in hypertrophic chondrocytes [27]. Indeed, a mouse model of MCDS that carries a causative mutation (p.Asn617Lys) in Col10a1 exhibits dwarfism as well as expanded hypertrophic zones [26]. This mutated type X collagen accumulates in the ER and causes ER stress specifically in growth plate hypertrophic chondrocytes. All of these results are comparable with our findings in G610C mice, suggesting that ER stress in hypertrophic chondrocytes may be involved in the mechanism underlying growth deficiency in OI.
It has been shown that a substantial number of hypertrophic chondrocytes transdifferentiate into osteoblasts and directly contribute to trabecular bone formation [14]. Given that chondrocytes are stagnated in the growth plate of G610C OI mice, ER stress in hypertrophic chondrocyte may also cause reduced trabecular bone formation, which is a typical musculoskeletal manifestation of OI. Moreover, hypertrophic chondrocytes are an essential source of receptor activator of nuclear factor-kB ligand (RANKL) for osteo/chondro-clastogenesis especially in growing bones [13]. Therefore, hypertrophic chondrocyte dysfunction could also be involved in the abnormal osteoclast activity observed in OI.
It has been demonstrated that cell stress in G610C OI osteoblasts is distinct from typical ER stress since BiP upregulation, one of ER stress indicators, does not occur [5]. In contrast, the expression of Hspa5, which encodes BiP, was significantly increased in G610C OI hypertrophic chondrocytes, suggesting that the accumulation of mutated type I collagen causes different types of cell stress in these cells. Given that chondrocytes express other matrix proteins that require modification in the ER such as type II collagen, type IX collagen, type X collagen, martrilin-3 and cartilage oligomeric matrix protein (COMP) [28], the accumulation of mutated type I collagen may cause misfolding of these other proteins which can trigger the typical ER stress response in hypertrophic chondrocytes. This may also suggest that the smaller amount of mutated type I collagen could be sufficient to induce ER stress in hypertrophic chondrocytes, accounting for relatively less expression of type I collagen in hypertrophic chondrocytes than in osteoblasts, although further investigation should be performed to fully understand how ER stress is induced in hypertrophic chondrocytes.
Our in vitro chondrocyte pellet culture demonstrated that maturation of G610C OI chondrocytes was significantly suppressed along with the increase of mutated Col1 expression compared to that of wild type chondrocytes, suggesting that G610C OI chondrocytes are cell-autonomously affected by ER stress. However, it remains unknown whether the abnormal skeletal environment created by osteoblasts expressing mutated type I collagen can affect chondrocyte activities in the growth plate. This discovery would be critical to determine the target cell types for new therapies in the treatment of growth deficiency. Since sclerostin antibody treatment failed to stimulate bone growth [29], the proper target may not be osteoblasts, but rather hypertrophic chondrocytes, that primarily cause growth deficiency in dominant forms of OI.
In conclusion, the current study demonstrated that growth plate hypertrophic chondrocytes in G610C OI mice were exposed to ER stress, which is associated with shorter bones and abnormal growth plates. Therefore, dysfunction of hypertrophic chondrocytes induced by ER stress may be a contributory mechanism underlying growth deficiency in G610C OI mice.
Supplementary Material
Highlights.
G610C OI mice exhibited shorter bones with elongated growth plates.
The ER in hypertrophic chondrocytes was significantly dilated in G610C OI mice.
ER stress was increased in hypertrophic chondrocytes along with suppression of chondrocyte maturation in G610C OI mice.
Acknowledgements
The authors thank the Animal Facility and Morphology Core for providing their services and resources. This research was supported by the institutional funds from both the University of Maryland, Baltimore and the Research Institute at Nationwide Children’s Hospital. This work was also supported in part by the Division of Intramural Research, National Institute of Child Health and Human Development, NIH.
Abbreviations
- OI
Osteogenesis imperfecta
- ER
endoplasmic reticulum
- GP
growth plate
- HZ
hypertrophic zone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Forlino A, Cabral WA, Barnes AM, Marini JC, New perspectives on osteogenesis imperfecta, Nature Reviews Endocrinology, 7 (2011) 540–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Forlino A, Marini JC, Osteogenesis imperfecta, The Lancet, 387 (2016) 1657–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Daley E, Streeten EA, Sorkin JD, Kuznetsova N, Shapses SA, Carleton SM, Shuldiner AR, Marini JC, Phillips CL, Goldstein SA, Leikin S, McBride DJ Jr., Variable bone fragility associated with an Amish COL1A2 variant and a knock-in mouse model, Journal of bone and mineral research, 25 (2010) 247–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ramshaw JA, Shah NK, Brodsky B, Gly-X-Y tripeptide frequencies in collagen: a context for host-guest triple-helical peptides, J Struct Biol, 122 (1998) 86–91. [DOI] [PubMed] [Google Scholar]
- [5].Mirigian LS, Makareeva E, Mertz EL, Omari S, Roberts-Pilgrim AM, Oestreich AK, Phillips CL, Leikin S, Osteoblast Malfunction Caused by Cell Stress Response to Procollagen Misfolding in α2(I)-G610C Mouse Model of Osteogenesis Imperfecta, Journal of Bone and Mineral Research, 31 (2016) 1608–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lisse TS, Thiele F, Fuchs H, Hans W, Przemeck GKH, Abe K, Rathkolb B, Quintanilla-Martinez L, Hoelzlwimmer G, Helfrich M, Wolf E, Ralston SH, de Angelis MH, ER Stress-Mediated Apoptosis in a New Mouse Model of Osteogenesis imperfecta, PLoS Genetics, 4 (2008) e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Makareeva E, Aviles NA, Leikin S, Chaperoning osteogenesis: new protein-folding disease paradigms, Trends in Cell Biology, 21 (2011) 168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hunziker EB, Mechanism of longitudinal bone growth and its regulation by growth plate chondrocytes, Microscopy research and technique, 28 (1994) 505–519. [DOI] [PubMed] [Google Scholar]
- [9].Wilsman NJ, Leiferman EM, Fry M, Farnum CE, Barreto C, Differential growth by growth plates as a function of multiple parameters of chondrocytic kinetics, Journal of orthopaedic research, 14 (1996) 927–936. [DOI] [PubMed] [Google Scholar]
- [10].Bowen ME, Ayturk UM, Kurek KC, Yang W, Warman ML, SHP2 Regulates Chondrocyte Terminal Differentiation, Growth Plate Architecture and Skeletal Cell Fates, PLoS Genetics, 10 (2014) e1004364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gerber H-P, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N, VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation, Nature medicine, 5 (1999) 623–628. [DOI] [PubMed] [Google Scholar]
- [12].Inada M, Wang Y, Byrne MH, Rahman MU, Miyaura C, López-Otín C, Krane SM, Critical roles for collagenase-3 (Mmp13) in development of growth plate cartilage and in endochondral ossification, Proceedings of the National Academy of Sciences of the United States of America, 101 (2004) 17192–17197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O'Brien CA, Matrix-embedded cells control osteoclast formation, Nature medicine, 17 (2011) 1235–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yang L, Tsang KY, Tang HC, Chan D, Cheah KS, Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation, Proc Natl Acad Sci U S A, 111 (2014) 12097–12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Golovchenko S, Hattori T, Hartmann C, Gebhardt M, Gebhard S, Hess A, Pausch F, Schlund B, von der Mark K, Deletion of beta catenin in hypertrophic growth plate chondrocytes impairs trabecular bone formation, Bone, 55 (2013) 102–112. [DOI] [PubMed] [Google Scholar]
- [16].Sanguinetti C, Greco F, De Palma L, Specchia N, Falciglia F, Morphological changes in growth-plate cartilage in osteogenesis imperfecta, The Journal of bone and joint surgery. British volume, 72 (1990) 475–479. [DOI] [PubMed] [Google Scholar]
- [17].Sarathchandra P, Cassella JP, Ali SY, Enzyme histochemical localisation of alkaline phosphatase activity in osteogenesis imperfecta bone and growth plate: a preliminary study, Micron, 36 (2005) 715–720. [DOI] [PubMed] [Google Scholar]
- [18].Stoss H, Cartilaginous changes in osteogenesis imperfecta, Prog Clin Biol Res, 200 (1985) 343–353. [PubMed] [Google Scholar]
- [19].Kalajzic I, Kalajzic Z, Kaliterna M, Gronowicz G, Clark SH, Lichtler AC, Rowe D, Use of type I collagen green fluorescent protein transgenes to identify subpopulations of cells at different stages of the osteoblast lineage, Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research, 17 (2002) 15–25. [DOI] [PubMed] [Google Scholar]
- [20].Foellmer HG, Kawahara K, Madri JA, Furthmayr H, Timpl R, Tuderman L, A monoclonal antibody specific for the amino terminal cleavage site of procollagen type I, Eur J Biochem, 134 (1983) 183–189. [DOI] [PubMed] [Google Scholar]
- [21].Otsuru S, Gordon PL, Shimono K, Jethva R, Marino R, Phillips CL, Hofmann TJ, Veronesi E, Dominici M, Iwamoto M, Horwitz EM, Transplanted bone marrow mononuclear cells and MSCs impart clinical benefit to children with osteogenesis imperfecta through different mechanisms, Blood, 120 (2012) 1933–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Otsuru S, Hofmann TJ, Raman P, Olson TS, Guess AJ, Dominici M, Horwitz EM, Genomic and functional comparison of mesenchymal stromal cells prepared using two isolation methods, Cytotherapy, 17 (2015) 262–270. [DOI] [PubMed] [Google Scholar]
- [23].Kato Y, Iwamoto M, Koike T, Suzuki F, Takano Y, Terminal differentiation and calcification in rabbit chondrocyte cultures grown in centrifuge tubes: regulation by transforming growth factor beta and serum factors, Proceedings of the National Academy of Sciences, 85 (1988) 9552–9556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kirsch T, Nah HD, Shapiro IM, Pacifici M, Regulated production of mineralization-competent matrix vesicles in hypertrophic chondrocytes, The Journal of cell biology, 137 (1997) 1149–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Linz A, Knieper Y, Gronau T, Hansen U, Aszodi A, Garbi N, Hämmerling GJ, Pap T, Bruckner P, Dreier R, ER Stress During the Pubertal Growth Spurt Results in Impaired Long-Bone Growth in Chondrocyte-Specific ERp57 Knockout Mice, Journal of Bone and Mineral Research, 30 (2015) 1481–1493. [DOI] [PubMed] [Google Scholar]
- [26].Rajpar MH, McDermott B, Kung L, Eardley R, Knowles L, Heeran M, Thornton DJ, Wilson R, Bateman JF, Poulsom R, Arvan P, Kadler KE, Briggs MD, Boot-Handford RP, Targeted Induction of Endoplasmic Reticulum Stress Induces Cartilage Pathology, PLoS Genetics, 5 (2009) e1000691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bateman JF, Wilson R, Freddi S, Lamande SR, Savarirayan R, Mutations of COL10A1 in Schmid metaphyseal chondrodysplasia, Hum Mutat, 25 (2005) 525–534. [DOI] [PubMed] [Google Scholar]
- [28].Patterson SE, Dealy CN, Mechanisms and models of endoplasmic reticulum stress in chondrodysplasia: ER Stress Mechanisms in Chondrodysplasia, Developmental Dynamics, 243 (2014) 875–893. [DOI] [PubMed] [Google Scholar]
- [29].Sinder BP, Salemi JD, Ominsky MS, Caird MS, Marini JC, Kozloff KM, Rapidly growing Brtl/+ mouse model of osteogenesis imperfecta improves bone mass and strength with sclerostin antibody treatment, Bone, 71 (2015) 115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.