Abstract
Background
Studies examining the association between hyperoxia exposure after resuscitation from cardiac arrest and clinical outcomes have reported conflicting results. Our objective was to test the hypothesis that early post-resuscitation hyperoxia is associated with poor neurological outcome.
Methods
Multi-center, prospective cohort study. We included adult, cardiac arrest patients who were mechanically ventilated and received targeted temperature management after return of spontaneous circulation (ROSC). We excluded patients with cardiac arrest due to trauma or sepsis. Per protocol, partial pressure of arterial oxygen (PaO2) was measured at one and six hours after ROSC. Hyperoxia was defined as a PaO2 > 300 mmHg during the initial six hours after ROSC. The primary outcome was poor neurological function at hospital discharge, defined as a modified Rankin Scale > 3. Multivariable generalized linear regression with a log link was used to test the association between PaO2 and poor neurological outcome. To assess if there was an association between other supranormal PaO2 levels and poor neurological outcome, we used other PaO2 cut points to define hyperoxia (i.e. 100, 150, 200, 250, 350, 400 mmHg).
Results
Of the 280 patients included, 105 (38%) had exposure to hyperoxia. Poor neurological function at hospital discharge occurred in 70% of patients in the entire cohort, and 77% vs. 65% among patients with and without exposure to hyperoxia respectively [absolute risk difference 12% (95% CI 1% – 23%)]. Hyperoxia was independently associated with poor neurological function, relative risk 1.23 (95% CI 1.11 - 1.35). On multivariable analysis, a one-hour longer duration of hyperoxia exposure was associated with a 3% increase in risk of poor neurological outcome [relative risk 1.03 (95% CI 1.02 - 1.05)]. We found the association with poor neurological outcome began at 300 mmHg or higher.
Conclusions
Early hyperoxia exposure after resuscitation from cardiac arrest was independently associated with poor neurological function at hospital discharge.
Keywords: Hyperoxia, cardiac arrest, neurological injury, brain injury, partial pressure of arterial oxygen
Introduction
Post-cardiac arrest syndrome is a unique pathophysiological condition characterized by systemic post-resuscitation ischemia/reperfusion injury commonly resulting in neurological damage.1, 2 The in-hospital mortality among individuals with post-cardiac arrest syndrome is over 50%, and among those that survive, many are left with permanent and severe neurological disability.3 The identification of new therapies to attenuate the ongoing brain injury in this patient population is of the utmost importance given that cardiac arrest occurs in over 400,000 people each year in the United States alone.4
Exposure to hyperoxia [supranormal partial pressure of arterial oxygen (PaO2) due to high fractions of inspired oxygen (FiO2)] following resuscitation is previously demonstrated to amplify the production of oxygen free radicals, resulting in neuronal injury and death via cellular metabolic failure and apoptosis.5, 6 Current post-resuscitation guidelines recommend titrating the FiO2 in post-cardiac arrest patients to avoid hypoxia, and prolonged exposure to hyperoxia (most commonly defined as PaO2 > 300 mmHg).7–9 Our group previously published a retrospective registry study demonstrating an association between post-resuscitation exposure to PaO2 > 300 mmHg and in-hospital mortality.10 However, subsequent observational studies examining the associations between hyperoxia and clinical outcomes have reported conflicting results.10–16 All previous studies have methodological limitations. They were mostly retrospective in nature, used varying methodologies to define PaO2 derangements, and most evaluated arterial blood gas (ABG) measurements over the first 24 hours after return of spontaneous circulation (ROSC) rather than focusing on the period immediately after ROSC when the brain is likely most susceptible to additional reperfusion injury. By their design, these previous studies were subject to measurement bias, as they relied on ABG results ordered at the discretion of treating physicians, as opposed to protocol-directed ABG measurements at specific time points.
We performed a fully prospective, multi-center study using protocol-directed ABG measurements in the early hours following resuscitation and protocol directed assessments of neurological disability, as opposed to chart review. Our main objective was to test the association between early post-resuscitation hyperoxia and poor neurological outcome among adult patients successfully resuscitated from cardiac arrest.
Methods
Setting
We performed a prospective cohort study across six hospitals in the United States: 1) Cooper University Hospital, Camden, NJ (coordinating center); 2) Hospital of the University of Pennsylvania, Philadelphia, PA; 3) Penn-Presbyterian Medical Center, Philadelphia, PA; 4) Methodist Hospital, Indianapolis, IN; 5) University of Mississippi Medical Center, Jackson, MS; and 6) Beth Israel Deaconess Medical Center, Boston, MA. We prospectively collected data pertaining to the index cardiac arrest event, and outcomes consistent with the Utstein style for reporting cardiac arrest research, including all post-ROSC variables recommended for post-resuscitation research.17, 18 Each of the participating centers had a mechanism in place for real-time notification of study personnel when an out-of-hospital cardiac arrest patient arrives in the emergency department (ED) or when a cardiac arrest occurs in-hospital. This study was approved by the institutional review board at each participating institution and each subject gave written informed consent. This study is reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement.19 After review and approval by our study data use committee, we will allow other researchers who submit to us a protocol to have unrestricted access to our complete de-identified database in comma separated value format, together with a data dictionary.
Participants
We enrolled adult post-cardiac arrest patients who were comatose after ROSC between July 2013 and March 2017. The inclusion criteria were: 1) age ≥ 18 years; 2) cardiac arrest, defined as a documented absence of pulse and cardiopulmonary resuscitation (CPR) initiated; 3) ROSC > 20 min; 4) mechanically ventilated after ROSC; and 5) clinician intent to perform targeted temperature management. We decided to include patients with both in- and out-of-hospital cardiac arrest, as this would generate a pragmatic study whose results could be broadly applicable to as many cardiac arrest patients as possible. We excluded patients with presumed etiology of arrest secondary to trauma, hemorrhage or sepsis; residents of a nursing home or other long-term care facility; pregnancy; prisoners; and terminal illness with no reasonable expectation to survive to hospital discharge or known lack of commitment to aggressive support by next of kin. We also excluded patients who died prior to an arterial blood gas analysis being obtained.
Standard Care
Routine post-cardiac arrest care across all sites consisted of standard elements recommended by the American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care and included: (1) targeted temperature management for 24 hours after ROSC; (2) controlled rewarming to avoid hyperpyrexia with targeted temperature management; (3) 24/7 capability for goal-directed hemodynamic support interventions; (4) 24/7 capability for interventional cardiac catheterization (if needed); (5) 24/7 capability for continuous electroencephalographic monitoring; and (6) evidence-based approach to neurological prognostication, specifically waiting >72 hours after ROSC before support limitations for poor neurological prognosis.7, 20
Data Collection
As part of our research protocol we obtained an initial arterial blood gas (ABG) one hour (± two hours) after ROSC and a second ABG six hours (± two hours) after ROSC. At the time of ABG collection we also recorded the plateau airway pressure during an inspiratory hold on the ventilator. We recorded all additional ABG analyses ordered by the treating physician as well as all ventilator changes and the time the changes were made. The arterial oxygen saturation (SaO2) and the fraction of inspired oxygen (FiO2) were continuously monitored and recorded every 15 min for the initial six hours after ROSC. For both SaO2 and FiO2 the time-weighted average was calculated. To calculate the time-weighted average for each subject, we multiplied the length of time that the patient spent at a specific SaO2 value by that SaO2 value, added all these values together, and then divided by the total length of post-resuscitation observation time.21 We performed the same calculation for FiO2. We prospectively captured all the components of the Sequential Organ Failure Assessment (SOFA) score (i.e. respiratory, coagulation, hepatic, renal, cardiovascular, and neurological) during the first 24 hours after ROSC.22 For calculation of the SOFA score, we used the worst value for each component during the initial six-hour period after ROSC and excluded the neurological and respiratory components.22–24 We abstracted clinical data from the medical record into a Research Electronic Data Capture (REDCap, Vanderbilt University, TN) database, and exported into Stata/SE 14.1 for Mac, StataCorp LP (College Station, TX, USA) for analysis.25
Outcome measures
The primary outcome was poor neurological function or death at hospital discharge, defined a priori as a modified Rankin Scale (mRS) > 3.26 The mRS is a well-validated scale of neurological disability, which is widely used to measure outcome in stroke clinical trials (0: no symptoms, 1: no significant disability, 2: slight disability, 3: moderate disability, 4: moderate severe disability, 5: severe disability, 6: death). All raters were trained and certified in mRS assessment27 and used a structured questionnaire and interview, which have been shown to produce strong interobserver reliability.28, 29 Secondary outcomes were in-hospital mortality and early neurological injury defined as a Full Outline of UnResponsiveness (FOUR) score ≤ 6 at 72 hours after ROSC, based on previous literature.30, 31 The FOUR score is a well-validated scale of neurological injury for comatose patients. The FOUR score has 4 components: eye responses, motor responses, brainstem reflexes, and respiration pattern, and ranges from 0 to 16 with lower scores demonstrating worse injury.32
Data Analysis
Categorical variables were compared using the chi-square test. Continuous variables were compared using student t-test or Wilcoxon rank-sum test, based on the distribution of the data. We used Spearman’s correlation coefficient (r) to assess the relationship between 1- and 6-hour PaO2, and the corresponding FiO2 and SaO2. We used multivariable logistic regression analysis to identify what patient and management characteristics were associated with hyperoxia (see Supplemental Methods).
For the primary outcome, we calculated relative risk (RR) using multivariable generalized linear regression with a log link33 to test if exposure to hyperoxia during the initial six hours after ROSC was an independent predictor of poor neurologic function at hospital discharge. We a priori defined hyperoxia as PaO2 > 300 mmHg on one or more ABG analyses, based on previously described definition for hyperoxia.6, 7, 10, 13 A priori, we selected the following candidate variables for the regression model on the grounds that they were previously demonstrated to predict outcome in post–cardiac arrest patients: (1) age (decile); (2) initial cardiac rhythm [asystole or pulseless electrical activity (PEA) vs. ventricular fibrillation/pulseless ventricular tachycardia (VF/VT)];34 (3) metabolic acidosis (defined as one or more recorded base deficit ≤ -6 during the initial six hours after ROSC, based on previously published literature);35 (4) arterial hypotension (mean arterial pressure < 70 mmHg during the initial six hours after ROSC);21 (5) pre-arrest comorbidities (i.e., Charlson comorbidities index);36 (6) prolonged duration of CPR (CPR duration > 20 min);37 and (7) location of cardiac arrest (in- vs. out-of-hospital).38–41 Backward elimination with a criterion of p < 0.05 for retention in the model was used. Statistical interactions and collinearity were assessed. Goodness of fit of the model was evaluated with the deviance test. This analysis was repeated for both secondary outcomes. For the main analyses listwise deletion was used for missing co-variables. We also report results using multiple imputation for missing co-variables. These models used robust standard error and took into account the random effects at the institution (i.e. site of enrollment) level.
We performed several additional pre-planned sensitivity analyses for the primary outcome. First, we entered additional covariates beyond those pre-specified into a multivariable generalized linear regression model with a log link. Second, we assessed whether cardiac arrest location (pre-hospital or in-hospital) had different results. Finally, we performed a sensitivity analysis limited to only patients who survived to hospital discharge (detailed description of sensitivity analyses is discussed in Supplemental Methods).
We also examined the association between PaO2 and outcome across different thresholds to define hyperoxia (i.e. PaO2 > 100, 150, 200, 250, 300, 350, and 400 mmHg on one or more ABG analyses). We entered each threshold into a multivariable generalized linear regression model with a log link and calculated relative risks with 95% confidence intervals (CI) for poor neurological outcome adjusting for candidate variables retained in the original model. We graphed the relative risks with 95% CI and inspected the graph to assess if there was a threshold signal for neurological outcome over increasing PaO2 cut points.
To reflect the duration of hyperoxia exposure during the initial six hours after ROSC, we used the first PaO2 measurement to represent the PaO2 exposure during the time from ROSC to the first ABG measurement. We then calculated the time intervals between ABG measurements and inferred that the PaO2 remained constant at the level observed in the earlier measurement until the time point of the subsequent measurement (i.e. last value carried forward). Similar methodology to estimate PaO2 exposure has been used previously.16 We then added up the total time patients had exposure to hyperoxia during the initial six hours after ROSC. To test the impact of duration of hyperoxia exposure, we entered duration of exposure as a continuous variable (calibrated for one hour) into a multivariable generalized linear regression model with a log link adjusting for the candidate variables retained in the original model. Given some subjects had ABG analyses ordered by treating physicians in addition to the protocol, we adjusted the model for the total number of ABG analyses obtained during the initial six hours after ROSC, as well as time to first ABG.
Sample size calculation
We estimated the necessary sample size based on the following assumptions: a) a predicted event (i.e. survival with good neurological function) rate of 29%;21 and b) an estimated event (survival with good neurological function) per covariate ratio of 10:1 necessary for multivariable modeling.42, 43 To accrue the necessary 80 survivors with good neurological function we estimated that a minimum of 276 total subjects would be necessary and we planned to enroll 280.
Results
A total of 2084 subjects were screened for inclusion and 280 were included in the final cohort (Figure 1). We were unable to obtain informed consent for 326 patients (e.g. no surrogate decision maker available or declined consent). Compared to those excluded due to lack of informed consent, the study sample had a similar mean (SD) age 59 (16) vs. 59 (15), respectively. However, we found our study sample had a higher rate of VF/VT [37% vs. 21%] and longer duration of CPR [median (IQR) 15 (8–23) vs. 10 (1–22)]. Of those included 105 (38%) had exposure to hyperoxia and 175 (62%) had no exposure to hyperoxia during the initial six hours after ROSC. The median (IQR) time from ROSC to the first ABG analysis was 59 (35–103) min and the median (IQR) number of ABG analyses during the initial six hours after ROSC was 2 (2–3).
Figure 1.
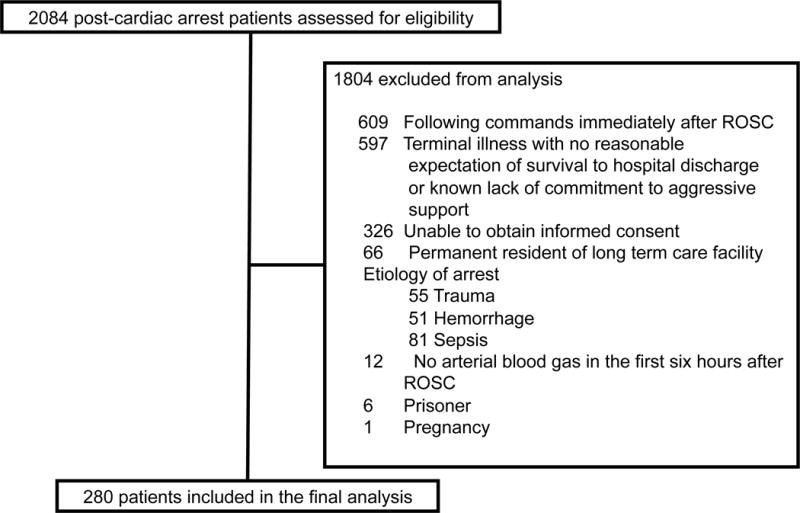
Study flow diagram
ROSC, return of spontaneous circulation
Table 1 displays the baseline data for all subjects in the cohort, as well as for subjects with and without exposure to hyperoxia. Out-of-hospital cardiac arrest with PEA/asystole as the initial rhythm was the most common type of cardiac arrest [109/280 (39%)], followed by out-of-hospital cardiac arrest with pulseless VF/VT as the initial rhythm [86/280 (31%)]. Initial rhythm was unclear for 23 (8%) and downtime was unknown for 5 (<2%) of patients. We found no differences in age, cardiac arrest characteristics or comorbidities between those exposed and unexposed to hyperoxia. Table 2 displays post-cardiac arrest data for all subjects. All patients were mechanically ventilated and received targeted temperature management after ROSC. Percutaneous coronary intervention (PCI) was performed within the first 36 hours in 22/86 (26%) of patients with out-of-hospital, pulseless VF/VT cardiac arrest. The median (IQR) SOFA score was lower among subjects with exposure to hyperoxia [4 (2–7)] compared to those without exposure [5 (3–7)]; however, this was not found to be statistically significant (Wilcoxon rank sum test p = 0.119). We found a poor correlation between PaO2 and SaO2 (r = 0.23), as well as PaO2 and FiO2 (r = 0.27). In addition, SaO2 could not reliably rule out the presence of hyperoxia exposure, and PaO2 as high as 295 mmHg occurred with FiO2 of 0.40 (Supplemental Figures 1–3). The only management characteristics found to be independent predictors of hyperoxia at 0- or 6-hours were FiO2 and PEEP, odds ratios 1.08 (95% CI 1.05–1.11) and 0.83 (95% CI 0.70–0.97) respectively (Supplemental Table 1).
Table 1.
Baseline data for all subjects at the time of cardiac arrest.
| Variables | All Subjects n = 280 |
No Hyperoxia n = 175 |
Hyperoxia* n = 105 |
p - value |
|---|---|---|---|---|
| Age [years (SD)] | 59 (15) | 59 (14) | 58 (16) | 0.803 |
| Female [n (%)] | 101 (36) | 70 (40) | 31 (30) | 0.077 |
| Pre-existing comorbidities [n (%)] | ||||
| Diabetes | 68 (24) | 42 (24) | 26 (25) | 0.886 |
| Known coronary artery disease | 75 (27) | 49 (28) | 26 (25) | 0.554 |
| Hypertension | 183 (65) | 120 (69) | 63 (60) | 0.144 |
| Malignancy | 20 (7) | 14 (8) | 6 (6) | 0.472 |
| Renal insufficiency | 43 (15) | 26 (15) | 17 (16) | 0.764 |
| Pulmonary disease | 65 (23) | 39 (22) | 26 (25) | 0.635 |
| Cerebral vascular disease | 24 (9) | 17 (10) | 7 (7) | 0.378 |
| Congestive heart failure | 69 (25) | 42 (24) | 27 (26) | 0.747 |
| Charlson comorbidity score36 [median (IQR)] | 1 (0–3) | 1 (0–3) | 1 (0–3) | 0.906 |
| Arrest location [n (%)] | ||||
| Out-of-hospital | 216 (77) | 138 (79) | 78 (74) | |
| In-hospital | 64 (23) | 37 (21) | 27 (26) | 0.378 |
| Initial arrest rhythm [n (%)] | ||||
| PEA/asystole | 154 (55) | 92 (53) | 62 (59) | |
| VF/VT | 103 (37) | 66 (37) | 37 (35) | 0.389 |
| Unknown | 23 (8) | 17 (10) | 6 (6) | |
| CPR duration [median (IQR)] | 15 (8–23) | 15 (7–25) | 15 (8–21) | 0.355 |
| CPR duration > 20 min [n (%)] | 80 (29) | 54 (31) | 26 (25) | 0.277 |
Partial pressure of arterial oxygen > 300 mmHg during the first six hours after return of spontaneous circulation; CPR, cardiopulmonary resuscitation; IQR, interquartile range; PEA, pulseless electrical activity; SD, standard deviation; VF, ventricular fibrillation; VT ventricular tachycardia
Table 2.
Post-cardiac arrest data for all subjects. All values are median interquartile range unless otherwise noted.
| Variables | All Subjects n = 280 |
No Hyperoxia n = 175 |
Hyperoxia* n = 105 |
p - value |
|---|---|---|---|---|
| Ventilator parameters | ||||
| TWA-FiO2 | 0.82 (0.66–0.97) | 0.87 (0.64–0.99) | 0.78 (0.68–0.92) | 0.162 |
| PEEP (cmH2O) | 5 (5–7) | 5 (5–8) | 5 (5–5) | <0.001 |
| Tidal volume (cc/kg PBW) | 7.4 (6.7–8.1) | 7.4 (6.8–8.1) | 7.3 (6.7–8.0) | 0.537 |
| Respiratory rate (breaths/min) | 17 (15–20) | 17 (15–21) | 16 (15–20) | 0.282 |
| Plateau pressure (cmH2O) | 20 (16–25) | 21 (17–26) | 20 (16–23) | 0.241 |
| Plateau pressure > 30 cmH2O [n (%)] | 42 (18) | 33 (22) | 9 (10) | 0.016 |
| PaO2 at 1 hr | 201 (99–343) | 121 (82–203) | 406 (304–488) | <0.001 |
| PaO2 at 6 hr | 106 (75–193) | 99 (71–156) | 128 (88–238) | <0.001 |
| TWA-SaO2 (%) | 98 (97–99) | 98 (96–99) | 99 (98–100) | <0.001 |
| ROSC to first ABG (min) | 59 (35–103) | 63 (39–122) | 48 (28–76) | 0.002 |
| Number of ABGs in first 6 hours | 2 (2–3) | 2 (2–3) | 3 (2–3) | 0.173 |
| PaCO2 (mmHg) | 44 (37–52) | 45 (38–54) | 43 (36–50) | 0.077 |
| pH | 7.27 (7.18–7.34) | 7.26 (7.18–7.34) | 7.28 (7.20–7.35) | 0.160 |
| Base excess | −8 (-11- -3) | −8 (-12- -4) | −6 (-10- -3) | 0.256 |
| Metabolic acidosis† [n (%)] | 202 (72) | 128 (73) | 74 (70) | 0.630 |
| MAP (mmHg) | 94 (82–105) | 93 (81–103) | 95 (84–106) | 0.209 |
| Arterial hypotension‡ [n (%)] | 142 (51) | 95 (54) | 47 (45) | 0.123 |
| Vasopressor infusion [n (%)] | 150 (54) | 99 (57) | 51 (49) | 0.194 |
| PCI [n (%)] | 31 (11) | 20 (11) | 11 (10) | 0.806 |
| Modified SOFA score | 5 (2–7) | 5 (3–7) | 4 (2–7) | 0.119 |
Partial pressure of arterial oxygen > 300 mmHg during the first six hours after return of spontaneous circulation.
Defined as a base deficit ≤ −6 during the first 6 hours after return of spontaneous circulation.
Defined as mean arterial pressure < 70 mmHg during the first 6 hours after return of spontaneous circulation. ABG, arterial blood gas; FiO2, fraction of inspired oxygen; MAP, mean arterial blood pressure; PaCO2, partial pressure of arterial carbon dioxide; PBW, predicted body weight; PCI, percutaneous coronary intervention; PEEP, positive end expiratory pressure, ROSC, return of spontaneous circulation; SaO2, arterial oxygen saturation; SOFA, sequential organ failure assessment; TWA, time weighted average.
Seventy percent of subjects had the primary outcome of poor neurological function or death at hospital discharge. Study subjects with exposure to hyperoxia had a higher incidence of poor neurological function at hospital discharge than patients with no exposure (77% vs. 65% respectively, absolute risk difference 12% (95% CI 1% - 23%, p = 0.035]. Figure 2 displays the proportion of subjects with each mRS score stratified by hyperoxia exposure (yes/no). The overall in-hospital mortality for the entire cohort was 55%. The mortality rate was 59% versus 52% among those with and without hyperoxia exposure respectively (p = 0.251). Two hundred and twenty five subjects survived to 72 hours and had a FOUR score measurement. The median (IQR) FOUR score at 72 hours was 8 (3–13) for the entire cohort, and 7 (3–13) vs. 10 (4–13) among patients with and without hyperoxia respectively (Wilcoxon rank sum test p = 0.148). Forty-seven percent vs. 35% had early neurological injury at 72 hours among patients with and without hyperoxia respectively (p = 0.073). The FOUR score among those who died after 72 hours was significantly lower compared to those who survived to hospital discharge [3 (0–7) vs. 13 (10–16) Wilcoxon rank sum test p < 0.001], suggesting those who died had significant neurological injury prior to death.
Figure 2.
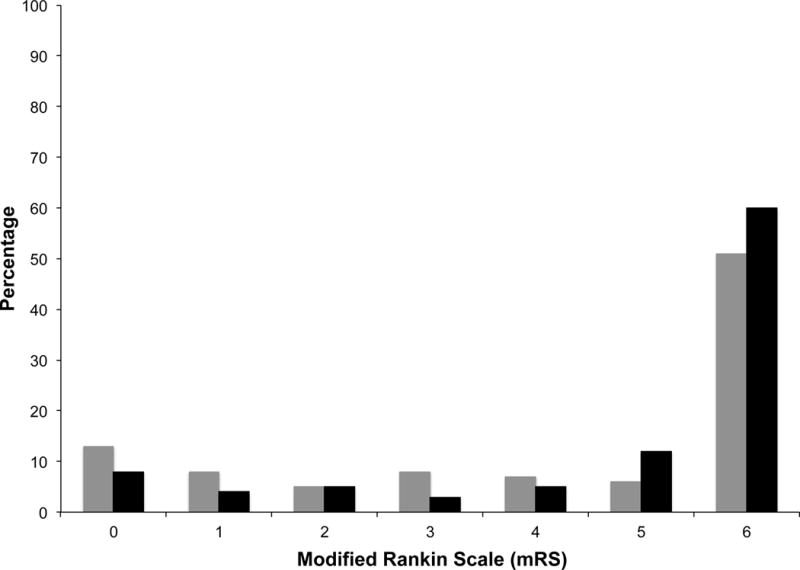
Modified Rankin Scale at hospital discharge stratified by no hyperoxia (gray columns) and hyperoxia (black columns).
mRS: 0, no symptoms; 1, no significant disability; 2, slight disability; 3, moderate disability; 4, moderate severe disability; 5, severe disability; 6, death
Table 3 displays the results of the multivariable regression models for the primary outcome as well as both secondary outcomes. After adjusting for potential baseline and post-cardiac arrest confounders, hyperoxia was an independent predictor of poor neurological function at hospital discharge, relative risk 1.23 (95% CI 1.11 - 1.35), as well as early neurological injury. Hyperoxia was found to be associated with in-hospital mortality when multiple imputation was used (see Supplemental Tables 2–7 for results of the full regression models).
Table 3.
Adjusted relative risks for hyperoxia (partial pressure of arterial oxygen > 300 mmHg) for the primary and secondary outcomes
| Outcome | Relative Risk | 95% CI | p-value |
|---|---|---|---|
| Primary Outcome: | |||
| Poor Neurological Outcome* | |||
| Listwise deletion | 1.23 | 1.11 – 1.35 | <0.001 |
| Multiple imputation | 1.24 | 1.13 – 1.35 | <0.001 |
| Secondary Outcomes: | |||
| In-hospital mortality | |||
| Listwise deletion | 1.24 | 0.99 – 1.55 | 0.060 |
| Multiple imputation | 1.25 | 1.01 – 1.54 | 0.040 |
| Early neurological injury† | |||
| Listwise deletion | 1.32 | 1.03 – 1.69 | 0.026 |
| Multiple imputation | 1.39 | 1.11 – 1.74 | 0.004 |
Defined as modified Rankin Scale (mRS) > 3 at hospital discharge;
Defined as a Full Outline of UnResponsiveness (FOUR) score ≤ 6 at 72 hours after return of spontaneous circulation. CI, confidence interval. Results of the full models are displayed in the supplemental material.
For the first sensitivity analysis of the primary outcome, several variables were statistically different at p < 0.10 when comparing hyperoxia and no hyperoxia groups: gender, mean positive end expiratory pressure (PEEP), plateau airway pressure > 30 cmH2O, time-weighted average SaO2, time from ROSC to first ABG analysis, and mean PaCO2 during the initial six hours after ROSC. After adjusting for these identified potential confounders, hyperoxia remained an independent predictor of poor neurological outcome, relative risk 1.23 (95% CI 1.05–1.44) (Supplemental Table 8). We did not find evidence that the association between hyperoxia and poor neurological outcome differed between cardiac arrest locations (Supplemental Table 9). Among patients who survived to hospital discharge, 33% had poor neurological outcome at hospital discharge. Hyperoxia remained an independent predictor of poor neurological outcome among survivors to hospital discharge, relative risk 1.42 (95% CI 1.09–1.87) (Supplemental Table 10).
Figure 3 displays adjusted relative risks with 95% CI for poor neurological outcome across ascending cut points used to define hyperoxia. Only PaO2 cut points of 300 mmHg and greater were found to be significantly associated with poor neurological outcome.
Figure 3.
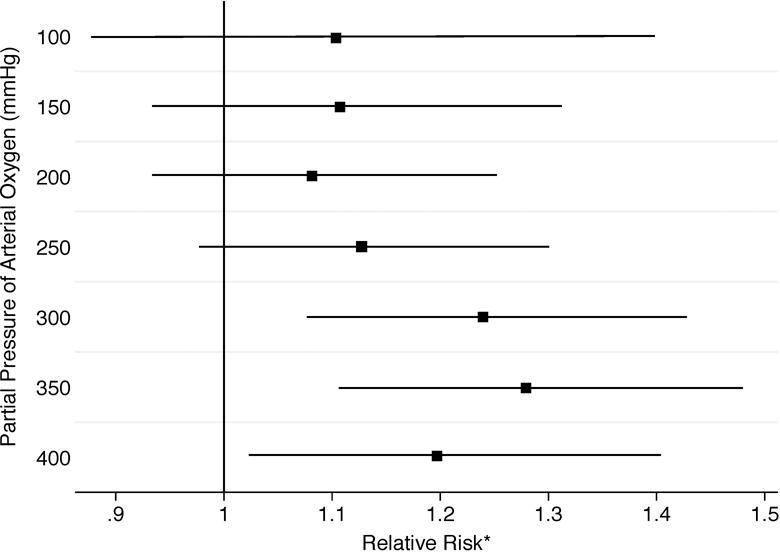
Adjusted relative risks (squares) with 95% confidence intervals (horizontal lines) for poor neurological outcome [defined as modified Rankin Scale (mRS) > 3] across ascending cut points to define hyperoxia.
| Cut point | No Hyperoxia n (Proportion with mRS > 3) |
Hyperoxia n (Proportion with mRS >3) |
| 100 | 40 (60%) | 240 (71%) |
| 150 | 77 (64%) | 203 (72%) |
| 200 | 114 (67%) | 166 (72%) |
| 250 | 139 (66%) | 141 (73%) |
| 300 | 175 (65%) | 105 (77%) |
| 350 | 199 (65%) | 81 (80%) |
| 400 | 216 (67%) | 64 (78%) |
During the initial six hours after ROSC, after adjusting for potential baseline and post-cardiac arrest confounders and total number of ABG analyses, a one-hour longer duration of hyperoxia exposure was associated with a 3% increase in risk of poor neurological outcome [relative risk 1.03 (95% CI 1.02 - 1.05)] (Supplemental Table 11).
Discussion
In this prospective, multi-center study, using a standardized protocol for ABG measurements, we tested whether exposure to hyperoxia after resuscitation from cardiac arrest was associated with poor neurological function at hospital discharge. We found 38% of patients had exposure to hyperoxia during the early hours following resuscitation, and that hyperoxia exposure after ROSC was an independent predictor of poor neurological function at hospital discharge. Our results suggest that the association between supranormal levels of PaO2 and poor neurological outcome begins at a PaO2 of 300 mmHg and higher. In addition, we found an association between duration of hyperoxia exposure and neurological outcome. In our multivariable models we also found hyperoxia to be independently associated with early neurological injury and our results suggest hyperoxia is associated with in-hospital mortality. Early neurological injury was common among subjects who died in hospital, suggesting that neurological injury was a major factor for mortality. Thus, it is reasonable to infer that hyperoxia’s association with mortality is mediated by early neurological injury. Finally, we found that SaO2 and FiO2 levels could not reliably rule out exposure to hyperoxia; therefore, frequent ABG measurements may be needed to avoid hyperoxia exposure. Although we found a weak correlation between FiO2 and PaO2 on univariable analysis, when adjusted for other ventilator settings and patient characteristics we found higher FiO2 was a predictor of hyperoxia exposure. We also found higher PEEP to have a negative association with hyperoxia exposure. Higher PEEP strategies are often employed in patients with lung injury who have higher oxygen requirements. Thus, elevated PEEP may be a marker for patients who are more difficult to oxygenate and thus less likely to develop hyperoxia. In summary, in this prospective, multi-center study using protocol-directed ABG measurements, we found that hyperoxia during the early period after ROSC is associated with poor neurological outcome.
Hyperoxia is postulated to cause harm in the context of reperfusion injury by increasing the formation of reactive oxygen species resulting in oxidative impairment of mitochondrial respiration and cerebral energy metabolism. Oxidative modification of mitochondrial proteins may disable brain pyruvate dehydrogenase complex activity,44 the only bridge between anaerobic and aerobic metabolism. In addition, oxidative stress activates the mitochondrial permeability transition pore to release NAD(H) into the cytosol, depleting a vital metabolic cofactor.45 Metabolic failure may ensue, resulting in decreased cerebral consumption of glucose and oxygen, increased production of lactate, and delayed neuronal cell death.46, 47 Furthermore, increased reactive oxygen species may impair electron transport chain activity by forming mitochondrial membrane pores that release cytochrome c into the cytosol,45 resulting in caspase-dependent apoptosis. Increased reactive oxygen species may also cause oxidation of brain lipids (i.e. lipid peroxidation), which may have physiologic (e.g. alteration of blood flow, neutrophil chemoattraction) and cellular toxic effects (e.g. excitotoxicity, neurodegeneration),48, 49 as well as promote cellular inflammatory reactions, specifically the activation of microglia and astrocytes in the neuronal microenvironment leading to increased neuronal cell death.50 Additionally, hyperoxia may also have a direct vasoconstrictor effect, which may reduce cerebral blood flow after ROSC, exacerbating ischemic injury.51, 52
Preclinical studies support the hypothesis that hyperoxia after ROSC worsens brain damage, as evidenced on functional neurological testing48, 50, 53 and histopathology,47, 54 and decreases survival.49 A recent randomized clinical trial of patients with acute myocardial infarction found no decrease in mortality at one year with the use of supplemental oxygen compared to room air.55 In addition, a randomized clinical trial of supplemental oxygen versus no supplemental oxygen in patients with ST-elevation myocardial infarction found supplemental oxygen increased the risk of recurrent myocardial infarction and cardiac arrhythmias, and increased myocardial infarct size at six months, suggesting higher PaO2 levels worsen myocardial reperfusion injury.56
Current post-resuscitation guidelines recommend that if the SaO2 is greater than 98% during the early period after cardiac arrest, the FiO2 should be titrated down to avoid prolonged exposure to hyperoxia.7–9 However, the current data on hyperoxia after resuscitation from cardiac arrest have significant limitations and are mostly from retrospective cohort studies, with conflicting results.10–16, 57, 58 Interpreting the current literature is difficult secondary to heterogeneity in methodologies used to define PaO2 derangements and outcomes. In addition, none of these previous studies used protocol-directed ABG measurements at specific time points causing concern for measurement bias. A recent systematic review and meta-analysis of observational studies found hyperoxia to be associated with in-hospital mortality; however, the authors of this meta-analysis warn these results should be interpreted with caution as there was significant heterogeneity between studies.59
A recent cohort study with historical controls found that initiating conservative oxygen therapy targeting a SaO2 of 88–92% using the lowest possible FiO2 among post-cardiac arrest patients admitted to the intensive care unit, was feasible and decreased ICU length of stay, but did not improve survival to hospital discharge.60 Of note, all patients in both the titrated and the conventional oxygen therapy groups in this previous study had PaO2 levels less than 200 mmHg, thus these results do not help inform the effects of PaO2 above 200 mmHg. To date there have been two randomized control trials evaluating the effects of supranormal PaO2 levels after resuscitation from cardiac arrest. One study randomized 28 subjects to a FiO2 of 0.30 versus 1.0 and found conservative oxygen therapy was safe.61 This trial found no difference in serum neuron specific enolase (NSE), a marker of neuronal injury, in the entire cohort, but found use of 0.30 oxygen was associated with decreased level of NSE at 24 hours in patients not treated with targeted temperature management. A second study in the pre-hospital setting randomized 18 subjects to standard care (highest possible oxygen flow rate) versus oxygen titration (targeting SaO2 90–94%).62 This trial was terminated early due to increased hypoxia episodes in the oxygen titration group. These data suggest titration of FiO2 is perhaps more appropriately managed in the hospital setting.
The results in this current study prospectively validate our previous findings that a PaO2 > 300 mmHg is associated with poor clinical outcomes.10 These findings, in conjunction with the current body of literature evaluating the association between PaO2/supplemental oxygen and clinical outcomes during reperfusion injury, support current post-cardiac arrest guideline recommendations to avoid prolonged exposure to hyperoxia. This study has important implications for future design of clinical trials aimed at identifying an optimal PaO2 after ROSC. Specifically, given the current evidence of an association with harm, and no evidence to suggest any potential benefit, such trials should focus on testing varying PaO2 ranges below 300 mmHg, as at this time there is currently insufficient equipoise to ethically randomize subjects to a PaO2 > 300 mmHg.
We acknowledge that this study has important limitations to consider. First, this was an observational study and thus we can only report association rather than infer causation. Second, although we used multivariable linear regression with a log link and multiple sensitivity analyses to adjust for potential confounders, there still exists the potential of unmeasured confounders. Third, in contrast to some resuscitation clinical investigations we included patients with both in- and out-of-hospital cardiac arrest. We felt this was necessary to allow for a more pragmatic study, in which the results can be applicable to the largest possible patient population. In the present study, arrest location was not associated with outcome. In addition, on sensitivity analysis we did not find evidence that the association between hyperoxia and poor neurological outcome differs between arrest locations (i.e. arrest location was not an effect modifier). Fourth, 326 subjects were excluded secondary to inability to obtain informed consent. Although we found similar mean ages between our study sample and those subjects excluded due to lack of informed consent, we found our study population had a higher rate of VT/VF and longer duration of CPR, suggesting some difference between our study sample and those excluded due to lack of informed consent, potentially introducing selection bias. Of note, 29% of subjects screened for inclusion who underwent CPR for cardiac arrest were excluded secondary to known lack of commitment to aggressive support. These patients likely underwent CPR secondary to the treatment team being unaware of the patient’s wishes (i.e. unavailable or no advanced directive) or family made the decision to withdraw care shortly after ROSC. Fifth, we found discordance between the measured PaO2 and corresponding SaO2. This discordance is likely secondary to poor SaO2 sensing, and demonstrates the limitations of pulse oximetry and underscores the importance of obtaining an ABG for PaO2 monitoring. Sixth, for estimating the duration of hyperoxia exposure we assumed the PaO2 level remained constant between ABG analyses. It is possible that the PaO2 level varied during this time allowing for potential measurement bias. Finally, it remains possible that hyperoxia reflects a patient population that is more ill and therefore has a higher likelihood to have poor neurological outcome. However, we did not observe any significant differences in the duration of CPR, post-resuscitation SOFA score, incidence of post-resuscitation arterial hypotension or vasopressor administration, or degree of metabolic acidosis between the two groups, suggesting this was not the case.
Conclusion
Early hyperoxia exposure after resuscitation from cardiac arrest is independently associated with death and poor neurological function at hospital discharge. The increased risk of poor neurological function appears to begin at a PaO2 of 300 mmHg.
Supplementary Material
Clinical Perspective.
What’s new
In this prospective multi-center protocol-directed cohort study that included 280 adult post-cardiac arrest patients, early hyperoxia exposure [partial pressure of arterial oxygen (PaO2) > 300 mmHg during the first six hours after return of spontaneous circulation (ROSC)] was an independent predictor of poor neurological function at hospital discharge after adjusting for potential baseline and post-cardiac arrest confounders, relative risk 1.23 (95% CI 1.11 - 1.35).
What are the clinical implications
Early hyperoxia exposure after resuscitation from cardiac arrest is independently associated with death and poor neurological function at hospital discharge. The increased risk of poor neurological function appears to begin at a PaO2 of 300 mmHg.
Acknowledgments
Funding sources
National Heart, Lung, and Blood Institute (U.S.), R01HL112815. The funding source had no role in study design, in collection, analysis, and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
Footnotes
Disclosures: None of the authors have potential financial conflicts of interest to disclose.
References
- 1.Negovsky VA, Gurvitch AM. Post-resuscitation disease–a new nosological entity. Its reality and significance. Resuscitation. 1995;30:23–27. doi: 10.1016/0300-9572(95)00861-m. [DOI] [PubMed] [Google Scholar]
- 2.Nolan JP, Neumar RW, Adrie C, Aibiki M, Berg RA, Bbttiger BW, Callaway C, Clark RS, Geocadin RG, Jauch EC, Kern KB, Laurent I, Longstreth WT, Merchant RM, Morley P, Morrison LJ, Nadkarni V, Peberdy MA, Rivers EP, Rodriguez-Nunez A, Sellke FW, Spaulding C, Sunde K, Vanden Hoek T, International Liaison Committee on R, Emergency Cardiovascular Care Committee AHA, Council on Cardiovascular S, Anesthesia, Council on Cardiopulmonary P, Critical C, Council on Clinical C, Council on S Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication: a scientific statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke (Part II) Int Emerg Nurs. 2010;18:8–28. doi: 10.1016/j.ienj.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Trzeciak S, Jones AE, Kilgannon JH, Milcarek B, Hunter K, Shapiro NI, Hollenberg SM, Dellinger P, Parrillo JE. Significance of arterial hypotension after resuscitation from cardiac arrest. Crit Care Med. 2009;37:2895–2903. doi: 10.1097/ccm.0b013e3181b01d8c. [DOI] [PubMed] [Google Scholar]
- 4.Kong MH, Fonarow GC, Peterson ED, Curtis AB, Hernandez AF, Sanders GD, Thomas KL, Hayes DL, Al-Khatib SM. Systematic review of the incidence of sudden cardiac death in the United States. J Am Coll Cardiol. 2011;57:794–801. doi: 10.1016/j.jacc.2010.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becker LB. New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovasc Res. 2004;61:461–470. doi: 10.1016/j.cardiores.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 6.Douzinas EE, Patsouris E, Kypriades EM, Makris DJ, Andrianakis I, Korkolopoulou P, Boursinos V, Papalois A, Sotiropoulou C, Davaris P, Roussos C. Hypoxaemic reperfusion ameliorates the histopathological changes in the pig brain after a severe global cerebral ischaemic insult. Intensive Care Med. 2001;27:905–910. doi: 10.1007/s001340100932. [DOI] [PubMed] [Google Scholar]
- 7.Callaway CW, Donnino MW, Fink EL, Geocadin RG, Golan E, Kern KB, Leary M, Meurer WJ, Peberdy MA, Thompson TM, Zimmerman JL. Part 8: Post-Cardiac Arrest Care: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132:S465–482. doi: 10.1161/CIR.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hazinski MF, Nolan JP, Aickin R, Bhanji F, Billi JE, Callaway CW, Castren M, de Caen AR, Ferrer JM, Finn JC, Gent LM, Griffin RE, Iverson S, Lang E, Lim SH, Maconochie IK, Montgomery WH, Morley PT, Nadkarni VM, Neumar RW, Nikolaou NI, Perkins GD, Perlman JM, Singletary EM, Soar J, Travers AH, Welsford M, Wyllie J, Zideman DA. Part 1: Executive Summary: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2015;132:S2–39. doi: 10.1161/CIR.0000000000000270. [DOI] [PubMed] [Google Scholar]
- 9.Nolan JP, Soar J, Cariou A, Cronberg T, Moulaert VR, Deakin CD, Bottiger BW, Friberg H, Sunde K, Sandroni C. European Resuscitation Council and European Society of Intensive Care Medicine Guidelines for Post-resuscitation Care 2015: Section 5 of the European Resuscitation Council Guidelines for Resuscitation 2015. Resuscitation. 2015;95:202–222. doi: 10.1016/j.resuscitation.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 10.Kilgannon JH, Jones AE, Shapiro NI, Angelos MG, Milcarek B, Hunter K, Parrillo JE, Trzeciak S, Emergency Medicine Shock Research Network I Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. Jama. 2010;303:2165–2171. doi: 10.1001/jama.2010.707. [DOI] [PubMed] [Google Scholar]
- 11.Bellomo R, Bailey M, Eastwood GM, Nichol A, Pilcher D, Hart GK, Reade MC, Egi M, Cooper DJ, Study of Oxygen in Critical Care G Arterial hyperoxia and in-hospital mortality after resuscitation from cardiac arrest. Crit Care. 2011;15:R90. doi: 10.1186/cc10090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helmerhorst HJ, Roos-Blom MJ, van Westerloo DJ, Abu-Hanna A, de Keizer NF, de Jonge E. Associations of arterial carbon dioxide and arterial oxygen concentrations with hospital mortality after resuscitation from cardiac arrest. Crit Care. 2015;19:348. doi: 10.1186/s13054-015-1067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson NJ, Dodampahala K, Rosselot B, Perman SM, Mikkelsen ME, Goyal M, Gaieski DF, Grossestreuer AV. The Association Between Arterial Oxygen Tension and Neurological Outcome After Cardiac Arrest. Ther Hypothermia Temp Manag. 2017;7:36–41. doi: 10.1089/ther.2016.0015. [DOI] [PubMed] [Google Scholar]
- 14.Kilgannon JH, Jones AE, Parrillo JE, Dellinger RP, Milcarek B, Hunter K, Shapiro NI, Trzeciak S, Emergency Medicine Shock Research Network I Relationship between supranormal oxygen tension and outcome after resuscitation from cardiac arrest. Circulation. 2011;123:2717–2722. doi: 10.1161/CIRCULATIONAHA.110.001016. [DOI] [PubMed] [Google Scholar]
- 15.Lee BK, Jeung KW, Lee HY, Lee SJ, Jung YH, Lee WK, Heo T, Min YI. Association between mean arterial blood gas tension and outcome in cardiac arrest patients treated with therapeutic hypothermia. Am J Emerg Med. 2014;32:55–60. doi: 10.1016/j.ajem.2013.09.044. [DOI] [PubMed] [Google Scholar]
- 16.Vaahersalo J, Bendel S, Reinikainen M, Kurola J, Tiainen M, Raj R, Pettila V, Varpula T, Skrifvars MB, Group FS Arterial blood gas tensions after resuscitation from out-of-hospital cardiac arrest: associations with long-term neurologic outcome. Crit Care Med. 2014;42:1463–1470. doi: 10.1097/CCM.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs I, Nadkarni V, Bahr J, Berg RA, Billi JE, Bossaert L, Cassan P, Coovadia A, D’Este K, Finn J, Halperin H, Handley A, Herlitz J, Hickey R, Idris A, Kloeck W, Larkin GL, Mancini ME, Mason P, Mears G, Monsieurs K, Montgomery W, Morley P, Nichol G, Nolan J, Okada K, Perlman J, Shuster M, Steen PA, Sterz F, Tibballs J, Timerman S, Truitt T, Zideman D. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update and simplification of the Utstein templates for resuscitation registries: a statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Councils of Southern Africa) Circulation. 2004;110:3385–3397. doi: 10.1161/01.CIR.0000147236.85306.15. [DOI] [PubMed] [Google Scholar]
- 18.Langhelle A, Nolan J, Herlitz J, Castren M, Wenzel V, Soreide E, Engdahl J, Steen PA. Recommended guidelines for reviewing, reporting, and conducting research on post-resuscitation care: the Utstein style. Resuscitation. 2005;66:271–283. doi: 10.1016/j.resuscitation.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 19.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Ann Intern Med. 2007;147:573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 20.Neumar RW, Otto CW, Link MS, Kronick SL, Shuster M, Callaway CW, Kudenchuk PJ, Ornato JP, McNally B, Silvers SM, Passman RS, White RD, Hess EP, Tang W, Davis D, Sinz E, Morrison LJ. Part 8: adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122:S729–767. doi: 10.1161/CIRCULATIONAHA.110.970988. [DOI] [PubMed] [Google Scholar]
- 21.Kilgannon JH, Roberts BW, Jones AE, Mittal N, Cohen E, Mitchell J, Chansky ME, Trzeciak S. Arterial blood pressure and neurologic outcome after resuscitation from cardiac arrest. Crit Care Med. 2014;42:2083–2091. doi: 10.1097/CCM.0000000000000406. [DOI] [PubMed] [Google Scholar]
- 22.Roberts BW, Kilgannon JH, Chansky ME, Mittal N, Wooden J, Parrillo JE, Trzeciak S. Multiple organ dysfunction after return of spontaneous circulation in postcardiac arrest syndrome. Crit Care Med. 2013;41:1492–1501. doi: 10.1097/CCM.0b013e31828a39e9. [DOI] [PubMed] [Google Scholar]
- 23.Trzeciak S, Dellinger RP, Parrillo JE, Guglielmi M, Bajaj J, Abate NL, Arnold RC, Colilla S, Zanotti S, Hollenberg SM. Early microcirculatory perfusion derangements in patients with severe sepsis and septic shock: relationship to hemodynamics, oxygen transport, and survival. Ann Emerg Med. 2007;49:88–98. 98 e1–2. doi: 10.1016/j.annemergmed.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 24.Trzeciak S, McCoy JV, Phillip Dellinger R, Arnold RC, Rizzuto M, Abate NL, Shapiro NI, Parrillo JE, Hollenberg SM. Early increases in microcirculatory perfusion during protocol-directed resuscitation are associated with reduced multi-organ failure at 24 h in patients with sepsis. Intensive Care Med. 2008;34:2210–2217. doi: 10.1007/s00134-008-1193-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, Horn J, Hovdenes J, Kjaergaard J, Kuiper M, Pellis T, Stammet P, Wanscher M, Wise MP, Aneman A, Al-Subaie N, Boesgaard S, Bro-Jeppesen J, Brunetti I, Bugge JF, Hingston CD, Juffermans NP, Koopmans M, Kober L, Langorgen J, Lilja G, Moller JE, Rundgren M, Rylander C, Smid O, Werer C, Winkel P, Friberg H, Investigators TTMT Targeted temperature management at 33 degrees C versus 36 degrees C after cardiac arrest. N Engl J Med. 2013;369:2197–2206. doi: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]
- 27.Quinn TJ, Lees KR, Hardemark HG, Dawson J, Walters MR. Initial experience of a digital training resource for modified Rankin scale assessment in clinical trials. Stroke. 2007;38:2257–2261. doi: 10.1161/STROKEAHA.106.480723. [DOI] [PubMed] [Google Scholar]
- 28.Wilson JT, Hareendran A, Grant M, Baird T, Schulz UG, Muir KW, Bone I. Improving the assessment of outcomes in stroke: use of a structured interview to assign grades on the modified Rankin Scale. Stroke. 2002;33:2243–2246. doi: 10.1161/01.str.0000027437.22450.bd. [DOI] [PubMed] [Google Scholar]
- 29.Wilson JT, Hareendran A, Hendry A, Potter J, Bone I, Muir KW. Reliability of the modified Rankin Scale across multiple raters: benefits of a structured interview. Stroke. 2005;36:777–781. doi: 10.1161/01.STR.0000157596.13234.95. [DOI] [PubMed] [Google Scholar]
- 30.Jalali R, Rezaei M. A comparison of the glasgow coma scale score with full outline of unresponsiveness scale to predict patients’ traumatic brain injury outcomes in intensive care units. Crit Care Res Pract. 2014;2014:289803. doi: 10.1155/2014/289803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nyam TE, Ao KH, Hung SY, Shen ML, Yu TC, Kuo JR. FOUR Score Predicts Early Outcome in Patients After Traumatic Brain Injury. Neurocritical care. 2017;26:225–231. doi: 10.1007/s12028-016-0326-y. [DOI] [PubMed] [Google Scholar]
- 32.Iyer VN, Mandrekar JN, Danielson RD, Zubkov AY, Elmer JL, Wijdicks EF. Validity of the FOUR score coma scale in the medical intensive care unit. Mayo Clin Proc. 2009;84:694–701. doi: 10.4065/84.8.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 34.Nadkarni VM, Larkin GL, Peberdy MA, Carey SM, Kaye W, Mancini ME, Nichol G, Lane-Truitt T, Potts J, Ornato JP, Berg RA. First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. Jama. 2006;295:50–57. doi: 10.1001/jama.295.1.50. [DOI] [PubMed] [Google Scholar]
- 35.Roberts BW, Kilgannon JH, Chansky ME, Mittal N, Wooden J, Trzeciak S. Association between postresuscitation partial pressure of arterial carbon dioxide and neurological outcome in patients with post-cardiac arrest syndrome. Circulation. 2013;127:2107–2113. doi: 10.1161/CIRCULATIONAHA.112.000168. [DOI] [PubMed] [Google Scholar]
- 36.Murray SB, Bates DW, Ngo L, Ufberg JW, Shapiro NI. Charlson Index is associated with one-year mortality in emergency department patients with suspected infection. Acad Emerg Med. 2006;13:530–536. doi: 10.1197/j.aem.2005.11.084. [DOI] [PubMed] [Google Scholar]
- 37.Hajbaghery MA, Mousavi G, Akbari H. Factors influencing survival after in-hospital cardiopulmonary resuscitation. Resuscitation. 2005;66:317–321. doi: 10.1016/j.resuscitation.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 38.Gaul GB, Gruska M, Titscher G, Blazek G, Havelec L, Marktl W, Muellner W, Kaff A. Prediction of survival after out-of-hospital cardiac arrest: results of a community-based study in Vienna. Resuscitation. 1996;32:169–176. doi: 10.1016/0300-9572(96)00956-2. [DOI] [PubMed] [Google Scholar]
- 39.Langhelle A, Tyvold SS, Lexow K, Hapnes SA, Sunde K, Steen PA. In-hospital factors associated with improved outcome after out-of-hospital cardiac arrest. A comparison between four regions in Norway. Resuscitation. 2003;56:247–263. doi: 10.1016/s0300-9572(02)00409-4. [DOI] [PubMed] [Google Scholar]
- 40.Peberdy MA, Kaye W, Ornato JP, Larkin GL, Nadkarni V, Mancini ME, Berg RA, Nichol G, Lane-Trultt T. Cardiopulmonary resuscitation of adults in the hospital: a report of 14720 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation. 2003;58:297–308. doi: 10.1016/s0300-9572(03)00215-6. [DOI] [PubMed] [Google Scholar]
- 41.Tok D, Keles GT, Toprak V, Topcu I. Assessment of in-hospital cardiopulmonary resuscitation using Utstein template in a university hospital. Tohoku J Exp Med. 2004;202:265–273. doi: 10.1620/tjem.202.265. [DOI] [PubMed] [Google Scholar]
- 42.Peduzzi P, Concato J, Feinstein AR, Holford TR. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol. 1995;48:1503–1510. doi: 10.1016/0895-4356(95)00048-8. [DOI] [PubMed] [Google Scholar]
- 43.Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–1379. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 44.Ikeda K, Liu X, Kida K, Marutani E, Hirai S, Sakaguchi M, Andersen LW, Bagchi A, Cocchi MN, Berg KM, Ichinose F, Donnino MW. Thiamine as a neuroprotective agent after cardiac arrest. Resuscitation. 2016;105:138–144. doi: 10.1016/j.resuscitation.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 45.Fiskum G, Danilov CA, Mehrabian Z, Bambrick LL, Kristian T, McKenna MC, Hopkins I, Richards EM, Rosenthal RE. Postischemic oxidative stress promotes mitochondrial metabolic failure in neurons and astrocytes. Ann N Y Acad Sci. 2008;1147:129–138. doi: 10.1196/annals.1427.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richards EM, Fiskum G, Rosenthal RE, Hopkins I, McKenna MC. Hyperoxic reperfusion after global ischemia decreases hippocampal energy metabolism. Stroke; a journal of cerebral circulation. 2007;38:1578–1584. doi: 10.1161/STROKEAHA.106.473967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vereczki V, Martin E, Rosenthal RE, Hof PR, Hoffman GE, Fiskum G. Normoxic resuscitation after cardiac arrest protects against hippocampal oxidative stress, metabolic dysfunction, and neuronal death. J Cereb Blood Flow Metab. 2006;26:821–835. doi: 10.1038/sj.jcbfm.9600234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Y, Rosenthal RE, Haywood Y, Miljkovic-Lolic M, Vanderhoek JY, Fiskum G. Normoxic ventilation after cardiac arrest reduces oxidation of brain lipids and improves neurological outcome. Stroke. 1998;29:1679–1686. doi: 10.1161/01.str.29.8.1679. [DOI] [PubMed] [Google Scholar]
- 49.Mickel HS, Vaishnav YN, Kempski O, von Lubitz D, Weiss JF, Feuerstein G. Breathing 100% oxygen after global brain ischemia in Mongolian Gerbils results in increased lipid peroxidation and increased mortality. Stroke; a journal of cerebral circulation. 1987;18:426–430. doi: 10.1161/01.str.18.2.426. [DOI] [PubMed] [Google Scholar]
- 50.Hazelton JL, Balan I, Elmer GI, Kristian T, Rosenthal RE, Krause G, Sanderson TH, Fiskum G. Hyperoxic reperfusion after global cerebral ischemia promotes inflammation and long-term hippocampal neuronal death. J Neurotrauma. 2010;27:753–762. doi: 10.1089/neu.2009.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dyson A, Stidwill R, Taylor V, Singer M. The impact of inspired oxygen concentration on tissue oxygenation during progressive haemorrhage. Intensive Care Med. 2009;35:1783–1791. doi: 10.1007/s00134-009-1577-2. [DOI] [PubMed] [Google Scholar]
- 52.Lu J, Dai G, Egi Y, Huang S, Kwon SJ, Lo EH, Kim YR. Characterization of cerebrovascular responses to hyperoxia and hypercapnia using MRI in rat. Neuroimage. 2009;45:1126–1134. doi: 10.1016/j.neuroimage.2008.11.037. [DOI] [PubMed] [Google Scholar]
- 53.Zwemer CF, Whitesall SE, D’Alecy LG. Cardiopulmonary-cerebral resuscitation with 100% oxygen exacerbates neurological dysfunction following nine minutes of normothermic cardiac arrest in dogs. Resuscitation. 1994;27:159–170. doi: 10.1016/0300-9572(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 54.Danilov CA, Fiskum G. Hyperoxia promotes astrocyte cell death after oxygen and glucose deprivation. Glia. 2008;56:801–808. doi: 10.1002/glia.20655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hofmann R, James SK, Jernberg T, Lindahl B, Erlinge D, Witt N, Arefalk G, Frick M, Alfredsson J, Nilsson L, Ravn-Fischer A, Omerovic E, Kellerth T, Sparv D, Ekelund U, Linder R, Ekstrom M, Lauermann J, Haaga U, Pernow J, Ostlund O, Herlitz J, Svensson L, Investigators DXS Oxygen Therapy in Suspected Acute Myocardial Infarction. N Engl J Med. 2017;377:1240–1249. doi: 10.1056/NEJMoa1706222. [DOI] [PubMed] [Google Scholar]
- 56.Stub D, Smith K, Bernard S, Nehme Z, Stephenson M, Bray JE, Cameron P, Barger B, Ellims AH, Taylor AJ, Meredith IT, Kaye DM, Investigators A Air Versus Oxygen in ST-Segment-Elevation Myocardial Infarction. Circulation. 2015;131:2143–2150. doi: 10.1161/CIRCULATIONAHA.114.014494. [DOI] [PubMed] [Google Scholar]
- 57.Elmer J, Scutella M, Pullalarevu R, Wang B, Vaghasia N, Trzeciak S, Rosario-Rivera BL, Guyette FX, Rittenberger JC, Dezfulian C, Pittsburgh Post-Cardiac Arrest S. The association between hyperoxia and patient outcomes after cardiac arrest: analysis of a high-resolution database. Intensive Care Med. 2015;41:49–57. doi: 10.1007/s00134-014-3555-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Janz DR, Hollenbeck RD, Pollock JS, McPherson JA, Rice TW. Hyperoxia is associated with increased mortality in patients treated with mild therapeutic hypothermia after sudden cardiac arrest. Crit Care Med. 2012;40:3135–3139. doi: 10.1097/CCM.0b013e3182656976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang CH, Chang WT, Huang CH, Tsai MS, Yu PH, Wang AY, Chen NC, Chen WJ. The effect of hyperoxia on survival following adult cardiac arrest: a systematic review and meta-analysis of observational studies. Resuscitation. 2014;85:1142–1148. doi: 10.1016/j.resuscitation.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 60.Eastwood GM, Tanaka A, Espinoza ED, Peck L, Young H, Martensson J, Zhang L, Glassford NJ, Hsiao YF, Suzuki S, Bellomo R. Conservative oxygen therapy in mechanically ventilated patients following cardiac arrest: A retrospective nested cohort study. Resuscitation. 2016;101:108–114. doi: 10.1016/j.resuscitation.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 61.Kuisma M, Boyd J, Voipio V, Alaspaa A, Roine RO, Rosenberg P. Comparison of 30 and the 100% inspired oxygen concentrations during early post-resuscitation period: a randomised controlled pilot study. Resuscitation. 2006;69:199–206. doi: 10.1016/j.resuscitation.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 62.Young P, Bailey M, Bellomo R, Bernard S, Dicker B, Freebairn R, Henderson S, Mackle D, McArthur C, McGuinness S, Smith T, Swain A, Weatherall M, Beasley R. HyperOxic Therapy OR NormOxic Therapy after out-of-hospital cardiac arrest (HOT OR NOT): a randomised controlled feasibility trial. Resuscitation. 2014;85:1686–1691. doi: 10.1016/j.resuscitation.2014.09.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


