Abstract
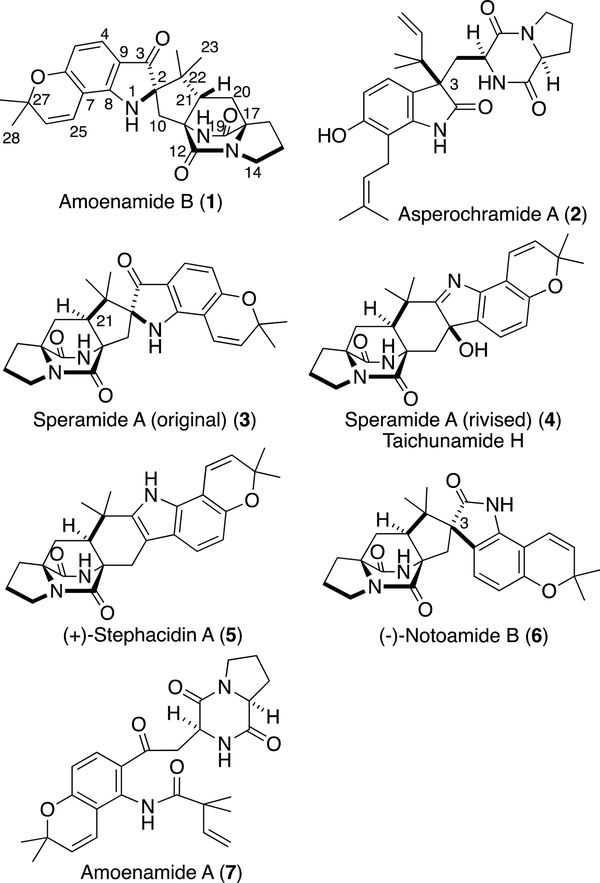
A new prenylated indoxyl alkaloid, Amoenamide B (1), was isolated from Aspergillus amoenus NRRL 35600 along with Asperochramide A (2). Although many prenylated oxyindole alkaloids, containing bicyclo[2.2.2]diazaoctane cores, have been isolated from the fungus of the genera Aspergillus and Penicillium to date, 1 is the fourth compound with the indoxyl unit containing the cores. During the structure elucidation of 1, we found that the planar structure matched to that of Speramide A (3), isolated from A. ochraceus KM007, but the reported structure of 3 was incorrect and turned out to be that of Taichunamide H (4), recently isolated from A. versicolor HDN11–84.
Keywords: Indoxyl alkaloid, Aspergillus, Fungus
Graphical Abstract
To create your abstract, type over the instructions in the template box below. Fonts or abstract dimensions should not be changed or altered.
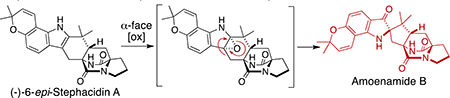
Introduction
In 2007, we reported the isolation of four new prenylated indole alkaloids, Notoamides A–D, from A. protuberus MF297–2.1 During our studies on the biosynthesis of the family of Notoamides in A. protuberus,2 the opposite enantiomers of Stephacidin A (5) and Notoamide B (6) (Figure 1) were isolated from A. amoenus (formerly A. versicolor) NRRL 35600.3 Compounds 5 and 6 contain bicyclo[2.2.2]diazaoctane cores, which may be formed through an intramolecular hetero Diels–Alder reaction from an isoprene unit and dioxopiperazine core. We are currently studying the mechanism of this fascinating construction. Recently, we isolated a new biosynthetically-interesting congener, Amoenamide A (7) (Figure 1), from A. amoenus along with five new enantiomers as minor metabolites.4 Herein, we report the isolation of a new congener, Amoenamide B (1), along with Asperochramide A (2), which was isolated from Aspergillus ochraceus (cgmcc 3.6281), recently.5
Fig. 1.
Structures of 1–7.
Results and Discussion
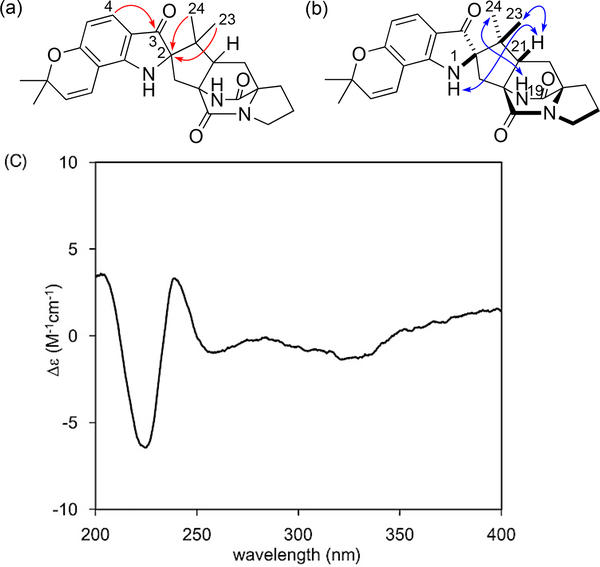
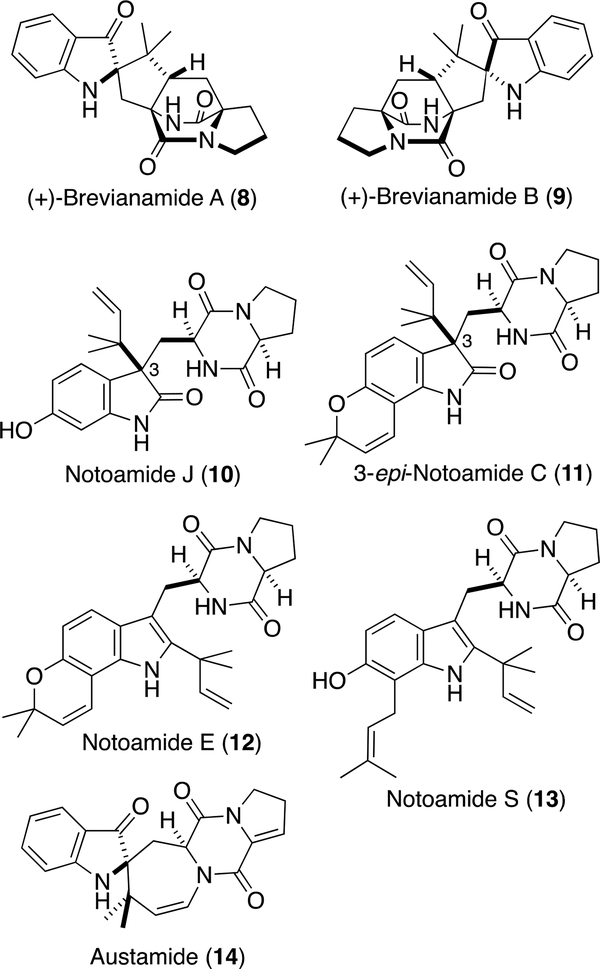
Aspergillus amoenus NRRL 35600 was cultured on rice and the metabolites were purified by column chromatography and HPLC to afford 1 and 2.6 The molecular formula of 1 was determined to be C26H29N3O4 by HRESIMS. The 1H NMR spectrum in DMSO-d6 (Table 1) showed four doublet olefinic and aromatic protons (δH 5.66 (d, J = 10.0 Hz, H-26), 6.77 (d, J = 10.0 Hz, H-25), 6.11 (d, J = 8.4 Hz, H-5), and 7.18 (d, J = 8.4 H-4)), two exchangeable protons (δH 7.73 (s, H-1) and 8.67 (s, H-19)), and four singlet methyl groups (δH 0.67 (3H, s, H3-23), 0.95 (3H, s, H3-24), 1.36 (3H, s, H3-28), and 1.40 (3H, s, H3-29)). The analysis of 13C and 2D NMR spectra readily indicated that the structure of 1 was similar to that of (–)-Notoamide B (6),1 a spiro-oxindole derivative. However, the HMBCs from H-4 to C-3 (δC 197.0) and from H3-23/H3-24 to C-2 (δC 79.7) (Figure 2a) clearly showed an indoxyl structure with a quaternary center at C-2 for 1. The detailed analysis of 2D NMR spectra are consistent with the planar structure of 1 corresponding to that of (+)-Brevianamides A (8) and B7 (9) (Figure 3), isolated from P. brevicompactum, but containing a 2,2-dimethylpyran ring fused to the indoxyl aromatic ring. NOE correlations, H-21 (δH 2.71, dd, J = 9.2, 5.8 Hz)/H-1, H-21/H3-23, and H-19/H3-24 (Figure 2b), indicated the relative configurations at C-2 and C-21, which corresponds to 8, but not 9. The CD spectrum of 1 showed the negative Cotton effects around 225 and 320 nm (Figure 2c), which were diagnostic for the dioxopiperazine amide bonds and the stereogenic center of the indoxyl core, respectively,8 and was superimposable on that of (+)-8.8 The absolute configuration of 1 appears to be consistent with that of (+)-8. HPLC analysis with a chiral-phase column (CHIRAL CELL OJ-H (4.6 × 250 mm), 93% n-hexane-2-PrOH) of 1 showed that 1 was optically pure. In addition, due to the presence of the indoxyl chromophore, 1 appears as a brilliant yellow color, characteristic of the indoxyl chromophore and both Brevianamides A (8) and B (9) exhibit this color.
Table 1.
1H and 13C NMR data for 1 in DMSO-d6.
| No. | δH, mult (J in Hz) | δC, type | HMBC |
|---|---|---|---|
| 1 | 7.73, s | 2, 3, 8, 9 | |
| 2 | 79.7, C | ||
| 3 | 197.0, C | ||
| 4 | 7.18, d (8.4) | 124.9, CH | 3, 6, 8 |
| 5 | 6.11, d (8.4) | 107.6, CH | 6, 7, 9 |
| 6 | 159.9, C | ||
| 7 | 102.7, C | ||
| 8 | 157.0, C | ||
| 9 | 113.7, C | ||
| 10 | 2.39, d (15.5) | 35.6, CH2 | 2, 3, 11, 12 |
| 2.44, d (15.5) | 2, 11, 22 | ||
| 11 | 66.6, C | ||
| 12 | 169.7, C | ||
| 14 | 3.25, m | 43.4, CH2 | |
| 3.30, m | |||
| 15 | 1.78, m | 24.7, CH2 | 17 |
| 1.95, m | 17 | ||
| 16 | 1.80, m | 28.5, CH2 | 15, 18 |
| 2.47, m | 15, 18 | ||
| 17 | 68.7, C | ||
| 18 | 172.3, C | ||
| 19 | 8.67, s | 11, 17 | |
| 20 | 1.66, dd (13.4, 5.8) | 27.3, CH2 | |
| 1.84, dd (13.4, 9.2) | 17 | ||
| 21 | 2.71, dd (9.2, 5.8) | 52.6, CH | 12, 20, 22 |
| 22 | 48.3, C | ||
| 23 | 0.67, s | 21.5, CH3 | 2, 21, 22, 24 |
| 24 | 0.95, s | 18.1, CH3 | 2, 21, 22, 23 |
| 25 | 6.77, d (10.0) | 116.4, CH | 6, 8, 27 |
| 26 | 5.66, d (10.0) | 127.3, CH | 7, 27 |
| 27 | 77.2, C | ||
| 28 | 1.36, s | 27.9, CH3 | 26, 27, 29 |
| 29 | 1.40, s | 27.3, CH3 | 26, 27, 28 |
Fig. 2.
Key HMBCs (a), NOEs (b), and ECD spectrum (c) of 1.
Fig. 3.
Structures of 8–14.
During the structure elucidation of 1, we noticed that the planar structure of 1 was identical to that of Speramide A (3) (Fig. 1), which was isolated from A. ochraceus KM007 by Hao.9 However, the chemical shifts (δC 113.7 (C-7), 134.7 (C-9), and 147.6 (C-8)) of the indoxyl moiety in 3 exhibited significant differences from those of 1 (δC 102.7 (C-7), 113.7 (C-9), and 157.0 (C-8)). Further, two four-bond HMBC correlations from H3-23 and H3-24 to C-3 reported by Hao9 are apparently inappropriate for the proposed structure of 3. Although 3 is an indoxyl derivative, Hao compared the CD spectrum with that of a oxindole derivative (–)-6,1 and these spectra were significantly different. These data clearly indicate that the proposed structure of 3 was incorrect. We analyzed the reported spectra in the literature9 and found that the correct structure should be 4, which is identical to that of Taichunamide H, recently isolated from A. versicolor HDN11–84.10
Asperochramide A (2) has the 3R-configuration (Fig. 1), although most prenylated indole alkaloids produced by fungi of the genera Aspergillus and Penicillium have the 3S-configuration (e.g. Notoamide C (Scheme 1)). Compound 2 is the third example with the 3R-configuration which includes Notoamide J (10),11 isolated from A. protuberus MF297–2, and 3-epi-Notoamide C (11),2a obtained by the feeding experiment of Notoamide E (12) (Figure 3) in the strain.
Scheme 1.
Proposed formation of 1 and 2 in A. amoenus.
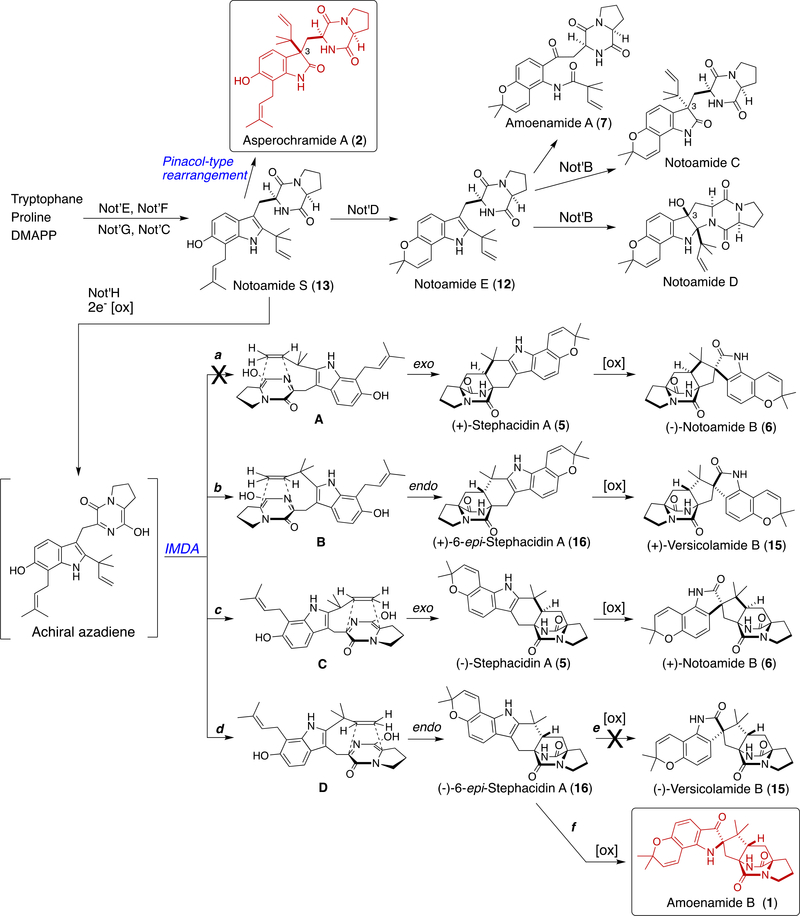
Amoenamide B (1) was discovered as the fourth compound containing an indoxyl structure with a quaternary center at C-2 among the family of prenylated indole alkaloids after the isolation of (+)-8, (+)-9, and austamide (14)12 (Figure 3) from A. ustus. In A. amoenus, (+)-Versicolamide B (15) and (+)-6 (Scheme 1) are the major and minor metabolites3 and are likely formed from Notoamide S (13) by the intra-molecular Diels– Alder (IMDA) reaction through intermediates B and C, respectively. Recently, we reported the isolation of an enantiomeric mixture of 16 enriched with the (–)-isomer together with (+)-15 from A. amoenus.2j This result strongly suggests that the fungus possesses the indole oxidase, which converts (+)-16 into (+)-15, but not for (–)-15. These experimental observations require further insight into the substrate specificity of the indole oxidases present in these fungi. We previously speculated that (–)-16 is a minor shunt metabolite in A. amoenus, but the isolation of 1 in this study clearly suggests that this fungus may possess the indoxyl oxidase (pathway f), which converts (–)-16 to 1, instead of the indole oxidase (pathway e) for (–)-15. Possibly, 1 is converted from (–)-16 through the oxidized intermediate 17 produced by α-face oxidation (Scheme 2). On the other hand, (–)-15 could be formed through intermediate 18 by β-face oxidation. Interestingly, the product afforded by a distinct pinacol rearrangement through 17 corresponds to (+)-Taichunamide E (19), whose (–)-antipode was isolated from A. taichungensis IBT 19404.13
Scheme 2.
Possible pathways of 1 and (+)-19 through 17 and (−)-15 through 18 from 16.
Biosynthetically, 2 may be formed from Notoamide S (13) by the Pinacol-type rearrangement (Scheme 1). IMDA reaction of the achiral azadiene derived from the oxidation of 13 affords the observed natural metabolites through intermediates A–D. Although we isolated both enantiomers of 5, 16, and 6 along with a single enantiomer of (+)-15 from three fungi, A. protuberus MF297–2,1 A. amoenus NRRL 35600,2j and A. taichungensis IBT 19404,13 only (–)-15 has yet to be isolated from any fungi. Biochemical investigations to address these subtle stereochemical anomalies are under intensive study in our laboratories.
Highlights.
A new prenylated alkaloid, amoenamide B, was isolated from Aspergillus amoenus.
Amoenamide B is the fourth compound with the indoxyl unit containing the cores.
Implication of the possible biosynthetic pathway of amoenamide B was described.
We corrected the structure of Speramide A, recently isolated from A. ochraceus.
Acknowledgments
This work was financially supported in part by JSPS KAKENHI Grant Numbers JP25108719 (S.T.) and JP24710252 (H.K.) of Japan and by the Yamada Science Foundation (S.T.). Financial support from the National Institutes of Health (Grant CA 070375 to RMW and DHS) is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Kato H, Yoshida T, Tokue T, Nojiri Y, Hirota H, Ohta T, Williams RM, Tsukamoto S. Angew Chem Int Ed. 2007;46:2254–2256. [DOI] [PubMed] [Google Scholar]
- 2.(a) Tsukamoto S, Kato H, Greshock TJ, Hirota H, Ohta T, Williams RM. J Am Chem Soc. 2000;131:3834–3835. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ding Y, de Wet JR, Cavalcoli J, Li S, Greshock TJ, Miller KA, Finefield JM, Sunderhaus JD, McAfoos TJ, Tsukamoto S, Williams RM, Sherman DH. J Am Chem Soc. 2010;132:12733–12740. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Finefield JM, Greshock TJ, Sherman DH, Tsukamoto S, Williams RM. Tetrahedron Lett. 2011, 52, 1987–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Finefield JM, Sherman DH, Tsukamoto S, Williams RM. J Org Chem. 2011;76:5954–5958. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Finefield JM, Kato H, Greshock TJ, Sherman DH, Tsukamoto S, Williams RM. Org Lett. 2011;13:3802–3805. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Kato H, Nakamura Y, Finefield JM, Umaoka H, Nakahara T, Williams RM, Tsukamoto S. Tetrahedron Lett. 2011;52:6923–6926. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Li S, Srinivasan K, Tran H, Yu F, Finefield JM, Sunderhaus JD, McAfoos TJ, Tsukamoto S, Williams RM, Sherman DH. MedChemComm 2012;3:987–996. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Sunderhaus JD, McAfoos TJ, Finefield JM, Kato H, Li S, Tsukamoto S, Sherman DH, Williams RM. Org Lett. 2013;15:22–25. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Kato H, Nakahara T, Yamaguchi M, Kagiyama I, Finefield JM, Sunderhaus JD, Sherman DH, Williams RM, Tsukamoto S. Tetrahedron Lett. 2015;56:247–251. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Kato H, Nakahara T, Sugimoto K, Matsuo K, Kagiyama I, Frisvad JC, Sherman DH, Williams RM, Tsukamoto S. Org Lett. 2015;17:700–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greshock TJ, Grubbs AW, Jiao P, Wicklow DT, Gloer JB, Williams RM. Angew Chem Int Ed. 2008;47:3573–3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sugimoto K, Sadahiro Y, Kagiyama I, Kato H, Sherman DH, Williams RM, Tsukamoto S. Tetrahedron Lett. 2017;58:2797–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wen H, Liu X, Zhang Q, Deng Y, Zang Y, Wang J, Liu J, Zhou Q, Hu L, Zhu H, Chen C, Zhang Y. Chem Biodivers. 2018;15:e1700550. [DOI] [PubMed] [Google Scholar]
- 6.The fungus, A. amoaenus NRRL 35600, which was obtained from the basidioma of Ganoderma australe collected in a Hawaiian forest. The fungus was cultured on rice media (100 g × 200) at 25 °C for a month. The culture was extracted with n-BuOH and the concentrated aqueous solution was extracted with n-BuOH. The n-BuOH extract was partitioned between n-hexane and 90% MeOH/H2O. The 90% MeOH/H2O fraction (202 g) was subjected to SiO2 chromatography with 5% MeOH/CH2Cl2 to yield a fraction (6.9 g) containing the prenylated indole alkaloids. The fraction was further purified by ODS chromatography with 75% MeOH/H2O and then SiO2 chromatography with EtOAc followed by HPLC (Develosil C30-UG-5, 45% CH3CN/H2O) to afford 1 (0.76 mg) and 2 (0.63 mg). Amoenamide B (1): [α]D21 +64° (c 0.64, MeOH); UV (MeOH) λmax (log ε) 228 (5.14), 264 (5.03), 320 (4.39), 334 (4.32) nm; ECD (200 μM, MeOH) λmax (Δε) 323 (−1.4), 257 (−0.96), 240 (3.2), 225 (−6.4), 205 (3.4) nm; IR (film) νmax 3320, 2925, 2854, 1670, 1601, 1439, 1401, 1312, 1114 cm−1; 1H and 13C NMR data (DMSO-d6), see Table 1.; HRESIMS m/z 448.2231 [M + H]+ (calcd for C26H30N3O4, 448.2231).
- 7.(a) Birch AJ, Wright JJ. J Chem Soc Chem Commun. 1969, 644–645. [Google Scholar]; (b) Birch AJ, Wright JJ. Tetrahedron 1970;26:2329–2344. [DOI] [PubMed] [Google Scholar]; (c) Birch AJ, Russell RA. Tetrahedron 1972;28:2999–3008. [Google Scholar]
- 8.Williams RM, Kwast E, Coffman H, Glinka T. J Am Chem Soc. 1989;111:3064–3065. [Google Scholar]
- 9.Chang Y-W, Yuan C-M, Zhang J, Liu S, Cao P, Hua H-M, Di Y-T, Hao X-J. Tetrahedron Lett. 2016;57:4952–4955. [Google Scholar]
- 10.Li F, Zhang Z, Zhang G, Che Q, Zhu T, Gu Q, Li D. Org Lett. 2018;20:1138–1141. [DOI] [PubMed] [Google Scholar]
- 11.(a) Tsukamoto S, Kato H, Samizo M, Nojiri Y, Onuki H, Hirota H, Ohta T. J Nat Prod. 2008;71:2064–2067. [DOI] [PubMed] [Google Scholar]; (b) Tsukamoto S, Kato H, Samizo M, Nojiri Y, Onuki H, Hirota H, Ohta T. J Nat Prod. 2013;76:1233–1233. [DOI] [PubMed] [Google Scholar]
- 12.Steyn PS. Tetrahedron Lett. 1971;12:3331–3334. [Google Scholar]
- 13.Kagiyama I, Kato H, Nehira T, Frisvad JC, Sherman DH, Williams RM, Tsukamoto S. Angew Chem Int Ed. 2016;55:1128–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]