Abstract
Aims
Calcific aortic valve stenosis (AS) is purportedly associated with less calcium burden in women than in men. We sought to examine sex-related differences and correlates of surgically excised aortic valve weight (AVW) in pure AS.
Methods and results
Clinical and echocardiographic characteristics of 888 consecutive patients who underwent aortic valve replacement for severe AS were correlated to AVW, and in 126 patients, AVW was also correlated to computed tomography aortic valve calcium (AVC) score. Women and men had similar indexed valve area (0.42 ± 0.09 vs. 0.42 ± 0.07 cm2/m2, P = 0.95) and mean systolic gradient (53 ± 15 vs. 52 ± 13 mmHg, P = 0.11), but women had higher New York Heart Association class (2.63 ± 0.70 vs. 2.50 ± 0.70, P = 0.01) and less prevalent coronary artery disease (38 vs. 52%, P < 0.0001). Aortic valve weight was lower in women (1.94 ± 0.88 vs. 3.08 ± 1.32 g, P < 0.0001) even when indexed to body surface area (1.09 ± 0.48 vs. 1.48 ± 0.62 g/m2, P < 0.0001) or left ventricular outflow tract (LVOT) area (0.54 ± 0.23 vs. 0.71 ± 0.29 g/cm2, P < 0.0001). Using multivariate analysis, male sex (P < 0.0001), bicuspid valve (P < 0.0001), and larger LVOT area (P < 0.0001) were the major determinants of increased AVW, along with current cigarette smoking (P = 0.007). Diabetes (P = 0.004) and hypertension (P = 0.03) were independently associated with lower AVW. Aortic valve calcium correlated well with AVW (r = 0.81, P < 0.0001) and was lower in women than in men (2520 ± 1199 vs. 3606 ± 1632 arbitrary units, P < 0.0001).
Conclusions
Despite the same degree of AS severity, women have less AVC and lower AVW compared with men, irrespective of valve morphology. Aortic valve calcium is correlated to excised AVW. Hypertension, diabetes, and current cigarette smoking were independently associated with AVW.
Keywords: Aortic stenosis, Sex differences, Aortic valve weight, Computed tomography
See page 700 for the editorial comment on this article (doi:10.1093/eurheartj/ehv577)
Introduction
Aortic valve stenosis (AS) is a common valve disease that increases in prevalence with ageing.1–3 The early lesion of aortic sclerosis is an active inflammatory process that involves endothelial cell disruption, lipid accumulation, and calcification and appears to share some mechanisms with atherosclerosis.4 This is followed by progressive calcium deposition, decreased cusp excursion, and stenosis, and the majority of the weight of an operatively excised valve with AS is thought to be the result of calcium.5 Sex-related differences in excised aortic valve weight (AVW) and the influence of valve morphology (unicuspid vs. bicuspid vs. tricuspid) on excised valve weight have been reported in seminal studies.5–7 However, these studies were not of pure AS and included patients with or without concomitant aortic valve regurgitation and did not account for sex differences in prevalent aortic regurgitation.
Studies of computed tomography (CT) calcium scoring have demonstrated that aortic valve calcium load correlates with AS severity,8–13 and some of these studies have suggested that women have a lower aortic valve calcium load compared with men.8,9 However, these studies did not examine how valve morphology influences the observed sex differences in CT calcium score. In addition, a small study found no differences in CT calcium scores for bicuspid and tricuspid aortic valves with severe stenosis,14 despite previous studies showing that bicuspid valves are heavier than tricuspid valves.5 Also, there are no studies to date correlating CT calcium load to excised AVW.
To address potential confounders in the observed sex differences in AVW and aortic valve calcium burden, we sought to (i) describe sex-related differences in clinical and echocardiographic characteristics of patients undergoing surgery for pure AS, (ii) determine clinical, echocardiographic, and valve morphology determinants of AVW, and (iii) correlate AVW with pre-operative aortic valve CT calcium score.
Methods
The study was approved by the Mayo Clinic Institutional Review Board. We identified consecutive patients who underwent aortic valve replacement for (i) severe AS or (ii) moderate AS and concomitant open heart surgery (e.g. coronary artery bypass grafting) at our institution between 1 January 2010 and 31 December 2012. Valve morphology (bicuspid or tricuspid) and aetiology of stenosis (degenerative or post-inflammatory) were determined by gross inspection of the excised valve by experienced cardiac pathologists (J.J.M. and W.D.E.). Following surgical excision and inspection, the valves were fixed in 10% neutral buffered formalin and stored in the institutional tissue registry. For the purpose of this study, between July 2013 and December 2013, valves were removed from storage, blotted dry, and weighed to the nearest 0.01 g. In addition, 17 grossly normal aortic valves were excised at autopsy from individuals with no cardiac disease and in whom permission had been obtained from the legal next-of-kin for the use of this tissue for research; these cases served as the control group, and the valves were weighed in the same fashion. Aortic valve weight was indexed by dividing the weight by body surface area or by estimated left ventricular outflow tract (LVOT) area as measured by two-dimensional echocardiography.
We excluded patients with coexistent moderate or more aortic regurgitation, a history of aortic valve endocarditis, prior balloon valvuloplasty, or hypertrophic obstructive cardiomyopathy. One hundred and seventy patients were excluded because they were not included in the institutional tissue registry or had incomplete valves. Only three unicommissural or unicuspid valves were excised and they were eliminated from the analysis. We excluded patients who were determined to have post-inflammatory AS (post-radiation or rheumatic) based on gross pathology.
A total of 998 patients with moderate (n = 110) or severe (n = 888) AS met inclusion criteria. Excised valve weights were compared between subjects with moderate vs. severe AS. However, subsequent analyses on clinical and echocardiographic correlates of excised valve weight, as well as correlation of excised valve weight to aortic valve CT calcium score, were performed only in patients with severe AS. Seventy-seven of the 888 patients with severe AS underwent concomitant mitral valve surgery.
All patients had a comprehensive transthoracic echocardiogram within 1 year of their surgical date (82% within 2 months, 96.7% within 4 months, and 99.5% within 6 months). The echocardiograms were performed at the Mayo Clinic, Rochester, MN, in accordance with American Society of Echocardiography (ASE) and European Association of Echocardiography (EAE) guidelines for the evaluation of cardiac chamber size and function and AS severity.15–17 The LVOT diameter was measured from the parasternal long axis zoomed view during early systole at the tissue blood interface at the insertion points of the coronary cusps to the septum and aortic–mitral intervalvular fibrosa. Aortic valve area was calculated using the continuity equation, and multiple imaging windows (e.g. apical, right parasternal) were interrogated to determine the peak aortic jet velocity. Indexed aortic valve area was calculated by dividing the aortic valve area by body surface area.
Patients with an aortic valve systolic mean Doppler gradient ≥40 mmHg or with an aortic valve area ≤1.0 cm2 were considered to have severe AS, whereas those with a valve area >1.0–1.5 cm2 and a mean gradient 25 to <40 mmHg were designated as moderate AS.18 Left ventricular mass index and relative wall thickness were calculated according to ASE/EAE guidelines.15 Systemic arterial compliance and valvulo-arterial impedance (an estimate of global left ventricular afterload) at the time of pre-operative transthoracic echocardiogram were calculated as follows:
A subset of 126 patients with severe AS had pre-operative non-contrast electrocardiogram-gated cardiac CT examinations obtained as part of their clinical evaluation. The aortic valve calcium score was calculated in this subset of patients. Calcification was defined as at least four contiguous pixels with a CT density of 130 Hounsfield units or greater using the method described by Agatston et al.19 and is reported in arbitrary units (AU). The calcification score was calculated using commercially available software (Syngo MMWP; Siemens AG, Berlin, Germany).
All patients underwent pre-operative coronary angiography. Significant coronary artery disease was considered present when there was luminal narrowing of ≥50% involving the left main or ≥70% involving the left anterior descending, intermediate, left circumflex, or right coronary arteries.
Statistical analysis was performed with JMP 9.0.1 (SAS, Cary, NC, USA). A P-value <0.05 was considered significant. Data were displayed as mean ± standard deviation or standard error, where appropriate. Continuous variables were tested for normality using the Shapiro–Wilk test. Valve weight, CT calcium score, aortic valve area, mean gradient, creatinine, and BMI were not normally distributed. Normally distributed continuous variables were compared with the use of two-tailed t-tests, while non-normally distributed continuous variables were compared using the Wilcoxon rank sum test. Nominal variables were compared using contingency tables and χ2 analysis. The association between valve weight and CT calcium scoring was measured using Pearson correlation. We used standard least squares regression for multivariate modelling of AVW. Variables with a P-value <0.05 on univariate analysis were included in the multivariate analysis.
Results
Baseline sex-related demographics and clinical characteristics are shown in Table 1. Of the 888 subjects, 63% were male, and mean age was 74 ± 10 years. On average, women were slightly older (75 ± 10 vs. 73 ± 10 years, P = 0.002), had smaller body surface area (1.79 ± 0.21 vs. 2.09 ± 0.20 m2, P < 0.0001), and were less likely to have coronary artery disease (38 vs. 52%, P = <0.0001) and prior coronary artery bypass graft surgery (6 vs. 15%, P < 0.0001) compared with men. Overall, women had a higher mean New York Heart Association (NYHA) functional class compared with men (2.63 ± 0.70 vs. 2.50 ± 0.70, P = 0.01) and were more likely to present with class 3–4 symptomatology (59 vs. 52%, P = 0.03).
Table 1.
Patient clinical characteristics
| All patients (n = 888) | Male (n = 559) | Female (n = 329) | P-value | |
|---|---|---|---|---|
| Age (years) | 74 ± 10 | 73 ± 10 | 75 ± 10 | 0.002 |
| Body surface area (m2) | 1.98 ± 0.25 | 2.09 ± 0.20 | 1.79 ± 0.21 | <0.0001 |
| Body mass index (m2/kg) | 30.2 ± 6.2 | 30.2 ± 5.6 | 30.2 ± 7.1 | 0.99 |
| Hypertension (%) | 680 (77) | 419 (75) | 261 (80) | 0.12 |
| Hyperlipidaemia (%) | 771 (87) | 494 (88) | 277 (84) | 0.10 |
| Diabetes mellitus (%) | 259 (29) | 164 (29) | 95 (29) | 0.94 |
| Tobacco use (%) | 49 (6) | 35 (6) | 14 (4) | 0.23 |
| Chronic lung disease (%) | 63 (7) | 47 (8) | 16 (5) | 0.06 |
| Coronary artery disease (%) | 417 (47) | 291 (52) | 126 (38) | <0.0001 |
| Prior myocardial infarction (%) | 125 (14) | 88 (16) | 37 (11) | 0.08 |
| Prior coronary artery bypass (%) | 103 (12) | 84 (15) | 19 (6) | <0.0001 |
| NYHA class | 2.55 ± 0.70 | 2.50 ± 0.70 | 2.63 ± 0.70 | 0.01 |
| Class 1 (%) | 47 (5) | 34 (6) | 13 (4) | |
| Class 2 (%) | 367 (41) | 243 (43) | 124 (38) | |
| Class 3 (%) | 410 (46) | 248 (44) | 162 (49) | |
| Class 4 (%) | 63 (7) | 34 (6) | 29 (9) |
NYHA, New York Heart Association.
Sex-related echocardiographic characteristics are shown in Table 2. Women and men had similar indexed aortic valve area (0.42 ± 0.09 vs. 0.42 ± 0.07 cm2/m2, P = 0.95) and aortic valve systolic mean Doppler gradient (53 ± 15 vs. 52 ± 15 mmHg, P = 0.11). Women had a lower left ventricular end-diastolic diameter (47 ± 6 vs. 52 ± 7 mm, P < 0.0001), lower left ventricular mass index (116 ± 30 vs. 127 ± 33 g/m2, P < 0.0001), and similar relative wall thickness (0.48 ± 0.10 vs. 0.48 ± 0.10, P = 0.62) compared with men. Women were more likely than men to have an E/e′ ≥15 (70 vs. 56%, P = 0.0002), but left atrial volume index was similarly increased in women and men (43 ± 14 vs. 43 ± 16 mL/m2, P = 0.70). Women had higher left ventricular ejection fraction (63 ± 10 vs. 60 ± 12%, P < 0.0001) but similar forward stroke volume index (48 ± 9 vs. 47 ± 9, P = 0.10), non-invasively measured systemic arterial compliance (0.90 ± 0.34 vs. 0.92 ± 0.32, P = 0.35), and valvulo-arterial impedance (3.92 ± 0.90 vs. 3.90 ± 0.79, P = 0.69), compared with men.
Table 2.
Pre-operative echocardiographic characteristics
| All patients (n = 888) | Male (n = 559) | Female (n = 329) | P-value | |
|---|---|---|---|---|
| Ejection fraction (%) | 61 ± 12 | 60 ± 12 | 63 ± 10 | <0.0001 |
| Ejection fraction <50% (%) | 108 (12) | 81 (15) | 27 (8) | 0.006 |
| LVEDD (mm) | 50 ± 7 | 52 ± 7 | 47 ± 6 | <0.0001 |
| LV mass index | 123 ± 32 | 127 ± 33 | 116 ± 30 | <0.0001 |
| Relative wall thickness | 0.48 ± 0.10 | 0.48 ± 0.10 | 0.48 ± 0.10 | 0.66 |
| Stroke volume index (mL/m2) | 47 ± 9 | 47 ± 9 | 48 ± 9 | 0.10 |
| Aortic valve area (cm2) | 0.83 ± 0.16 | 0.88 ± 0.15 | 0.75 ± 0.15 | <0.0001 |
| Aortic valve area index (cm2/m2) | 0.42 ± 0.08 | 0.42 ± 0.07 | 0.42 ± 0.09 | 0.95 |
| Mean gradient (mmHg) | 52 ± 14 | 52 ± 13 | 53 ± 15 | 0.11 |
| LVOT diameter (cm) | 2.3 ± 0.2 | 2.3 ± 0.2 | 2.1 ± 0.1 | <0.0001 |
| LVOT area (cm2) (2D echo) | 4.03 ± 0.73 | 4.32 ± 0.69 | 3.53 ± 0.49 | <0.0001 |
| LAVI (mL/m2) | 43 ± 15 | 43 ± 16 | 43 ± 14 | 0.70 |
| E/e′ ≥15 (%) | 473 (61) | 280 (56) | 193 (70) | 0.0002 |
| Diastolic function | 0.06 | |||
| Normal (%) | 147 (17) | 100 (18) | 47 (14) | |
| Grade 1 (%) | 282 (32) | 183 (33) | 99 (30) | |
| Grade 2 (%) | 183 (21) | 102 (18) | 81 (25) | |
| Grade 3–4 (%) | 75 (8) | 54 (10) | 21 (6) | |
| Indeterminate (%) | 201 (23) | 120 (21) | 81 (25) | |
| RVSP >40 mmHg (%) | 237 (32) | 144 (32) | 93 (33) | 0.87 |
| Systemic compliance | 0.91 ± 0.32 | 0.92 ± 0.32 | 0.90 ± 0.34 | 0.35 |
| Valvulo-arterial impedance | 3.90 ± 0.83 | 3.90 ± 0.79 | 3.92 ± 0.90 | 0.69 |
LVEDD, left ventricular end-diastolic diameter; LV, left ventricular; LVOT, left ventricular outflow tract; LAVI, left atrial volume index; E, early mitral inflow; e′, early diastolic mitral annular velocity; RVSP, right ventricular systolic pressure.
Sex-related valve morphologic characteristics and weight of excised aortic valves are shown in Table 3. Severely stenotic valves were on average approximately five times heavier than control valves (2.66 ± 1.30 vs. 0.50 ± 0.13 g, P < 0.0001) and ranged in weight from 0.43 to 8.92 g (Figure 1) and were heavier than valves with moderate stenosis (2.63 ± 1.29 vs. 2.09 ± 1.08 g, P < 0.0001). There were 32 valves (3.6%) that weighed <1 g. Subjects with a valve weight <1 g had similar indexed aortic valve area (0.43 ± 0.07 vs. 0.42 ± 0.08 cm2/m2, P = 0.24), but lower mean transvalvular gradient (44 ± 11 vs. 53 ± 14 mmHg, P < 0.0001). These individuals were also more likely to be female (89 vs. 35%, P < 0.0001) with a tricuspid valve (92 vs. 72%, P = 0.007) and had smaller LVOT diameter (2.1 ± 0.1 vs. 2.3 ± 0.2 cm, P < 0.0001).
Table 3.
Sex differences in valve weight after stratification by valve morphology and correcting for body surface area and left ventricular outflow tract area
| Male | Female | P-value | |
|---|---|---|---|
| All patients | |||
| Valve morphology | 0.08 | ||
| Bicuspid (%) | 165 (30) | 79 (24) | |
| Tricuspid (%) | 394 (70) | 250 (76) | |
| Valve weight (g) | 3.08 ± 1.32 | 1.94 ± 0.88 | <0.0001 |
| Valve weight indexed to BSA (g/m2) | 1.48 ± 0.62 | 1.09 ± 0.48 | <0.0001 |
| Valve weight indexed to LVOT area (g/cm2) | 0.71 ± 0.29 | 0.54 ± 0.23 | <0.0001 |
| Bicuspid aortic valves only | |||
| Valve weight (g) | 3.89 ± 1.44 | 2.39 ± 1.01 | <0.0001 |
| Valve weight indexed to BSA (g/m2) | 1.84 ± 0.66 | 1.33 ± 0.53 | <0.0001 |
| Valve weight indexed to LVOT area (g/cm2) | 0.85 ± 0.32 | 0.62 ± 0.24 | <0.0001 |
| Tricuspid aortic valves only | |||
| Valve weight (g) | 2.75 ± 1.10 | 1.79 ± 0.78 | <0.0001 |
| Valve weight indexed to BSA (g/m2) | 1.33 ± 0.53 | 1.01 ± 0.44 | <0.0001 |
| Valve weight indexed to LVOT area (g/cm2) | 0.66 ± 0.25 | 0.52 ± 0.23 | <0.0001 |
BSA, body surface area; LVOT, left ventricular outflow tract.
Figure 1.
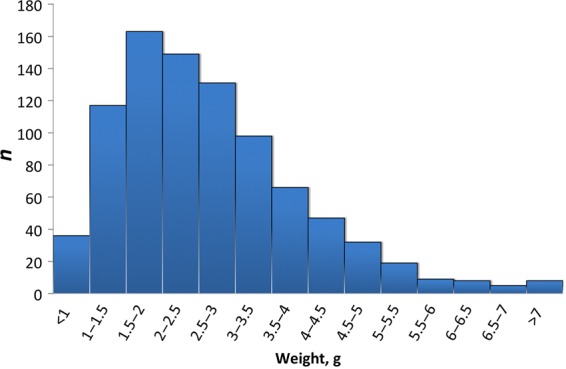
Valve weights in patients with severe stenosis. Mean valve weight was 2.66 ± 1.30 g with considerable range (0.43–8.92 g).
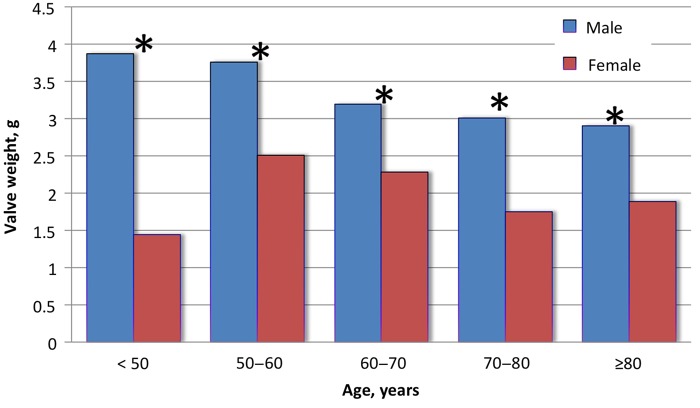
Mean valve weight was greater in men compared with women (3.08 ± 1.32 vs. 1.94 ± 0.88 g, P < 0.0001) (Figure 2). Mean valve weight was increased in bicuspid vs. tricuspid aortic valves (3.41 ± 1.49 vs. 2.38 ± 1.09 g, P < 0.0001), but men had heavier valves even after stratification into bicuspid (3.89 ± 1.44 vs. 2.40 ± 1.01 g, P < 0.0001; Table 3) and tricuspid aortic valves (2.75 ± 1.10 vs. 1.79 ± 0.78 g, P < 0.0001; Table 3). The prevalence of bicuspid valve was not different between men and women (30 vs. 24%, P = 0.09).
Figure 2.
Sex-related valve weights according to decade. Males had heavier valves than females during every decade of life. The largest magnitude of difference was seen in young patients <50 years of age. (*P-value <0.001)
Left ventricular outflow tract size and body surface area correlated positively with valve weight (R = 0.51 and 0.37, respectively; P < 0.0001 for both). However, men had consistently heavier valves after indexing to body surface area (all valves: 1.49 ± 0.62 vs. 1.09 ± 0.48 g/m2, P < 0.0001; bicuspid valves: 1.84 ± 0.66 vs. 1.33 ± 0.53 g/m2, P < 0.0001; tricuspid valves: 1.33 ± 0.53 vs. 1.01 ± 0.44 g/m2, P < 0.0001) and LVOT area (all valves: 0.71 ± 0.29 vs. 0.55 ± 0.23 g/cm2, P < 0.0001; bicuspid valves: 0.85 ± 0.32 vs. 0.62 ± 0.24 g/cm2, P < 0.0001; tricuspid valves: 0.66 ± 0.25 vs. 0.52 ± 0.23 g/cm2, P < 0.0001) (Table 3). There was not a statistically significant correlation between body mass index and valve weight.
There were multiple univariate correlates, but on multivariate analysis, age, sex, valve morphology, LVOT area, current cigarette use, diabetes, and hypertension remained independent predictors of AVW (Table 4). Older age was associated with increased valve weight, but the effect size was only 0.08 g per decade, which corresponds to only 3% of the average weight of a stenotic valve. Diabetes and hypertension showed an independent negative association with valve weight. Subjects with diabetes had a slightly larger aortic valve area (0.85 ± 0.17 vs. 0.83 ± 0.16 cm2, P = 0.04) and lower mean transvalvular gradient (50 ± 12 vs. 53 ± 14 mmHg, P = 0.03) but similar mean NYHA class (2.61 ± 0.70 vs. 2.53 ± 0.71, P = 0.11) compared with those without diabetes. Diabetics also had decreased systemic compliance (0.84 ± 0.29 vs. 0.94 ± 0.33, P < 0.0001) and increased valvulo-arterial impedance (4.06 ± 0.85 vs. 3.84 ± 0.81, P = 0.004). Subjects with hypertension had a similar valve area (0.84 ± 0.16 vs. 0.82 ± 0.17 cm2, P = 0.33) but slightly lower mean gradient (52 ± 14 vs. 54 ± 14 mmHg, P = 0.02) and worse NYHA class (2.59 ± 0.71 vs. 2.41 ± 0.67, P = 0.0007), lower systemic compliance (0.89 ± 0.31 vs. 0.99 ± 0.36, P = 0.0003) but similar valvulo-arterial impedance to subjects without hypertension (3.93 ± 0.83 vs. 3.83 ± 0.82, P = 0.15).
Table 4.
Univariate and multivariate regression of aortic valve weight (r2 = 0.37)
| Valve weight (g), univariate
analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| Term | Yes | No | P-value | Term | Estimate | P-value |
| Male | 3.1 ± 1.3 | 1.9 ± 0.9 | <0.0001 | Male | 0.34 | <0.0001 |
| Age >70 | 2.5 ± 1.2 | 3.0 ± 1.4 | <0.0001 | Age (per decade)a | 0.08 | 0.05 |
| Bicuspid valve | 3.4 ± 1.5 | 2.4 ± 1.1 | <0.0001 | Bicuspid valve | 0.35 | <0.0001 |
| Cigarette smoker | 3.3 ± 1.5 | 2.6 ± 1.3 | 0.003 | Cigarette smoker | 0.21 | 0.007 |
| Diabetes | 2.4 ± 1.1 | 2.8 ± 1.3 | <0.0001 | Diabetes | −0.11 | 0.004 |
| Hypertension | 2.5 ± 1.2 | 3.1 ± 1.5 | <0.0001 | Hypertension | −0.09 | 0.03 |
| Dyslipidaemia | 2.6 ± 1.3 | 2.9 ± 1.5 | 0.03 | Dyslipidaemia | −0.10 | 0.07 |
| CAD | 2.6 ± 1.2 | 2.8 ± 1.4 | 0.02 | CAD | 0.04 | 0.31 |
| Dialysis | 2.2 ± 1.2 | 2.6 ± 1.3 | 0.36 | |||
| LVOT diameter ≥2.3 cm | 3.2 ± 1.3 | 2.1 ± 1.0 | <0.0001 | LVOT diametera (per mm increase) | 0.21 | <0.0001 |
| BSA >2.0 | 3.1 ± 1.3 | 2.3 ± 1.2 | <0.0001 | |||
CAD, coronary artery disease; LVOT, left ventricular outflow tract; BSA, body surface area.
aIncluded as a continuous variable in the multivariate analysis.
A subset of 126 patients (78 men and 48 women) had CT calcium scoring within 6 months of surgery (mean: 42 ± 40 days). They were slightly older (77 ± 9 vs. 73 ± 10 years, P < 0.0001) and had worse stenosis by aortic valve area (0.77 ± 0.15 vs. 0.84 ± 0.16 cm2, P < 0.0001) and mean systolic gradient (55 ± 15 vs. 52 ± 13 mmHg, P = 0.04) compared with patients without CT scan. Otherwise, the two groups were similar. Among subjects who underwent CT scan, women had similar indexed valve area (0.39 ± 0.09 vs. 0.40 ± 0.06 cm2, P = 0.75) and mean transvalvular gradient (58 ± 18 vs. 53 ± 13 mmHg, P = 0.08) compared with men. This group included 100 subjects with tricuspid valves and 26 subjects with bicuspid valves. Calcium scores ranged from 59 to 11 000 AU (mean: 3192 ± 1569, median: 2859 AU), and mean calcium score was significantly lower in women compared with men (2520 ± 1199 vs. 3606 ± 1632 AU, P < 0.0001), even when stratified into tricuspid (2619 ± 1199 vs. 3517 ± 1397 AU, P = 0.0009) and bicuspid (1938 ± 1107 vs. 3886 ± 2237 AU, P = 0.008) valve groups separately. Correlation between CT calcium scoring and excised valve weight was good (r = 0.81, P < 0.0001; Figure 3). The ratio of CT calcium to valve weight was similar in women and men (1187 ± 591 vs. 1178 ± 379 AU/g, P = 0.93), suggesting a similar volume of calcification per gram of valve weight.
Figure 3.
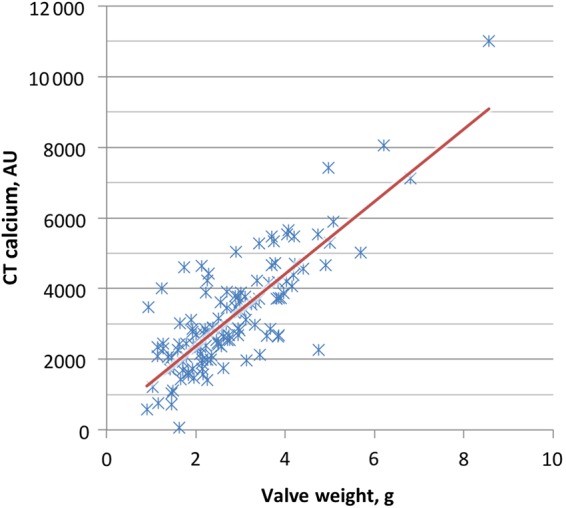
Valve weight vs. computed tomography calcium score. A good correlation was seen between post-operative valve weight and computed tomography calcium score in 126 patients with computed tomography calcium scoring within 6 months of surgery (R = 0.81, P < 0.0001).
Discussion
This study is among the largest contemporary cohorts examined to determine sex-related differences in the clinical profile of patients undergoing surgical aortic valve replacement for pure AS. It is also the first study to correlate CT aortic valve calcium score to excised AVW. Our cohort was older, with a high burden of comorbidities at the time of surgery, compared with previous studies,5,20–23 reflecting the changing profile of patients needing aortic valve surgery for AS. There were some differences in the clinical profile but not severity of AS between men and women, where women were slightly more symptomatic at the time of aortic valve replacement with more prevalent diastolic dysfunction, but much less coronary artery disease. There was no sex-related difference in the prevalence of bicuspid valves in our cohort likely related to the exclusion of patients with coexistent moderate or severe regurgitation since males are more likely to suffer from regurgitation in the setting of a bicuspid valve.5,24 Also, this may explain the very few unicommissural or unicuspid valves in our cohort, which were excluded from the analysis.
Valve weight correlated well with CT calcium scoring in the 126 patients who had CT calcium scoring within 6 months of surgery. To our knowledge, there are no prior studies evaluating the relationship between gross post-operative valve weight and CT calcium score. One previous study showed an excellent relationship between CT calcium score and aortic valve calcium weight after removal of soft tissue from formalin fixed valves in a small cohort of patients.10 Similarly, we observed a strong linear correlation between CT calcium score and gross weight of the excised valve, supporting the hypothesis that excised valve weight can be used as a surrogate for valve calcification. Additionally, our data show a similar ratio of calcification to valve weight in men and women, suggesting that men may undergo a greater degree of both calcification and fibrosis for a similar severity of stenosis, compared with women. However, it is interesting to note the wide range of CT calcium score as well as that of excised valve weight in these patients with severe AS.
Previous studies have reported increased valve weight in males compared with females and have shown a correlation between body mass index and AVW,5 but larger body surface area and LVOT area have not been explored as a potential explanation for the increased valve weight observed in men. We observed a progressively increasing valve weight as LVOT size or body surface area increased consistent with prior observations in patients without aortic valve pathology that as the heart size increases, cusp area and valve weight also increase.25 On average, males have larger body surface area and LVOT size than females, which could potentially account for differences in valve weight. However, even after indexing to body surface area and LVOT size, males had consistently heavier valves than females. This observation remained consistent in patients with tricuspid and bicuspid aortic valves separately.
The severity of AS at the time of surgery does not explain sex-related differences in excised AVW since the indexed valve area and mean gradient were similar in men and women. Despite this similar degree of stenosis, valve weight is on average 59% higher in men compared with women. Interestingly, hypertension and diabetes were associated with decreased valve weight, while current tobacco use was associated with increased valve weight.
Further studies examining molecular mechanisms underlying sex-related differences in the development and progression of AS are needed since a better understanding of these mechanisms could ultimately lead to medical therapy that could prevent or reverse AS.
Limitations
This is a retrospective study. Valves were stored in 10% neutral buffered formalin prior to being weighed. However, all valves were stored for a similar length of time, and potential error introduced would be systematic and not influence the observed sex-related correlates of excised valve weight. Not all patients had CT calcium scoring introducing potential for bias within this subset. Patients for whom only a partial valve was received by the pathologist were excluded from the analysis. However, it is possible even in intact valves that some calcific debris was lost in the process of valve excision. Reassuring was the fact that the weight of the excised valve did correlate with severity of stenosis where moderately stenotic valves weighed less than severely stenotic valves. Lastly, the underlying histologic determinants of the sex-related difference in the weight of the calcium deposition and weight of excised aortic valve are beyond the scope of the current study.
Authors' contributions
J.J.T. and V.T.N. performed statistical analysis; V.T.N., J.J.M., W.D.E., and M.E.-S. handled funding and supervision; J.J.T., V.T.N., R.M.S., J.J.M., D.J.S., S.V.P., J.F.M., T.A.F., J.K.O., W.D.E., and M.E.-S. acquired the data; V.T.N., R.M.S., J.J.M., W.D.E., and M.E.-S. conceived and designed the research; J.J.T., V.T.N., R.M.S., J.J.M., M.-A.C., W.D.E., and M.E.-S. drafted the manuscript; and J.J.T., V.T.N., R.M.S., J.J.M., M.-A.C., S.V.P., J.F.M., T.A.F., J.K.O., J.D.M., W.D.E., and M.E.-S. made critical revision of the manuscript for key intellectual content.
Conflict of interest: none declared.
References
- 1. Lin SL, Liu CP, Young ST, Lin M, Chiou CW. Age-related changes in aortic valve with emphasis on the relation between pressure loading and thickened leaflets of the aortic valves. Int J Cardiol 2005;103:272–279. [DOI] [PubMed] [Google Scholar]
- 2. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005–1011. [DOI] [PubMed] [Google Scholar]
- 3. Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, Kitzman DW, Otto CM. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol 1997;29:630–634. [DOI] [PubMed] [Google Scholar]
- 4. Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O'Brien KD. Characterization of the early lesion of ‘degenerative’ valvular aortic stenosis. Histological and immunohistochemical studies. Circulation 1994;90:844–853. [DOI] [PubMed] [Google Scholar]
- 5. Roberts WC, Ko JM. Weights of operatively-excised stenotic unicuspid, bicuspid, and tricuspid aortic valves and their relation to age, sex, body mass index, and presence or absence of concomitant coronary artery bypass grafting. Am J Cardiol 2003;92:1057–1065. [DOI] [PubMed] [Google Scholar]
- 6. Roberts WC, Ko JM. Relation of weights of operatively excised stenotic aortic valves to preoperative transvalvular peak systolic pressure gradients and to calculated aortic valve areas. J Am Coll Cardiol 2004;44:1847–1855. [DOI] [PubMed] [Google Scholar]
- 7. Roberts WC, Ko JM, Hamilton C. Comparison of valve structure, valve weight, and severity of the valve obstruction in 1849 patients having isolated aortic valve replacement for aortic valve stenosis (with or without associated aortic regurgitation) studied at 3 different medical centers in 2 different time periods. Circulation 2005;112:3919–3929. [DOI] [PubMed] [Google Scholar]
- 8. Aggarwal SR, Clavel MA, Messika-Zeitoun D, Cueff C, Malouf J, Araoz PA, Mankad R, Michelena H, Vahanian A, Enriquez-Sarano M. Sex differences in aortic valve calcification measured by multidetector computed tomography in aortic stenosis. Circ Cardiovasc Imaging 2013;6:40–47. [DOI] [PubMed] [Google Scholar]
- 9. Clavel MA, Messika-Zeitoun D, Pibarot P, Aggarwal SR, Malouf J, Araoz PA, Michelena HI, Cueff C, Larose E, Capoulade R, Vahanian A, Enriquez-Sarano M. The complex nature of discordant severe calcified aortic valve disease grading: new insights from combined Doppler echocardiographic and computed tomographic study. J Am Coll Cardiol 2013;62:2329–2338. [DOI] [PubMed] [Google Scholar]
- 10. Messika-Zeitoun D, Aubry MC, Detaint D, Bielak LF, Peyser PA, Sheedy PF, Turner ST, Breen JF, Scott C, Tajik AJ, Enriquez-Sarano M. Evaluation and clinical implications of aortic valve calcification measured by electron-beam computed tomography. Circulation 2004;110:356–362. [DOI] [PubMed] [Google Scholar]
- 11. Shavelle DM, Budoff MJ, Buljubasic N, Wu AH, Takasu J, Rosales J, Otto CM, Zhao XQ, O'Brien KD. Usefulness of aortic valve calcium scores by electron beam computed tomography as a marker for aortic stenosis. Am J Cardiol 2003;92:349–353. [DOI] [PubMed] [Google Scholar]
- 12. Kizer JR, Gefter WB, deLemos AS, Scoll BJ, Wolfe ML, Mohler ER III. Electron beam computed tomography for the quantification of aortic valvular calcification. J Heart Valve Dis 2001;10:361–366. [PubMed] [Google Scholar]
- 13. Kaden JJ, Freyer S, Weisser G, Willingstorfer W, Bilbal A, Pfleger S, Suselbeck T, Haase KK, Dempfle CE, Borggrefe M. Correlation of degree of aortic valve stenosis by Doppler echocardiogram to quantity of calcium in the valve by electron beam tomography. Am J Cardiol 2002;90:554–557. [DOI] [PubMed] [Google Scholar]
- 14. Ferda J, Linhartova K, Kreuzberg B. Comparison of the aortic valve calcium content in the bicuspid and tricuspid stenotic aortic valve using non-enhanced 64-detector-row-computed tomography with prospective ECG-triggering. Eur J Radiol 2008;68:471–475. [DOI] [PubMed] [Google Scholar]
- 15. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 16. Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, Iung B, Otto CM, Pellikka PA, Quinones M. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. Eur J Echocardiogr 2009;10:1–25. [DOI] [PubMed] [Google Scholar]
- 17. Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 2009;22:107–133. [DOI] [PubMed] [Google Scholar]
- 18. Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Baron-Esquivias G, Baumgartner H, Borger MA, Carrel TP, De Bonis M, Evangelista A, Falk V, Iung B, Lancellotti P, Pierard L, Price S, Schafers HJ, Schuler G, Stepinska J, Swedberg K, Takkenberg J, Von Oppell UO, Windecker S, Zamorano JL, Zembala M. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012;33:2451–2496. [DOI] [PubMed] [Google Scholar]
- 19. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15:827–832. [DOI] [PubMed] [Google Scholar]
- 20. Kulik A, Lam BK, Rubens FD, Hendry PJ, Masters RG, Goldstein W, Bedard P, Mesana TG, Ruel M. Gender differences in the long-term outcomes after valve replacement surgery. Heart 2009;95:318–326. [DOI] [PubMed] [Google Scholar]
- 21. Bech-Hanssen O, Wallentin I, Houltz E, Beckman Suurkula M, Larsson S, Caidahl K. Gender differences in patients with severe aortic stenosis: impact on preoperative left ventricular geometry and function, as well as early postoperative morbidity and mortality. Eur J Cardiothorac Surg 1999;15:24–30. [DOI] [PubMed] [Google Scholar]
- 22. Fuchs C, Mascherbauer J, Rosenhek R, Pernicka E, Klaar U, Scholten C, Heger M, Wollenek G, Czerny M, Maurer G, Baumgartner H. Gender differences in clinical presentation and surgical outcome of aortic stenosis. Heart 2010;96:539–545. [DOI] [PubMed] [Google Scholar]
- 23. Rosenhek R, Binder T, Porenta G, Lang I, Christ G, Schemper M, Maurer G, Baumgartner H. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med 2000;343:611–617. [DOI] [PubMed] [Google Scholar]
- 24. Roberts WC, Morrow AG, McIntosh CL, Jones M, Epstein SE. Congenitally bicuspid aortic valve causing severe, pure aortic regurgitation without superimposed infective endocarditis. Analysis of 13 patients requiring aortic valve replacement. Am J Cardiol 1981;47:206–209. [DOI] [PubMed] [Google Scholar]
- 25. Silver MA, Roberts WC. Detailed anatomy of the normally functioning aortic valve in hearts of normal and increased weight. Am J Cardiol 1985;55:454–461. [DOI] [PubMed] [Google Scholar]