Watch a video presentation of this article
Abbreviations
- ASK1
apoptosis signal‐regulating kinase 1
- BA
bile acid
- CAD
coronary artery disease
- CCR
chemokine (C‐C motif) receptor
- CKD
chronic kidney disease
- CVC
cenicriviroc
- EV
esophageal varices
- FXR
farnesoid X receptor
- HbA1C
“hemoglobin A1c”
- HCC
hepatocellular carcinoma
- HSC
hepatic stellate cell
- KC
Kupffer cell
- NAFL
nonalcoholic fatty liver
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- OCA
obeticholic acid
- PPAR
peroxisome proliferator‐activated receptor
- RCT
randomized controlled trial
- SEL
selonsertib
- T2DM
type 2 diabetes
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of liver disease in Western countries, affecting approximately 20% to 30% of individuals in the United States.1, 2 NAFLD has a histological spectrum that ranges from the relatively benign nonalcoholic fatty liver (NAFL), to the aggressive form of nonalcoholic steatohepatitis (NASH), to NASH with advanced fibrosis/cirrhosis leading to end‐stage liver disease.2, 3
Because there is no US Food and Drug Administration (FDA)–approved treatment for NAFLD, lifestyle modifications are recommended for patients. In a prospective study on the effects of weight loss on NAFLD, weight loss ≥10% led to a regression of fibrosis in 45% of patients and resolution of NASH in 90% of patients.4 However, in that study only 10% of patients were able to lose ≥10% weight, indicating the urgent need to develop new pharmacological treatments beyond lifestyle modifications.
The recent increased understanding of the disease pathogenesis has led to the development of numerous medical therapies for NAFLD that target various disease pathways. This review will discuss four medications that are in phase III randomized controlled trials (RCTs): elafibranor, obeticholic acid (OCA), cenicriviroc (CVC), and selonsertib (SEL). It is important to note that the histological endpoints for each phase III study are slightly different, which preclude head‐to‐head comparison of the results. Finally, we will attempt to provide the readers with a new way of thinking that compares the NAFLD spectrum with that of type 2 diabetes (T2D) to risk‐stratify patients with NAFLD and decide on the appropriate treatment course.
Peroxisome Proliferator‐Activated Receptor Agonist
Peroxisome proliferator‐activated receptors (PPARs) are ligand‐activated transcription factors that regulate metabolic processes.2, 5 PPARα is ubiquitously expressed5 and regulates lipid metabolism and energy homeostasis in multiple organs.2 PPARδ is expressed in metabolically active tissues including the liver, where it plays a role in shifting hepatic metabolism toward lipid oxidation.2, 5
Elafibranor is a dual PPARα/δ agonist that improves glucose homeostasis, increases insulin metabolism, and reduces inflammation. A phase IIb RCT (NCT01694849/GOLDEN‐505) assessed the effects of elafibranor (120 mg/day, 80 mg/day, or placebo) for 52 weeks.6 The primary endpoint was the reversal of NASH without worsening fibrosis; however, the results were not statistically significant. After post hoc analyses with a modified definition of the primary outcome, a larger proportion of patients in the elafibranor (120 mg/day) group saw a resolution of NASH compared with those in the placebo group (19% versus 12%, P = 0.045). The updated definition of NASH resolution emphasized hepatocyte ballooning and defined worsening of fibrosis as any one‐stage increase.
A phase III RCT (NCT02704403/RESOLVE‐IT) is enrolling patients to evaluate the effects of elafibranor (120 mg/day or placebo) on histological improvement defined as resolution of NASH without worsening of fibrosis at 72 weeks with longer follow‐up to assess its effects on liver‐related morbidity and mortality.
Farnesoid X Receptor Agonist
Farnesoid X receptors (FXRs) are nuclear receptor transcription factors, expressed in the liver, that regulate insulin sensitivity and participate in lipid metabolism.7 Bile acids (BAs), natural ligands of the FXRs,7 are synthesized in the liver and promote insulin sensitivity and decrease gluconeogenesis and circulating triglycerides when bound to FXRs.8
OCA (6‐ethylchenodeoxycholic acid) is a synthetic BA and an FXR activator.9 A phase IIb RCT (NCT01265498/FLINT) evaluated OCA (25 mg/day or placebo) for 72 weeks for the treatment of histologically proven NASH. The primary outcome was improvement in liver histology without worsening fibrosis.9 Histological improvement was achieved in 45% of patients in the OCA group compared with 21% of the placebo group (P = 0.0002); improvement in fibrosis was seen in 35% of the OCA group compared with 19% of the placebo (P = 0.004). However, resolution of NASH did not differ in the OCA group (22%) and the placebo group (13%) (P = 0.08).9
A phase III RCT (NCT02548351/REGENERATE) is currently enrolling patients with biopsy‐proven NASH to evaluate the effect of OCA (10 mg/day, 25 mg/day, or placebo) for 72 weeks on liver histology. Patients will also be followed for 6 years to assess hard outcomes such as progression to cirrhosis, need for liver transplantation, and death.
Chemokine (C‐C Motif) Receptor Type 2/5 Antagonist
The inflammatory response to hepatocyte injury results in hepatic fibrogenesis. This response activates Kupffer cells (KCs) and hepatic stellate cells (HSCs) in addition to recruiting macrophages and monocytes. KCs, monocytes, and HSCs all express chemokine (C‐C motif) receptor types 2 (CCR2) and 5 (CCR5), which promote the inflammatory response in hepatic injury.10
CVC, a dual antagonist of CCR2 and CCR5, demonstrated antifibrotic effects in preclinical models. A phase IIb RCT (NCT02217475/CENTAUR) is currently evaluating the effects of CVC (150 mg/day or placebo) on the treatment of NASH with liver fibrosis. The primary endpoint is histological improvement without worsening fibrosis.11 After 1 year of treatment, the difference in histological improvement was not statistically significant between the CVC group and placebo group, 16% versus 19%, respectively (P = 0.52). When analyzing one of the secondary endpoints—improvement in fibrosis by at least one stage—individually, more of the CVC group (20%) achieved improvement in fibrosis compared with the placebo group (10%) (P = 0.02).11
A phase III RCT is enrolling patients with NASH to assess the effects of CVC (150 mg/day or placebo) on liver fibrosis (NCT03028740/AURORA).
Apoptosis Signal‐Regulating Kinase 1 Inhibitor
Apoptosis signal‐regulating kinase 1 (ASK1) is a member of the mitogen‐activated protein kinase kinase kinase family that plays a role in stress responses.12 Activation of ASK1 by oxidative stress leads to hepatic inflammation, hepatocyte apoptosis, and fibrosis.2
SEL (GS‐4997) is a selective inhibitor of ASK1. A phase II RCT (NCT02466516) was conducted to evaluate the effects of SEL (6 or 18 mg/day) alone or in combination with simtuzumab (125 mg/week) in patients with NASH and fibrosis for 24 weeks. Simtuzumab, a humanized monoclonal antibody, has been indicated as ineffective in fibrosis treatment and was considered a placebo.13 Patients treated with SEL had more fibrosis improvement; 43% of the 18 mg SEL group and 30% of the 6 mg SEL group improved compared with 20% of the simtuzumab (placebo) group alone.
Two phase III studies are currently enrolling patients with NASH and bridging fibrosis (NCT03053050/STELLAR‐3) and compensated cirrhosis (NCT03053063/STELLAR‐4) to evaluate the effects of SEL (6 mg/day, 18 mg/day, or placebo) with a planned interim analysis at 48 weeks to assess histological improvement.
Redefining the NAFLD Spectrum: NAFLD Is the New Type 2 Diabetes
Even with the development of effective FDA‐approved therapies for NAFLD, several issues may delay their routine use in clinical practice. The main issue is that NAFLD is very common, and the majority of patients have the relatively less progressive form, NAFL. This leads many primary care physicians to believe that NAFLD is not a serious illness that requires treatment. Identifying patients with NASH/advanced fibrosis still requires a liver biopsy, which is not a feasible option for a disease that affects one‐third of the general population in the United States.
We believe that the future management of the NAFLD spectrum will evolve in a similar pattern to the current management of the T2DM spectrum (Fig. 1). We would like to compare NAFL with prediabetes, which is not a disease per se, but a risk factor for development of T2DM. The mainstay for the management of both NAFL and prediabetes is lifestyle modifications. NASH is considered the driving force behind the development of liver fibrosis and eventually cirrhosis; therefore, we consider NASH a serious illness that warrants aggressive medical management with different pharmacological agents similar to what is required for patients with T2DM. Finally, the development of NASH cirrhosis with portal hypertension complications indicates end organ damage to the liver. This is similar to the development of insulin‐dependent diabetes and its macrovascular and microvascular complications, and requires the most aggressive approach to treatment.
Figure 1.
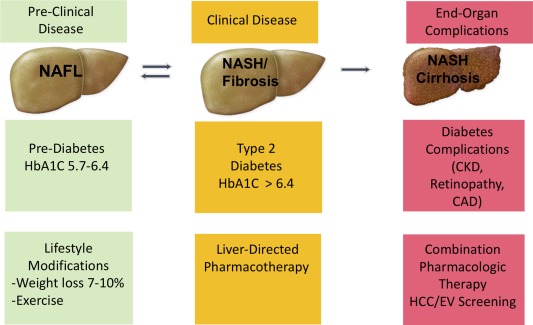
Similarities in disease spectrum and management between NAFLD and T2DM. Patients with NAFL should be treated with lifestyle modifications similar to the current standard of care in patients with prediabetes. Patients with NASH have serious potential for disease progression to cirrhosis and its complications and should be considered for pharmacological treatments when available. Finally, patients with NASH cirrhosis require the most aggressive treatment approach with possibly combination therapies and monitoring for the development of hepatocellular carcinoma (HCC) and esophageal varices (EV). Abbreviations: CAD, coronary artery disease; CKD, chronic kidney disease.
New imaging technologies now allow point‐of‐care diagnosis of NAFLD and staging of liver fibrosis, which most likely will become the hepatologist's new “hemoglobin A1c” (HbA1C) to risk‐stratify patients into different NAFLD severity categories.
Conclusion
As the prevalence and clinical burden of NAFLD increases, the need for an FDA‐approved treatment intensifies. Currently, dozens of medications are in clinical trials to identify the most effective treatment. Therapies vary in their mechanism of action, focusing on metabolic targets, anti‐inflammatory effects, or antifibrotic effects. Elafibranor, OCA, CVC, and SEL are four of the drugs undergoing phase III RCTs (summarized in Table 1). Continued research efforts ensure that treatment options will become available soon.
Table 1.
Medications Undergoing Phase III Clinical Trials for the Treatment of NAFLD
| Phase II Efficacy Data | ||||||
|---|---|---|---|---|---|---|
| Medication | Mechanism | Resolution of NASH | Decrease in Fibrosis Stage | Phase III RCT | Effective Dosage | Planned Interim Analysis Duration |
| Elafibranor | PPARα/δ agonist | Yes | No | RESOLVE‐IT | 120 mg/day | 72 weeks |
| OCA | FXR agonist | No | Yes | REGENERATE | 10‐25 mg/day | 72 weeks |
| CVC | CCR2/CCR5 antagonist | No | Yes | AURORA | 150 mg/day | 52weeks |
| SEL | ASK1 inhibitor | No | Yesa | STELLAR 3 and 4 | 6 and 18 mg/day | 48 weeks |
Numerically higher rates of fibrosis improvement that did not reach statistical significance. This was a proof‐of‐concept study that was not powered to detect histological changes in fibrosis stage.
Potential conflict of interest: N.A. received research funding from Intercept Pharmaceuticals, Allergan, and Gilead Sciences. He is on the speaker bureau for Intercept Pharmaceuticals and Gilead Sciences.
REFERENCES
- 1. Townsend SA, Newsome PN. Review article: new treatments in non‐alcoholic fatty liver disease. Aliment Pharmacol Ther 2017;46:494‐507. [DOI] [PubMed] [Google Scholar]
- 2. Rotman Y, Sanyal AJ. Current and upcoming pharmacotherapy for non‐alcoholic fatty liver disease. Gut 2017;66:180‐190. [DOI] [PubMed] [Google Scholar]
- 3. Alkhouri N, Feldstein AE. Noninvasive diagnosis of nonalcoholic fatty liver disease: are we there yet? Metabolism 2016;65:1087‐1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vilar‐Gomez E, Martinez‐Perez Y, Calzadilla‐Bertot L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 149:367‐378.e5. [DOI] [PubMed] [Google Scholar]
- 5. Grygiel‐Górniak B. Peroxisome proliferator‐activated receptors and their ligands: nutritional and clinical implications – a review. Nutr J 2014;13:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ratziu V, Harrison SA, Francque S, et al. Elafibranor, an agonist of the peroxisome proliferator‐activated receptor‐alpha and ‐delta, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology 2016;150:1147‐1159.e5. [DOI] [PubMed] [Google Scholar]
- 7. Ali AH, Carey EJ, Lindor KD. Recent advances in the development of farnesoid X receptor agonists. Ann Transl Med 2015;3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carr RM, Reid AE. FXR agonists as therapeutic agents for non‐alcoholic fatty liver disease. Curr Atheroscler Rep 2015;17:500. [DOI] [PubMed] [Google Scholar]
- 9. Neuschwander‐Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non‐cirrhotic, non‐alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo‐controlled trial. Lancet 2015;385:956‐965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lefebvre E, Moyle G, Reshef R, et al. Antifibrotic effects of the dual CCR2/CCR5 antagonist cenicriviroc in animal models of liver and kidney fibrosis. PLoS ONE 2016;11:e0158156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Friedman SL, Ratziu V, Harrison SA, et al. A randomized, placebo‐controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology; doi: 10.1002/hep.29477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hayakawa R, Hayakawa T, Takeda K, et al. Therapeutic targets in the ASK1‐dependent stress signaling pathways. Proc Jpn Acad Ser B Phys Biol Sci 2012;88:434‐453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Loomba R, Lawitz E, Mantry PS, et al. The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: a randomized, phase 2 trial. Hepatology 2018;67:549‐559. [DOI] [PMC free article] [PubMed] [Google Scholar]


