Abstract
Cellular proteostasis is a highly dynamic process and is primarily carried out by the degradation tools of ubiquitin-proteasome system (UPS). Abnormalities in UPS function result in the accumulation of damaged or misfolded proteins which can form intra- and extracellular aggregated proteinaceous deposits leading to cellular dysfunction and/or death. Deposition of abnormal protein aggregates and the cellular inability to clear them have been implicated in the pathogenesis of a number of neurodegenerative disorders such as Alzheimer’s and Parkinson’s. Contrary to the upregulation of proteasome function in oncogenesis and the use of proteasome inhibition as a therapeutic strategy, activation of proteasome function would serve therapeutic objectives of treatment of neurodegenerative diseases. This review describes the current understanding of the role of the proteasome in neurodegenerative disorders and potential utility of proteasomal modulation therein.
Keywords: Ubiquitin-proteasome system, Neurodegenerative disorders, Proteasome modulators, Brain pathologies, Proteostasis
Introduction
The Ubiquitin-proteasome system (UPS) is the key intracellular molecular machinery for protein degradation and maintenance of protein homeostasis in eukaryotic cells. Although originally dismissed as a “garbage disposal” system, in the last two decades, UPS has been recognized as a central player in the regulation of essential cellular functions including cell cycle, cell differentiation, antigen processing, stress signaling, inflammatory responses, and apoptosis. UPS also exhibits important functions in normal brain development by controlling cell fate and specification [1]. Apparently, the presence of functional UPS components in both pre- and postsynaptic compartments of neurons is required for their proper function [2]. Alterations in UPS activity have been shown to induce pathological changes and abnormal brain function. Many brain pathologies, especially neurodegenerative diseases, are characterized by the accumulation of toxic levels of protein aggregates which challenges the proteostatic mechanisms to the point of collapse. Modulating UPS mechanisms has therefore emerged as a promising adjunct treatment strategy for diverse brain pathologies including brain cancer, neurodegeneration, brain-associated autoimmune disorders and inherited brain disorders associated with protein misfolding and toxic gain of functions [3–5]. During the last two decades many mechanisms that regulate the UPS have been unraveled, and depending on the disorder in question, both proteasome inhibition and activation present significant potential in pharmacotherapeutic development. As the general nature of UPS [6–8] and its participation in aging and cancer [9–13] have been widely reviewed in the published literature, this short review primarily covers the role of proteasome and investigational use of proteasome modulation in the therapy of neurodegenerative disorders.
The Ubiquitin-Proteasome System (UPS)
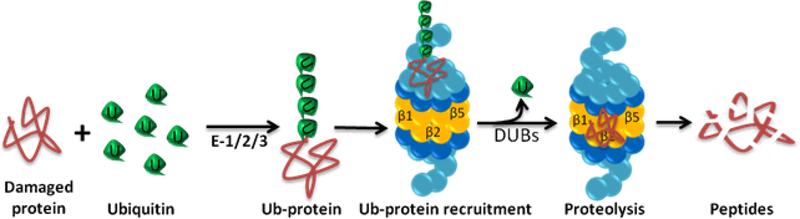
UPS is the primary degradation system in eukaryotic cells, which mediates the degradation of short-lived regulatory proteins and the removal of damaged soluble proteins [3]. It involves the implementation of two sequential steps: ubiquitination and proteolytic degradation of ubiquitinated proteins (Figure 1). The ubiquitination step covalently attaches an ubiquitin chain to the lysine residues in substrate proteins, which serves as a recognition signal for further processing of proteins by proteasome. The formation of an isopeptide bond between the ε-amino group of lysine residues and the carboxyl group of the C-terminal glycine of ubiquitin is an ATP-dependent process. It is achieved via a cascade involving three distinct classes of enzymes: ubiquitin-activating enzymes (E1), ubiquitin-conjugating enzymes (E2) and ubiquitin ligases (E3) [14]. An E1 enzyme mediates the ATP-dependent activation of ubiquitin. The activated ubiquitin is translocated to an E2-conjugating enzyme, followed by E3 ligase-mediated facilitation of ubiquitin transfer to a specific lysine residue to the target substrate [15]. In this process, the substrate specificity is achieved through the availability of a large number of different E3s that interact with specific target substrates [16]. To date, at least 35 E2s and over 600 E3s have been discovered to exist in mammalian cells, which suggests a system with a high degree of substrate specificity [17]. When a proper ubiquitin chain consisting of at least four ubiquitin moieties is assembled on a substrate protein, it becomes a subject for the proteasome wherein it is degraded into short peptides and amino acids which are recycled for new protein synthesis.
Figure 1: Ubiquitin-proteasome system (UPS).
UPS involves ubiquitination and proteolytic degradation of ubiquitinated proteins. Ubiquitin is first attached to the target protein via a cascade involving three distinct enzymes: ubiquitin-activating enzymes (E1), ubiquitin-conjugating enzymes (E2), and ubiquitin ligases (E3). The ubiquitinated substrate is recognized, unfolded, and deubiquitinated by the 19S regulatory particle. The unfolded protein enters the 20S catalytic particle where it is degraded by the β1 (trypsin-like activity), β2 (caspase-like activity) and β5 (chymotrypsin-like activity) subunits.
The Proteasome Assemblies
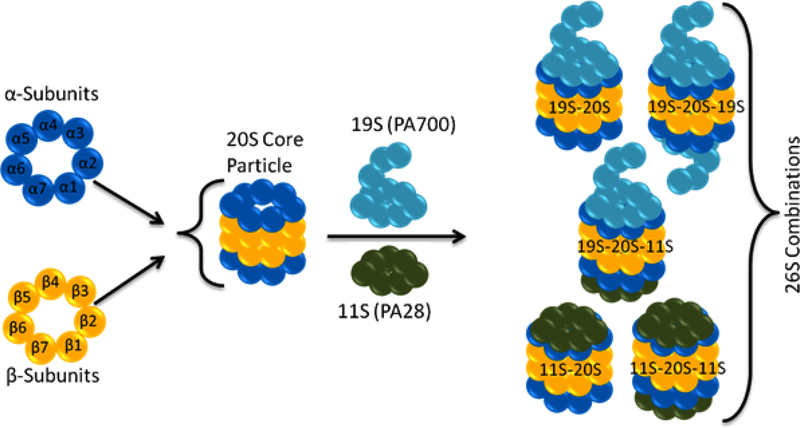
In its constitutive presentation, the proteasome is a mono-capped or bi-capped cylindrical structure housing the proteolytic active sites. The cylindrical core (also known as core particle 20S or CP) is formed by two different types of protein subunits, α and β, which are arranged in four stacked heptameric rings enclosing a central cavity. In eukaryotes, seven distinct α subunits are located in the two outer rings of the barrel, and seven distinct β subunits form the two inner rings [18]. The β subunits face the interior cavity of the cylinder and house the active sites for proteolytic activity: β1 cleaves after acidic residues (caspase-like activity), β2 cleaves after basic residues (trypsin-like activity), and β5 cleaves after hydrophobic residues (chymotrypsin-like activity).Access to the active sites is regulated by a consisting of N-terminal protrusions of the subunits [19,20]
The default status of the CP gate is closed. Thus, for substrate access and proteasomal degradation to occur, the N-termini need to be displaced from their axial position to reveal a continuous channel leading into the catalytic cavity. Modulation of the gate is a prerequisite for substrate entry into the proteolytic chamber and is mediated by proteasome activators. Several endogenous modulators have been described, including the regulatory particle (RP/19S/PA700), activator of the PA28 protein family (11S) and Blm10/PA200 activator [21]. As shown in Figure 2, these regulator assemblies cap the two ends of CP and modify the function of constitutively active 20S CP.
Figure 2: Various assemblies of proteasome.
The proteasome is a mono-capped or bi-capped cylindrical structure. The cylindrical core (20S or CP) is formed by two different types of protein subunits, α and β, which are arranged in four stacked heptameric rings enclosing a central cavity. The proteasome core particle can be capped with 19S or 11S activator complexes (a third activator complex Blm10/PA200 is not shown).
The dominant partner of 20S CP in the assembly of 26S proteasome is RP/19S/PA700, which can connect to one or both ends of the core by binding to the terminal α-rings of the 20S cylinder. It is composed of 19 integral subunits that form two biochemically separable sub-complexes, the lid and base [22]. The base sub-complex is situated proximal to the CP gate region. It contains six homologous ATPases (Regulatory particle triphosphatase proteins (Rpt) 1–6), which form a hexameric ring. They belong to the family of ATPases associated with diverse cellular activities (AAA). The lid, on the other hand, consists of nine non-ATPase subunits [3]. When the 20S core is bound by two 19S modules (19S–20S–19S proteasome complex), the assembly is categorized as the classic 26S proteasome. The 19S ATP-dependent proteasome activator (PA700) recognizes the ubiquitinated protein substrates for deubiquitination, unfolding, and threading them into the catalytic chamber of the proteasome in an ATP- and ubiquitin-dependent manner [23].
Like the 19S regulator, the 11S regulator (PA28 complex) also activates the 20S proteasome by binding to the α-rings of the 20S proteasome, but its function is ATP-independent [24]. PA28 responds to stress by increased expression [25]. It is expressed as PA28α, PA28β, and PA28γ isoforms, the exact functions of which are not clearly understood [25]. PA28α and PA28β have been shown to form hetero-heptameric rings in cytosol [26,27], while PA28γ forms homo-heptameric rings and is found in the nuclei of vertebrates as well as invertebrates [26,27].
Both PA28α and PA28β units are inducible by interferon-γ, which suggests a potential role of PA28α/β in major histocompatibility complex (MHC) Class I-mediated antigen presentation [28]. They are also expressed in organs involved in non-immune functions. In eukaryotic cells, PA28α/β can generate hybrid 26S proteasomes with enhanced proteolytic efficiency [29]. It can also facilitate heat shock protein (HSP) 90-mediated protein refolding [30]. The role of PA28γ is not entirely clear, but the mice deficient in PA28γ exhibit reduced body-size, and embryonic fibroblasts derived from these mice shows cell cycle defects [31]. It is thought that 11S–20S–11S complex can only degrade simple unstructured proteins, whereas 19S–20S–11S and 19S–20S–19S complexes can hydrolyze large complex proteins [29].
The Blm10/PA200 family can also form pure or hybrid complexes in which Blm10/PA200 binds to one end and the 19S to the other end of the CP cylinder [32,33]. It is conserved from yeast to humans and is populated by monomeric proteins of ~ 250 kDa. Blm10 binds to the proteasome during the late phases of CP assembly and contributes to the final maturation of CP complexes [34,35]. One physiological target for Blm10–proteasome complexes is the transcription factor split finger protein 1 (Sfp1), which regulates ribosomal protein genes [36]. Additional studies suggest a potential role for Blm10 in mitochondrial homeostasis [37,38]. Furthermore, it may be an important participant in DNA or oxidative damage repair processes and chromosome stability [32,39,40], most likely through ATP- and ubiquitin-independent degradation of acetylated histones in somatic cells [41].
Immunoproteasome and thymoproteasome
In addition to the constitutive proteasome assemblies discussed above, adaptive processes can induce the cells to express alternative proteasomal phenotypes. The immunoproteasome and thymoproteasome are the two known alternative proteasome forms. Their expression is primarily regulated by the prevailing cytokine environment [42–45]. In the immunoproteasome, β1, β2, and β5 subunits are replaced by LMP2 (low-molecular mass polypeptide 2, also known as β1i), MECL-1 (multicatalytic endopeptidase complex-like-1, β2i), and LMP7 (β5i). LMP7 and MECL-1 immuno-subunits display essentially the same cleavage specificity as their constitutive counterparts, but LMP2 shows more chymotrypsin-like activity than the caspase-like activity of β1 [46]. Recent studies have suggested a role of the immunoproteasome in inflammation [47], tumor development [48], lipid metabolism [49] and NF-κB signaling [50]. Constitutive proteasome and immunoproteasome usually coexist in cells, but the ratio between the two isoforms varies on the basis of cell type and environment [51]. The reports suggest that the triggering factor for the conversion of constitutive proteasome into immunoproteasome is interferon-γ [52–54], and the immunoproteasome is assembled four times faster than the constitutive proteasome, but exhibits greatly reduced stability [55,56]. Unlike the ubiquitous expression of immunoproteasome, thymoproteasome is exclusively present in the thymus [57]. Thymoproteasome contains the β1i and β2i immune-subunits as well as a thymic proteasome subunit β5t (also known as proteasome subunit beta type-11 or PSMB11). The incorporation of β5t results in the reduction of the chymotrypsin-like activity of the proteasome [57], and its expression is essential for positive selection of T cells in the thymus [58].
UPS dysfunction in Neurodegenerative disorders
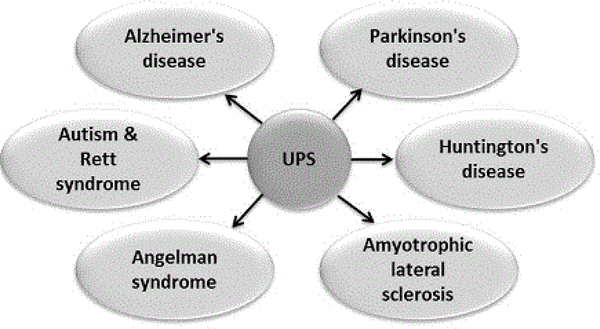
The high metabolic activity in the brain makes intracellular neuronal content particularly vulnerable to oxidative damage. Apart from the recently discovered glymphatic system [59], the brain’s only known method for disposal is to break down and recycle the proteins within individual cells. The glymphatic system is a paravascular transport system that allows for cerebrospinal fluid (CSF) and interstitial fluid (ISF) exchange, facilitating the efficient clearance of solutes and waste from the brain [60]. On the other hand, as the primary proteolytic complex responsible for the elimination of damaged and misfolded intracellular proteins, UPS plays an important role in preventing accumulation of proteinaceous trash in brain cells. Given the importance of housekeeping function delivered by UPS, its potential impact on several neuronal dysfunctions is significant. Both the constitutive and immunoproteasome participate in normal neuronal physiology [61–63], and their aberration is linked to various brain pathologies (Figure 3). Evidence suggests that a unifying characteristic of several neurodegenerative disorders is the inability of cells to dispose of aggregated and misfolded proteins. In the text below, we briefly discuss the identified role of UPS in various neurodegenerative disorders.
Figure 3: UPS dysregulation in neurodegenerative disorders.
Almost all neurodisorders of contemporary interest are characterized by imbalanced UPS function, indicating the possible role of modulators of UPS as adjunct therapy.
Alzheimer’s disease (AD): AD is characterized by dementia and loss of cognitive function, resulting in memory impairment, personality changes, psychosis, and language disturbances [3]. The progressive intellectual decline in AD patients is accompanied by an increase in the deposition of protein aggregates that eventually form intracellular neurofibrillary tangles (NFT) and extracellular senile/amyloid plaques [64]. The chronic neuroinflammation observed in the brain samples from AD patients has been found to be associated with increased immunoproteasome (LMP2) expression [63].
Reports suggest that UPS may be involved in the degradation of amyloid precursor protein (APP) via the endoplasmic reticulum-associated degradation (ERAD) arm of the UPS [65]. Amyloid-β also interacts with the molecular pathways that regulate the phosphorylation of microtubule-associated tau protein which is the second major protein associated with AD plaques [66]. It increases the expression of the regulator for the calcineurin gene (RCAN1), which inhibits tau dephosphorylation by a serine-threonine phosphatase calcineurin [67]. Hyperphosphorylation of tau disrupts its normal function and results in the accumulation of neurofibrillary tangles. The fibrillar tau co-precipitates with the proteasome, and proteasome activity is significantly reduced in AD patients, as compared to age-matched controls [68]. Amyloid-β aggregates have also been shown to block the UPS function [69]. The degradation of tau can be accelerated by proteasome activator Blm10 [37], as well as by inhibiting the proteasome-associated deubiquitinating enzyme Usp14 [70].
Parkinson’s disease (PD): Parkinson’s disease is a chronic progressive neurodegenerative disorder, clinically characterized by resting tremor, rigidity, and bradykinesia, as well as cognitive deficit and autonomic dysfunction [71]. The role of UPS in PD was first revealed by the discovery of E3 ligase activity of parkin and mutation in parkin gene [72,73]. Parkin mutations account for up to 77% of the familial cases with an age of onset <30 yr [74], and for 10%–20% of early-onset PD (EOPD) patients [75]. Parkin, a 53 kDa protein, is normally expressed diffusely in neurons throughout the brain [76], but is absent in the brain of patients with autosomal recessive juvenile parkinsonism [77]. Mutant parkin fails to effectively function as a ligase, resulting in toxic accumulation of its substrates, such as cell division control-related protein (CDCrel-1) and parkin-associated endothelial-like receptor (Pael-R). CDCrel-1 is a septin protein that regulates synaptic vesicle release and is found to be toxic to dopaminergic neurons [78]. Pael-R, on other hand, accumulates in Lewy bodies [79] which are eosinophilic intracytoplasmic inclusions present in dopaminergic neurons of the substantia nigra in the brains of PD patients.
Parkin itself has been found to be a component of Lewy bodies [80], but the major structural protein associated with Lewy bodies is a 14.5 kDa protein called α-synuclein,. Based on the reports that depletion of the proteasome subunit Rpt2 results in accumulation of α-synuclein and the development of Lewy body-like inclusions in mice [81–83], α-synuclein is identified as a substrate for the 26S proteasome. However, α-synuclein monomers could also be degraded by 20S core particle without prior ubiquitination and in the absence of 19S RP [84]. The aggregates of misfolded α-synuclein perpetuate the UPS defect by interacting with the regulatory 19S unit and inhibiting the function of the 26S proteasome [85]. More evidence supporting the role of UPS-mediated α-synuclein degradation in the genesis of PD comes from a recent mice study where proteasomal inhibition in the nigrostriatal pathway by lactacystin resulted in partial dopaminergic cell loss and concurrent striatal dopamine depletion, accompanied by increased expression of Ser129-phosphorylated α-synuclein [86]. It is important to note that α-synuclein is also affected at the genetic level in PD, characterized by mutations, as well as duplication or triplication of the synuclein gene [87,88]. Furthermore, the role of chaperone-mediated autophagy (CMA) and macroautophagy in the degradation of α-synuclein is also critical [89] and two familial mutations (A30P and A53T) of α-synuclein have been found to impair CMA degradation [90]. Another implication of the UPS in PD comes from the observation associating PD with a mis-sense mutation (I93M) in a deubiquitinase enzyme, ubiquitin carboxyl-terminal hydrolase L1 (UCHL1), which decreases its deubiquitinating activity [91,92]. Several other instances of familial PD being associated with genetic defects in UPS have been discussed elsewhere in greater detail [6,93–95].
Huntington’s disease (HD): HD is an autosomal dominant disease, which is characterized by motor dysfunction, cognitive decline, and psychosis. The disease is caused by an expansion of a CAG (cytosine-adenine-guanine) triplet repeat region in huntingtin (Htt) gene through out-of-register recombination between repeat elements. The result is an expansion of a poly-glutamine (poly Q) stretch in the N-terminal domain of the Htt protein [96]. At the structural level, such an expansion (more than 40 glutamines repeats) results in fibril formation and aggregation [97,98].
Although proteasome activity is reduced in HD brains [99], the origin of proteasome dysfunction remains unclear. Studies have shown that proteasomes are sequestered in Htt inclusion bodies, which results in an overall reduction in UPS function [100]. In a striatal cell culture model of HD, the chymotrypsin-like and caspase-like activity were found to be reduced, while the trypsin-like activity was markedly enhanced [101]. These changes in enzyme activities were associated with reduction in the ability to recognize and degrade ubiquitinated substrates [101]. In HD94 conditional mouse model of HD as well as in the post-mortem brain of HD patients, Díaz-Hernández et al observed an induction of immunoproteasome subunits (LMP2 and LMP7) [61]. Despite an incomplete understanding of the role of proteasome in HD, evidence is accumulating to suggest that enhancement of proteasome activity may be beneficial in cells challenged by polyQ-Htt, since upregulation of PA28γ transcription improved cell survival in a cellular HD model [102].
Amyotrophic lateral sclerosis (ALS): ALS is a progressive neurodegenerative disorder affecting motor neurons. Ubiquitinated inclusion bodies are found within the motor-neurons in both familial and sporadic forms of the disease, suggesting that UPS dysfunction is a possible contributor to the genesis of ALS [3]. Accordingly, mice with a conditional knockout of proteasome subunit Rpt3 in motor neurons exhibited ALS-like pathology, particularly the accumulation of protein aggregates with signature components of ALS inclusion bodies, such as the transactive response (TAR )DNA-binding protein 43 (TDP-43) and fused in sarcoma (FUS) RNA-binding protein [103]. Induction of immunoproteasome subunits (LMP2, MECL-1, and LMP7) has been observed in ALS [104], and pyrrolidine dithiocarbamate treatment, which completely blocked the induction of immunoproteasome expression, led to decreased survival in the mutant superoxide dismutase 1 (SOD1-G93A) rat model of ALS [105]. These results suggest that induction of immunoproteasome may help the nervous system to cope with ALS caused by SOD1 mutation.
Disorders associated with mutation or loss of function of proteasomal gene
Angelman syndrome (AS), Rett syndrome (RS), and autism are neurodegenerative disorders, where UPS dysfunction has been implicated. AS is a neurodevelopmental disorder whose main features are intellectual disability, lack of speech, seizures, and a behavioral profile characterized by a happy demeanor, easily provoked laughter, short attention span, hypermotoric behavior, mouthing of objects, sleep disturbance, and an affinity for water [107]. RS is an X-linked dominant disorder predominantly affecting females, which is classified as an autism spectrum disorder (ASD). Clinically, it is characterized by psychomotor regression with loss of volitional hand use and spoken language, the development of repetitive hand stereotypes, and gait impairment. Classical autism, on the other hand, is marked by distinct impaired social interaction. It is suggested that these diseases are associated with loss of function of the ubiquitin protein ligase UBE3A, also known as E6AP ubiquitin-protein ligase (E6AP) [106, 107], and the disease manifestation appears to be associated with the severity of UBE3A loss [108]. For instance, the occurrence of autism has been correlated with significantly dysregulated ubiquitin protein ligase E3A gene in the isodicentric chromosome 15 (Idic15) of autistic subjects [109–111]. The UBE3A gene product, E6-AP, has been shown to function both as an E3 ligase in the ubiquitin proteasome pathway and as a transcriptional co-activator. Thus, induction of UBE3A may provide a therapeutic means to treat autism and similar disorders. The proteasome system also plays a role in controlling mutated neuroligins and cholinesterases in ASD [112,113].
Recently, the potential role of γ -aminobutyric acid (GABAA) receptors in the development of autism has been suggested [114], mostly because of the co-morbid association between autism and epilepsy and GABAergic mechanisms responsible for epilepsy [115,116]. GABAA-mediated neurotransmission is known to play a crucial role in synaptic tuning and neuronal wiring in pre and early postnatal days [117]. In a recent study on postmortem middle frontal gyrus tissues from ASD patients, Crider et al. found a significant decrease in GABAAα1 protein accompanied by an increased expression of synovial apoptosis inhibitor 1 (SYVN1), an endoplasmic reticulum (ER)-associated degradation (ERAD) E3 ubiquitin ligase [118]. In a simulated in vitro cortical neuron culture model, the authors collected evidence of polyubiquitination and proteasomal degradation of GABAAα1, a phenomenon which was inhbited by proteasome inhibitor MG132 and SYVN1 siRNA [118].
UPS as a Target in Neurodegenerative Diseases
The prevailing views on proteasome function have changed radically over the last two decades. It appears that the regulation of proteasomal levels and activity at various steps including its assembly, localization, and function is highly complex, which might serve to fine-tune proteasome function to specific cellular environments and demands. Although we still do not know whether the proteasomal defects in brain pathologies are the primary cause or are secondary to an alternate etiology such as mitochondrial damage, ER stress or oxidative stress [119–125], modulation of proteasome function as a therapeutic strategy in neurodegenerative disorders is beginning to gain momentum. Some of the investigational drugs in this respect are listed in Table 1.
Table 1:
Proteasome modulators in neurodegenerative disorders. Proteasome inhibitors (unshaded rows) have been mostly employed to create models of neurodegenerative diseases, whereas proteasome activators (shaded rows) have been tested for therapeutic application in neurodegeneration.
| Drug | Classification | Target pathology |
|---|---|---|
| PSI (Z-Ile-Glu(OtBu)-Ala-Leu-al) | Reversible β5 inhibitor | PD [132] |
| AD [133] | ||
| Epoxomicin | Irreversible β5=β2> β1 inhibitor | PD [134] |
| HD [135] | ||
| Lactacystin/Clasto-lactacystin β-lactone | Irreversible β5=β2= β1 inhibitor | PD [2] |
| AD [136] | ||
| HD [137], | ||
| ALS [138–141] | ||
| Autism [109, 118] | ||
| MG101 or ALLN (N-acetyl-Leu-Leu-Norleu-al) | Reversible β5 inhibitor | PD [142] |
| AD [143] | ||
| MG115 (Z-Leu-Leu-Nva-al) | Reversible β5 and β1 inhibitor | PD and AD [144] |
| MG132 (Z-Leu-Leu-Leu-al) | Covalently binds to the active site of the β subunits | PD [142] |
| ALS [145] | ||
| Autism [109, 118] | ||
| AD [133, 136] | ||
| MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine hydrochloride) | Mitochondrial Complex I inhibitor | PD [144, 146] |
| PDTC (Pyrrolidine dithiocarbamate) | NF-κB inhibitor, antioxidant, immunoproteasome inhibitor | ALS [105] |
| Clioquinol | 20S inhibitor (Cu-mediated interaction) | HD [147] |
| Cyclosporin A | Non-competitive β5 inhibitor | PD [148, 149] |
| Paraquat (pesticide) | 20S inhibitor | PD [124, 150, 151] |
| Betulinic acid | β5 activator | Autism [118] |
| Benzamil | Inhibition of acid-sensing ion channels (ASIC1a) | HD [126] |
It must be noted, however, that neurodegenerative disorders are caused by UPS downregulation, and only few instances appear in the literature where the therapeutic induction of UPS has been tested. For example, betulinic acid, a proteasome activator, may have therapeutic implications in ASD which has been found to be associated with enhanced GABAergic activity. Betulinic acid has been shown to significantly suppress the induction of GABAAα1 protein levels in an ASD model of cortical neurons [118]. Benzamil is another example of a proteasome activator with the potential to act against neurodegenerative diseases (Table 1) [126]. Most of the examples of UPS modulators enlisted in Table 1 are proteasome inhibitors which have been employed to create in vivo and in vitro models of neurodegenerative disorders. Regardless, as our understanding of the various players involved in the UPS, in particular, the ligases and deubiquitinases which are known to be directly involved in various brain pathologies, becomes clearer, therapeutic strategies to upregulate UPS function will evolve. Nevertheless, it is well recognized that maintaining steady-state levels of proteasome composition and function is important for the maintenance of proteostasis in neuronal cells which depend heavily on balanced UPS functioning. As discussed above, proteostatic imbalances are common among neurodegenerative diseases leading to increased damage to the cellular protein pool, intracellular protein aggregation, and reduced proteasome activity. Therefore, pharmacotherapeutic strategies aimed at modulating the proteasome system might prove beneficial for neurodegenerative disease treatment. The discovery of small molecule inhibitors of deubiquitinating ubiquitin-specific proteases (USP) and other deubiquitinating enzymes will provide more specific targets than the 20S inhibitors presently available. Moreover, many neurodegenerative cytoplasmic inclusions can also be cleared by autophagy, and upregulation of autophagy has also been proposed as a general treatment for Parkinson’s disease, polyglutamine repeat disorders, and tauopathies [127]. It is noteworthy that impairment of the UPS results in upregulation of autophagy [128,129] and in some cases, this upregulation can compensate for diminished UPS function [128,130].
In pathologies where the UPS components are mutated, as in autosomal recessive forms of Parkinson’s and Angelman disease, gene therapy to replace the loss of E3 ligase activity may be possible in the future. Furthermore, it has been demonstrated that overexpression of UBE3A protects against the toxicity of polyglutamine repeat proteins in models of Huntington’s disease and spinocerebellar ataxia [131]. In another interesting study, anti-acidosis drug benzamil was found to enhance UPS activity and decrease mutant huntingtin aggregation in the brains of a mouse model of Huntington’s disease [126].
Conclusion
Although the interest in investigating the potential utility of proteasome modulation is increasing, current applications are limited by an incomplete understanding of the various players involved in the UPS, in particular the ligases and deubiquitinases which have been directly implicated in various brain pathologies. The future research will allow revelation of more specific targets and may provide potential insight for the treatment of neurodegenerative disorders. However, the eventual therapeutic targeting of UPS in neurodegenerative diseases will solely depend on the discovery and development of specific activators of the proteasome system.
Acknowledgment
The authors acknowledge the funding support from National Heart, Lung, and Blood Institute [R01HL104286].
References:
- 1.Hede SM, Savov V, Weishaupt H, Sangfelt O, Swartling FJ (2014) Oncoprotein stabilization in brain tumors. Oncogene 33: 4709–4721. [DOI] [PubMed] [Google Scholar]
- 2.Caldeira MV, Salazar IL, Curcio M, Canzoniero LM, Duarte CB5 (2014) Role of the ubiquitin-proteasome system in brain ischemia: friend or foe? Prog Neurobiol 112: 50–69. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt M, Finley D (2014) Regulation of proteasome activity in health and disease. Biochim Biophys Acta 1843: 13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amelio I, Landré V, Knight RA, Lisitsa A, Melino G, et al. (2015) Polypharmacology of small molecules targeting the ubiquitin-proteasome and ubiquitin-like systems. Oncotarget 6: 9646–9656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciechanover A, Kwon YT2 (2015) Degradation of misfolded proteins in neurodegenerative diseases: therapeutic targets and strategies. Exp Mol Med 47: e147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jansen AH, Reits EA, Hol EM (2014) The ubiquitin proteasome system in glia and its role in neurodegenerative diseases. Front Mol Neurosci 7: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ristic G, Tsou WL, Todi SV (2014) An optimal ubiquitin-proteasome pathway in the nervous system: the role of deubiquitinating enzymes. Front Mol Neurosci 7: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jung T, Catalgol B, Grune T (2009) The proteasomal system. Mol Aspects Med 30: 191–296. [DOI] [PubMed] [Google Scholar]
- 9.Jankowska E, Stoj J, Karpowicz P, Osmulski PA, Gaczynska M (2013) The proteasome in health and disease. Curr Pharm Des 19: 1010–1028. [PubMed] [Google Scholar]
- 10.Martinez-Vicente M, Sovak G, Cuervo AM (2005) Protein degradation and aging. Exp Gerontol 40: 622–633. [DOI] [PubMed] [Google Scholar]
- 11.D’Arcy P, Wang X, Linder S (2015) Deubiquitinase inhibition as a cancer therapeutic strategy. Pharmacol Ther 147: 32–54. [DOI] [PubMed] [Google Scholar]
- 12.Deshaies RJ (2014) Proteotoxic crisis, the ubiquitin-proteasome system, and cancer therapy. BMC Biol 12: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pal A, Young MA, Donato NJ3 (2014) Emerging potential of therapeutic targeting of ubiquitin-specific proteases in the treatment of cancer. Cancer Res 74: 4955–4966. [DOI] [PubMed] [Google Scholar]
- 14.Finley D (2009) Recognition and processing of ubiquitin-protein conjugates by the proteasome. Annu Rev Biochem 78: 477–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clague MJ, Coulson JM, Urbé S (2012) Cellular functions of the DUBs. J Cell Sci 125: 277–286. [DOI] [PubMed] [Google Scholar]
- 16.Weissman AM (2001) Themes and variations on ubiquitylation. Nat Rev Mol Cell Biol 2: 169–178. [DOI] [PubMed] [Google Scholar]
- 17.Rubio MD, Wood K, Haroutunian V, Meador-Woodruff JH (2013) Dysfunction of the ubiquitin proteasome and ubiquitin-like systems in schizophrenia. Neuropsychopharmacology 38: 1910–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groll M, Ditzel L, Löwe J, Stock D, Bochtler M, et al. (1997) Structure of 20S proteasome from yeast at 2.4 A resolution. Nature 386: 463–471. [DOI] [PubMed] [Google Scholar]
- 19.Groll M, Bajorek M, Köhler A, Moroder L, Rubin DM, et al. (2000) A gated channel into the proteasome core particle. Nat Struct Biol 7: 1062–1067. [DOI] [PubMed] [Google Scholar]
- 20.Whitby FG, Masters EI, Kramer L, Knowlton JR, Yao Y, et al. (2000) Structural basis for the activation of 20S proteasomes by 11S regulators. Nature 408: 115–120. [DOI] [PubMed] [Google Scholar]
- 21.Stadtmueller BM, Hill CP (2011) Proteasome activators. Mol Cell 41: 8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glickman MH, Rubin DM, Coux O, Wefes I, Pfeifer G, et al. (1998) A subcomplex of the proteasome regulatory particle required for ubiquitin-conjugate degradation and related to the COP9-signalosome and eIF3. Cell 94: 615–623. [DOI] [PubMed] [Google Scholar]
- 23.Braun BC, Glickman M, Kraft R, Dahlmann B, Kloetzel PM, et al. (1999) The base of the proteasome regulatory particle exhibits chaperone-like activity. Nat Cell Biol 1: 221–226. [DOI] [PubMed] [Google Scholar]
- 24.Li Z, Arnaud L, Rockwell P, Figueiredo-Pereira ME (2004) A single amino acid substitution in a proteasome subunit triggers aggregation of ubiquitinated proteins in stressed neuronal cells. J Neurochem 90: 19–28. [DOI] [PubMed] [Google Scholar]
- 25.Ordway GA, Neufer PD, Chin ER, DeMartino GN (2000) Chronic contractile activity upregulates the proteasome system in rabbit skeletal muscle. J Appl Physiol (1985) 88: 1134–1141. [DOI] [PubMed] [Google Scholar]
- 26.Dubiel W, Pratt G, Ferrell K, Rechsteiner M (1992) Purification of an 11 S regulator of the multicatalytic protease. J Biol Chem 267: 22369–22377. [PubMed] [Google Scholar]
- 27.Ma CP, Slaughter CA, DeMartino GN (1992) Identification, purification, and characterization of a protein activator (PA28) of the 20 S proteasome (macropain). J Biol Chem 267: 10515–10523. [PubMed] [Google Scholar]
- 28.Realini C, Jensen CC, Zhang Z, Johnston SC, Knowlton JR, et al. (1997) Characterization of recombinant REGalpha, REGbeta, and REGgamma proteasome activators. J Biol Chem 272: 25483–25492. [DOI] [PubMed] [Google Scholar]
- 29.Tanahashi N, Murakami Y, Minami Y, Shimbara N, Hendil KB, et al. (2000) Hybrid proteasomes. Induction by interferon-gamma and contribution to ATP-dependent proteolysis. J Biol Chem 275: 14336–14345. [DOI] [PubMed] [Google Scholar]
- 30.Minami Y, Kawasaki H, Minami M, Tanahashi N, Tanaka K, et al. (2000) A critical role for the proteasome activator PA28 in the Hsp90-dependent protein refolding. J Biol Chem 275: 9055–9061. [DOI] [PubMed] [Google Scholar]
- 31.Murata S, Kawahara H, Tohma S, Yamamoto K, Kasahara M, et al. (1999) Growth retardation in mice lacking the proteasome activator PA28gamma. J Biol Chem 274: 38211–38215. [DOI] [PubMed] [Google Scholar]
- 32.Blickwedehl J, Agarwal M, Seong C, Pandita RK, Melendy T, et al. (2008) Role for proteasome activator PA200 and postglutamyl proteasome activity in genomic stability. Proc Natl Acad Sci U S A 105: 16165–16170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt M, Haas W, Crosas B, Santamaria PG, Gygi SP, et al. (2005) The HEAT repeat protein Blm10 regulates the yeast proteasome by capping the core particle. Nat Struct Mol Biol 12: 294–303. [DOI] [PubMed] [Google Scholar]
- 34.Marques AJ, Glanemann C, Ramos PC, Dohmen RJ (2007) The Cterminal extension of the beta7 subunit and activator complexes stabilize nascent 20 S proteasomes and promote their maturation. J Biol Chem 282: 34869–34876. [DOI] [PubMed] [Google Scholar]
- 35.Fehlker M, Wendler P, Lehmann A, Enenkel C (2003) Blm3 is part of nascent proteasomes and is involved in a late stage of nuclear proteasome assembly. EMBO Rep 4: 959–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lopez AD, Tar K, Krügel U, Dange T, Ros IG, et al. (2011) Proteasomal degradation of Sfp1 contributes to the repression of ribosome biogenesis during starvation and is mediated by the proteasome activator Blm10. Mol Biol Cell 22: 528–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dange T, Smith D, Noy T, Rommel PC, Jurzitza L, et al. (2011) Blm10 protein promotes proteasomal substrate turnover by an active gating mechanism. J Biol Chem 286: 42830–42839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sadre-Bazzaz K, Whitby FG, Robinson H, Formosa T, Hill CP (2010) Structure of a Blm10 complex reveals common mechanisms for proteasome binding and gate opening. Mol Cell 37: 728–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doherty KM, Pride LD, Lukose J, Snydsman BE, Charles R, et al. (2012) Loss of a 20S proteasome activator in Saccharomyces cerevisiae downregulates genes important for genomic integrity, increases DNA damage, and selectively sensitizes cells to agents with diverse mechanisms of action. G3 (Bethesda) 2: 943–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blickwedehl J, McEvoy S, Wong I, Kousis P, Clements J, et al. (2007) Proteasomes and proteasome activator 200 kDa (PA200) accumulate on chromatin in response to ionizing radiation. Radiat Res 167: 663–674. [DOI] [PubMed] [Google Scholar]
- 41.Qian MX, Pang Y, Liu CH, Haratake K, Du BY, et al. (2013) Acetylation-mediated proteasomal degradation of core histones during DNA repair and spermatogenesis. Cell 153: 1012–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown MG, Driscoll J, Monaco JJ (1991) Structural and serological similarity of MHC-linked LMP and proteasome (multicatalytic proteinase) complexes. Nature 353: 355–357. [DOI] [PubMed] [Google Scholar]
- 43.Ortiz-Navarrete V, Seelig A, Gernold M, Frentzel S, Kloetzel PM, et al. (1991) Subunit of the ‘20S’ proteasome (multicatalytic proteinase) encoded by the major histocompatibility complex. Nature 353: 662–664. [DOI] [PubMed] [Google Scholar]
- 44.Groettrup M, Kraft R, Kostka S, Standera S, Stohwasser R, et al. (1996) A third interferon-gamma-induced subunit exchange in the 20S proteasome. Eur J Immunol 26: 863–869. [DOI] [PubMed] [Google Scholar]
- 45.Nandi D, Jiang H, Monaco JJ (1996) Identification of MECL-1 (LMP-10) as the third IFN-gamma-inducible proteasome subunit. J Immunol 156: 2361–2364. [PubMed] [Google Scholar]
- 46.Huber EM, Basler M, Schwab R, Heinemeyer W, Kirk CJ, et al. (2012) Immuno- and constitutive proteasome crystal structures reveal differences in substrate and inhibitor specificity. Cell 148: 727–738. [DOI] [PubMed] [Google Scholar]
- 47.Seifert U, Bialy LP, Ebstein F, Bech-Otschir D, Voigt A, et al. (2010) Immunoproteasomes preserve protein homeostasis upon interferon-induced oxidative stress. Cell 142: 613–624. [DOI] [PubMed] [Google Scholar]
- 48.Bellavista E, Andreoli F, Parenti MD, Martucci M, Santoro A, et al. (2013) Immunoproteasome in cancer and neuropathologies: a new therapeutic target? Curr Pharm Des 19: 702–718. [PubMed] [Google Scholar]
- 49.Kitamura A, Maekawa Y, Uehara H, Izumi K, Kawachi I, et al. (2011) A mutation in the immunoproteasome subunit PSMB8 causes autoinflammation and lipodystrophy in humans. J Clin Invest 121: 4150–4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pickering AM, Davies KJ (2012) Differential roles of proteasome and immunoproteasome regulators Pa28αβ, Pa28γ and Pa200 in the degradation of oxidized proteins. Arch Biochem Biophys 523: 181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Noda C, Tanahashi N, Shimbara N, Hendil KB, Tanaka K (2000) Tissue distribution of constitutive proteasomes, immunoproteasomes, and PA28 in rats. Biochem Biophys Res Commun 277: 348–354. [DOI] [PubMed] [Google Scholar]
- 52.Gaczynska M, Goldberg AL, Tanaka K, Hendil KB, Rock KL (1996) Proteasome subunits X and Y alter peptidase activities in opposite ways to the interferon-gamma-induced subunits LMP2 and LMP7. J Biol Chem 271: 17275–17280. [DOI] [PubMed] [Google Scholar]
- 53.Eleuteri AM, Kohanski RA, Cardozo C, Orlowski M (1997) Bovine spleen multicatalytic proteinase complex (proteasome). Replacement of X, Y, and Z subunits by LMP7, LMP2, and MECL1 and changes in properties and specificity. J Biol Chem 272: 11824–11831. [DOI] [PubMed] [Google Scholar]
- 54.Griffin TA, Nandi D, Cruz M, Fehling HJ, Kaer LV, et al. (1998) Immunoproteasome assembly: cooperative incorporation of interferon gamma (IFN-gamma)-inducible subunits. J Exp Med 187: 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heink S, Ludwig D, Kloetzel PM, Krüger E (2005) IFN-gamma-induced immune adaptation of the proteasome system is an accelerated and transient response. Proc Natl Acad Sci U S A 102: 9241–9246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yewdell JW (2005) Immunoproteasomes: regulating the regulator. Proc Natl Acad Sci U S A 102: 9089–9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murata S, Sasaki K, Kishimoto T, Niwa S, Hayashi H, et al. (2007) Regulation of CD8+ T cell development by thymus-specific proteasomes. Science 316: 1349–1353. [DOI] [PubMed] [Google Scholar]
- 58.Xing Y, Jameson SC, Hogquist KA (2013) Thymoproteasome subunit-Î25T generates peptide-MHC complexes specialized for positive selection. Proc Natl Acad Sci U S A 110: 6979–6984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jessen NA, Munk AS, Lundgaard I, Nedergaard M (2015) The Glymphatic System: A Beginner’s Guide. Neurochem Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iliff JJ, Lee H, Yu M, Feng T, Logan J, et al. (2013) Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest 123: 1299–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Díaz-Hernández M, Hernández F, Martín-Aparicio E, Gómez-Ramos P, Morán MA, et al. (2003) Neuronal induction of the immunoproteasome in Huntington’s disease. J Neurosci 23: 11653–11661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Piccinini M, Mostert M, Croce S, Baldovino S, Papotti M, et al. (2003) Interferon-gamma-inducible subunits are incorporated in human brain 20S proteasome. J Neuroimmunol 135: 135–140. [DOI] [PubMed] [Google Scholar]
- 63.Mishto M, Bellavista E, Santoro A, Stolzing A, Ligorio C, et al. (2006) Immunoproteasome and LMP2 polymorphism in aged and Alzheimer’s disease brains. Neurobiol Aging 27: 54–66. [DOI] [PubMed] [Google Scholar]
- 64.Selkoe DJ (2011) Alzheimer’s disease. Cold Spring Harb Perspect Biol 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kaneko M, Koike H, Saito R, Kitamura Y, Okuma Y, et al. (2010) Loss of HRD1-mediated protein degradation causes amyloid precursor protein accumulation and amyloid-beta generation. J Neurosci 30: 3924–3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Takei Y, Teng J, Harada A, Hirokawa N (2000) Defects in axonal elongation and neuronal migration in mice with disrupted tau and map1b genes. J Cell Biol 150: 989–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lloret A, Badia MC, Giraldo E, Ermak G, Alonso MD, et al. (2011) Amyloid-Î2 toxicity and tau hyperphosphorylation are linked via RCAN1 in Alzheimer’s disease. J Alzheimers Dis 27: 701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Keck S, Nitsch R, Grune T, Ullrich O (2003) Proteasome inhibition by paired helical filament-tau in brains of patients with Alzheimer’s disease. J Neurochem 85: 115–122. [DOI] [PubMed] [Google Scholar]
- 69.Tseng BP, Green KN, Chan JL, Blurton-Jones M, LaFerla FM (2008) Abeta inhibits the proteasome and enhances amyloid and tau accumulation. Neurobiol Aging 29: 1607–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee MJ, Lee JH, Rubinsztein DC (2013) Tau degradation: the ubiquitin-proteasome system versus the autophagy-lysosome system. Prog Neurobiol 105: 49–59. [DOI] [PubMed] [Google Scholar]
- 71.Fiala O, Zahorakova D, Pospisilova L, Kucerova J, Matejckova M, et al. (2014) Parkin (PARK 2) mutations are rare in Czech patients with early-onset Parkinson’s disease. PLoS One 9: e107585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, et al. (1998) Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392: 605–608. [DOI] [PubMed] [Google Scholar]
- 73.Shimura H, Hattori N, Kubo Si, Mizuno Y, Asakawa S, et al. (2000) Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat Genet 25: 302–305. [DOI] [PubMed] [Google Scholar]
- 74.Lücking CB, Dürr A, Bonifati V, Vaughan J, De Michele G, et al. (2000) Association between early-onset Parkinson’s disease and mutations in the parkin gene. N Engl J Med 342: 1560–1567. [DOI] [PubMed] [Google Scholar]
- 75.Klein C, Lohmann-Hedrich K (2007) Impact of recent genetic findings in Parkinson’s disease. Curr Opin Neurol 20: 453–464. [DOI] [PubMed] [Google Scholar]
- 76.Solano SM, Miller DW, Augood SJ, Young AB, Penney JB Jr (2000) Expression of alpha-synuclein, parkin, and ubiquitin carboxy-terminal hydrolase L1 mRNA in human brain: genes associated with familial Parkinson’s disease Ann Neurol 47: 201–10. [PubMed] [Google Scholar]
- 77.Shimura H, Hattori N, Kubo S, Yoshikawa M, Kitada T, et al. (1999) Immunohistochemical and subcellular localization of Parkin protein: absence of protein in autosomal recessive juvenile parkinsonism patients. Ann Neurol 45: 668–672. [DOI] [PubMed] [Google Scholar]
- 78.Jung AE, Fitzsimons HL, Bland RJ, During MJ, Young D (2008) HSP70 and constitutively active HSF1 mediate protection against CDCrel-1mediated toxicity. Mol Ther 16: 1048–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Murakami T, Shoji M, Imai Y, Inoue H, Kawarabayashi T, et al. (2004) Pael-R is accumulated in Lewy bodies of Parkinson’s disease. Ann Neurol 55: 439–442. [DOI] [PubMed] [Google Scholar]
- 80.Fahn S, Sulzer D (2004) Neurodegeneration and neuroprotection in Parkinson disease. NeuroRx 1: 139–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bedford L, Hay D, Devoy A, Paine S, Powe DG, et al. (2008) Depletion of 26S proteasomes in mouse brain neurons causes neurodegeneration and Lewy-like inclusions resembling human pale bodies. J Neurosci 28: 8189–8198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Paine SM, Anderson G, Bedford K, Lawler K, Mayer RJ et al. (2013) Pale body-like inclusion formation and neurodegeneration following depletion of 26S proteasomes in mouse brain neurones are independent of alpha-synuclein. PLoS One 8: e54711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wahl C, Kautzmann S, Krebiehl G, Strauss K, Woitalla D, et al. (2008) A comprehensive genetic study of the proteasomal subunit S6 ATPase in German Parkinson’s disease patients. J Neural Transm 115: 1141–1148. [DOI] [PubMed] [Google Scholar]
- 84.Tofaris GK, Layfield R, Spillantini MG (2001) alpha-synuclein metabolism and aggregation is linked to ubiquitin-independent degradation by the proteasome. FEBS Lett 509: 22–26. [DOI] [PubMed] [Google Scholar]
- 85.Snyder H, Mensah K, Theisler C, Lee J, Matouschek A, et al. (2003) Aggregated and monomeric alpha-synuclein bind to the S6’ proteasomal protein and inhibit proteasomal function. J Biol Chem 278: 11753–11759. [DOI] [PubMed] [Google Scholar]
- 86.Bentea E, Van der Perren A, Van Liefferinge J3, El Arfani A, Albertini G, et al. (2015) Nigral proteasome inhibition in mice leads to motor and non-motor deficits and increased expression of Ser129 phosphorylated Î ±-synuclein. Front Behav Neurosci 9: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, et al. (1997) Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 276: 2045–2047. [DOI] [PubMed] [Google Scholar]
- 88.Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, et al. (2004) Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet 364: 1167–1169. [DOI] [PubMed] [Google Scholar]
- 89.Lopes da Fonseca T, Villar-Piqué A, Outeiro TF (2015) The Interplay between Alpha-Synuclein Clearance and Spreading. Biomolecules 5: 435–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D (2004) Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science 305: 1292–1295. [DOI] [PubMed] [Google Scholar]
- 91.Schulte C, Gasser T (2011) Genetic basis of Parkinson’s disease: inheritance, penetrance, and expression. Appl Clin Genet 4: 67–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Biskup S, Gerlach M, Kupsch A, Reichmann H, Riederer P, et al. (2008) Genes associated with Parkinson syndrome. J Neurol 255 Suppl 5: 8–17. [DOI] [PubMed] [Google Scholar]
- 93.Lim KL, Tan JM (2007) Role of the ubiquitin proteasome system in Parkinson’s disease. BMC Biochem 8 Suppl 1: S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Leroy E, Boyer R, Auburger G, Leube B, Ulm G, et al. (1998) The ubiquitin pathway in Parkinson’s disease. Nature 395: 451–452. [DOI] [PubMed] [Google Scholar]
- 95.Betarbet R, Sherer TB, Greenamyre JT (2005) Ubiquitin-proteasome system and Parkinson’s diseases. Exp Neurol 191 Suppl 1: S17–27. [DOI] [PubMed] [Google Scholar]
- 96.(1993) A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disease Collaborative Research Group. Cell 72: 971–983. [DOI] [PubMed] [Google Scholar]
- 97.Sanchez I, Mahlke C Yuan J (2003) Pivotal role of oligomerization in expanded polyglutamine neurodegenerative disorders. Nature 421: 373–379. [DOI] [PubMed] [Google Scholar]
- 98.Ordway JM, Tallaksen-Greene S, Gutekunst CA, Bernstein EM, Cearley JA, et al. (1997) Ectopically expressed CAG repeats cause intranuclear inclusions and a progressive late onset neurological phenotype in the mouse. Cell 91: 753–763. [DOI] [PubMed] [Google Scholar]
- 99.Ortega Z, Lucas JJ1 (2014) Ubiquitin-proteasome system involvement in Huntington’s disease. Front Mol Neurosci 7: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Holmberg CI, Staniszewski KE, Mensah KN, Matouschek A, Morimoto RI (2004) Inefficient degradation of truncated polyglutamine proteins by the proteasome. EMBO J 23: 4307–4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hunter JM, Lesort M, Johnson GV (2007) Ubiquitin-proteasome system alterations in a striatal cell model of Huntington’s disease. J Neurosci Res 85: 1774–1788. [DOI] [PubMed] [Google Scholar]
- 102.Seo H, Sonntag KC, Kim W, Cattaneo E, Isacson O (2007) Proteasome activator enhances survival of Huntington’s disease neuronal model cells. PLoS One 2: e238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tashiro Y, Urushitani M, Inoue H, Koike M, Uchiyama Y, et al. (2012) Motor neuron-specific disruption of proteasomes, but not autophagy, replicates amyotrophic lateral sclerosis. J Biol Chem 287: 42984–42994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Puttaparthi K, Elliott JL (2005) Non-neuronal induction of immunoproteasome subunits in an ALS model: possible mediation by cytokines. Exp Neurol 196: 441–451. [DOI] [PubMed] [Google Scholar]
- 105.Ahtoniemi T, Goldsteins G, Keksa-Goldsteine V, Malm T, Kanninen K, et al. (2007) Pyrrolidine dithiocarbamate inhibits induction of immunoproteasome and decreases survival in a rat model of amyotrophic lateral sclerosis. Mol Pharmacol 71: 30–37. [DOI] [PubMed] [Google Scholar]
- 106.Shi SQ, Bichell TJ, Ihrie RA, Johnson CH (2015) Ube3a imprinting impairs circadian robustness in Angelman syndrome models. Curr Biol 25: 537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lehman NL (2009) The ubiquitin proteasome system in neuropathology. Acta Neuropathol 118: 329–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Glessner JT, Wang K, Cai G, Korvatska O, Kim CE, et al. (2009) Autism genome-wide copy number variation reveals ubiquitin and neuronal genes. Nature 459: 569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tsai NP, Wilkerson JR, Guo W, Maksimova MA, DeMartino GN, et al. (2012) Multiple autism-linked genes mediate synapse elimination via proteasomal degradation of a synaptic scaffold PSD-95. Cell 151: 1581–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Baron CA, Tepper CG, Liu SY, Davis RR, Wang NJ, et al. (2006) Genomic and functional profiling of duplicated chromosome 15 cell lines reveal regulatory alterations in UBE3A-associated ubiquitin-proteasome pathway processes. Hum Mol Genet 15: 853–869. [DOI] [PubMed] [Google Scholar]
- 111.Lee SY, Ramirez J, Franco M, Lectez B, Gonzalez M, et al. (2014) Ube3a, the E3 ubiquitin ligase causing Angelman syndrome and linked to autism, regulates protein homeostasis through the proteasomal shuttle Rpn10. Cell Mol Life Sci 71: 2747–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.De Jaco A, Comoletti D, King CC, Taylor P (2008) Trafficking of cholinesterases and neuroligins mutant proteins. An association with autism. Chem Biol Interact 175: 349–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.De Jaco A, Lin MZ, Dubi N, Comoletti D, Miller MT, et al. (2010) Neuroligin trafficking deficiencies arising from mutations in the alpha/beta-hydrolase fold protein family. J Biol Chem 285: 28674–28682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cellot G, Cherubini E (2014) GABAergic signaling as therapeutic target for autism spectrum disorders. Front Pediatr 2: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Snodgrass SR (1992) GABA and epilepsy: their complex relationship and the evolution of our understanding. J Child Neurol 7: 77–86. [DOI] [PubMed] [Google Scholar]
- 116.Gabis L, Pomeroy J, Andriola MR (2005) Autism and epilepsy: cause, consequence, comorbidity, or coincidence? Epilepsy Behav 7: 652–656. [DOI] [PubMed] [Google Scholar]
- 117.Ben-Ari Y, Woodin MA, Sernagor E, Cancedda L, Vinay L, et al. (2012) Refuting the challenges of the developmental shift of polarity of GABA actions: GABA more exciting than ever! Front Cell Neurosci 6: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Crider A, Pandya CD, Peter D, Ahmed AO2, Pillai A1 (2014) Ubiquitinproteasome dependent degradation of GABAAα1 in autism spectrum disorder. Mol Autism 5: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Olanow CW, McNaught KS (2006) Ubiquitin-proteasome system and Parkinson’s disease. Mov Disord 21: 1806–1823. [DOI] [PubMed] [Google Scholar]
- 120.Sullivan PG, Dragicevic NB, Deng JH, Bai Y, Dimayuga E, et al. (2004) Proteasome inhibition alters neural mitochondrial homeostasis and mitochondria turnover. J Biol Chem 279: 20699–20707. [DOI] [PubMed] [Google Scholar]
- 121.Shamoto-Nagai M,Maruyama W, Kato Y, Isobe K, Tanaka M, et al. (2003) An inhibitor of mitochondrial complex I, rotenone, inactivates proteasome by oxidative modification and induces aggregation of oxidized proteins in SH-SY5Y cells. J Neurosci Res 74: 589–597. [DOI] [PubMed] [Google Scholar]
- 122.Höglinger GU, Carrard G, Michel PP, Medja F, Lombès A, et al. (2003) Dysfunction of mitochondrial complex I and the proteasome: interactions between two biochemical deficits in a cellular model of Parkinson’s disease. J Neurochem 86: 1297–1307. [DOI] [PubMed] [Google Scholar]
- 123.Jha N, Kumar MJ, Boonplueang R, Andersen JK (2002) Glutathione decreases in dopaminergic PC12 cells interfere with the ubiquitin protein degradation pathway: relevance for Parkinson’s disease? J Neurochem 80: 555–561. [DOI] [PubMed] [Google Scholar]
- 124.Ding Q, Keller JN (2001) Proteasome inhibition in oxidative stress neurotoxicity: implications for heat shock proteins. J Neurochem 77: 1010–1017. [DOI] [PubMed] [Google Scholar]
- 125.Okada K, Wangpoengtrakul C, Osawa T, Toyokuni S, Tanaka K, et al. (1999) 4-Hydroxy-2-nonenal-mediated impairment of intracellular proteolysis during oxidative stress. Identification of proteasomes as target molecules. J Biol Chem 274: 23787–93. [DOI] [PubMed] [Google Scholar]
- 126.Wong HK, Bauer PO, Kurosawa M, Goswami A, Washizu C, et al. (2008) Blocking acid-sensing ion channel 1 alleviates Huntington’s disease pathology via an ubiquitin-proteasome system-dependent mechanism. Hum Mol Genet 17: 3223–3235. [DOI] [PubMed] [Google Scholar]
- 127.Williams A, Jahreiss L, Sarkar S, Saiki S, Menzies FM, et al. (2006) Aggregate-prone proteins are cleared from the cytosol by autophagy: therapeutic implications. Curr Top Dev Biol 76: 89–101. [DOI] [PubMed] [Google Scholar]
- 128.Pandey UB, Nie Z, Batlevi Y, McCray BA, Ritson GP, et al. (2007) HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS. Nature 447: 859–863. [DOI] [PubMed] [Google Scholar]
- 129.Iwata A, Riley BE, Johnston JA, Kopito RR (2005) HDAC6 and microtubules are required for autophagic degradation of aggregated huntingtin. J Biol Chem 280: 40282–40292. [DOI] [PubMed] [Google Scholar]
- 130.Pan T, Kondo S, Zhu W, Xie W, Jankovic J, et al. (2008) Neuroprotection of rapamycin in lactacystin-induced neurodegeneration via autophagy enhancement. Neurobiol Dis 32: 16–25. [DOI] [PubMed] [Google Scholar]
- 131.Mishra A, Dikshit P, Purkayastha S, Sharma J, Nukina N, et al. (2008) E6-AP promotes misfolded polyglutamine proteins for proteasomal degradation and suppresses polyglutamine protein aggregation and toxicity. J Biol Chem 283: 7648–7656. [DOI] [PubMed] [Google Scholar]
- 132.Figueiredo-Pereira ME, Berg KA, Wilk S (1994) A new inhibitor of the chymotrypsin-like activity of the multicatalytic proteinase complex (20S proteasome) induces accumulation of ubiquitin-protein conjugates in a neuronal cell. J Neurochem 63: 1578–1581. [DOI] [PubMed] [Google Scholar]
- 133.Delobel P, Leroy O, Hamdane M, Sambo AV, Delacourte A, et al. (2005) Proteasome inhibition and Tau proteolysis: an unexpected regulation. FEBS Lett 579: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Meng L, Mohan R, Kwok BH, Elofsson M, Sin N, et al. (1999) Epoxomicin, a potent and selective proteasome inhibitor, exhibits in vivo antiinflammatory activity. Proc Natl Acad Sci U S A 96: 10403–10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ravikumar B, Duden R, Rubinsztein DC (2002) Aggregate-prone proteins with polyglutamine and polyalanine expansions are degraded by autophagy. Hum Mol Genet 11: 1107–1117. [DOI] [PubMed] [Google Scholar]
- 136.Yew EH, Cheung NS, Choy MS, Qi RZ, Lee AY, et al. (2005) Proteasome inhibition by lactacystin in primary neuronal cells induces both potentially neuroprotective and pro-apoptotic transcriptional responses: a microarray analysis. J Neurochem 94: 943–956. [DOI] [PubMed] [Google Scholar]
- 137.Wyttenbach A, Carmichael J, Swartz J, Furlong RA, Narain Y, et al. (2000) Effects of heat shock, heat shock protein 40 (HDJ-2), and proteasome inhibition on protein aggregation in cellular models of Huntington’s disease. Proc Natl Acad Sci U S A 97: 2898–2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Di Noto L, Whitson LJ, Cao X, Hart PJ, Levine RL (2005) Proteasomal degradation of mutant superoxide dismutases linked to amyotrophic lateral sclerosis. J Biol Chem 280: 39907–39913. [DOI] [PubMed] [Google Scholar]
- 139.Urushitani M, Sik A, Sakurai T, Nukina N, Takahashi R, et al. (2006) Chromogranin-mediated secretion of mutant superoxide dismutase proteins linked to amyotrophic lateral sclerosis. Nat Neurosci 9: 108–18. [DOI] [PubMed] [Google Scholar]
- 140.Martín-Aparicio E, Yamamoto A, Hernández F, Hen R, Avila J, et al. (2001) Proteasomal-dependent aggregate reversal and absence of cell death in a conditional mouse model of Huntington’s disease. J Neurosci 21: 8772–8781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Waelter S, Boeddrich A, Lurz R, Scherzinger E, Lueder G, et al. (2001) Accumulation of mutant huntingtin fragments in aggresome-like inclusion bodies as a result of insufficient protein degradation. Mol Biol Cell 12: 1393–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Biasini E, Fioriti L, Ceglia I, Invernizzi R, Bertoli A, et al. (2004) Proteasome inhibition and aggregation in Parkinson’s disease: a comparative study in untransfected and transfected cells. J Neurochem 88: 545–553. [DOI] [PubMed] [Google Scholar]
- 143.Jana NR, Zemskov EA, Wang G, Nukina N (2001) Altered proteasomal function due to the expression of polyglutamine-expanded truncated N-terminal huntingtin induces apoptosis by caspase activation through mitochondrial cytochrome c release. Hum Mol Genet 10: 1049–59. [DOI] [PubMed] [Google Scholar]
- 144.Ding Q, Dimayuga E, Martin S, Bruce-Keller AJ, Nukala V, et al. (2003) Characterization of chronic low-level proteasome inhibition on neural homeostasis. J Neurochem 86: 489–497. [DOI] [PubMed] [Google Scholar]
- 145.Cheroni C, Marino M, Tortarolo M, Veglianese P, De Biasi S, et al. (2009) Functional alterations of the ubiquitin-proteasome system in motor neurons of a mouse model of familial amyotrophic lateral sclerosis. Hum Mol Genet 18: 82–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zeng BY, Iravani MM, Lin ST, Irifune M, Kuoppamäki M, et al. (2006) MPTP treatment of common marmosets impairs proteasomal enzyme activity and decreases expression of structural and regulatory elements of the 26S proteasome. Eur J Neurosci 23: 1766–1774. [DOI] [PubMed] [Google Scholar]
- 147.Nguyen T, Hamby A, Massa SM (2005) Clioquinol down-regulates mutant huntingtin expression in vitro and mitigates pathology in a Huntington’s disease mouse model. Proc Natl Acad Sci U S A 102: 11840–11845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Meyer S, Kohler NG, Joly A (1997) Cyclosporine A is an uncompetitive inhibitor of proteasome activity and prevents NF-kappaB activation. FEBS Lett 413: 354–358. [DOI] [PubMed] [Google Scholar]
- 149.Matsuura K, Kabuto H, Makino H, Ogawa N (1996) Cyclosporin A attenuates degeneration of dopaminergic neurons induced by 6hydroxydopamine in the mouse brain. Brain Res 733: 101–104. [DOI] [PubMed] [Google Scholar]
- 150.Wills J, Credle J, Oaks AW, Duka V, Lee JH, et al. (2012) Paraquat, but not maneb, induces synucleinopathy and tauopathy in striata of mice through inhibition of proteasomal and autophagic pathways. PLoS One 7: e30745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cicchetti F, Lapointe N, Roberge-Tremblay A, Saint-Pierre M, Jimenez L, et al. (2005) Systemic exposure to paraquat and maneb models early Parkinson’s disease in young adult rats. Neurobiol Dis 20: 360–371. [DOI] [PubMed] [Google Scholar]