Abstract
Despite the use of surgical resection and aggressive chemotherapy, nearly 50% of patients with colorectal carcinoma develop recurrent disease, highlighting the need for improved therapies. Curcumin (diferuloylmethane), the major active ingredient of turmeric (curcuma longa) with no discernable toxicity, has been shown to inhibit the growth of transformed cells and colon carcinogenesis at the initiation, promotion, and progression stages in carcinogen-induced rodent models. In a Phase I clinical trial, curcumin has been found to be extremely well tolerated and effective. In this review, we summarized the current status of our knowledge about the effectiveness of curcumin when given in combination with current chemotherapeutics such as 5-fluorouracil, oxaliplatin, and gemcitabine in treatment of gastrointestinal cancers with particular reference to colorectal cancer. Existing data suggest that curcumin in combination with chemotherapy is a superior strategy for treatment of gastrointestinal cancer.
INTRODUCTION
Colorectal cancer is the third most common cancer in both men and women, constituting 10% of new cancer cases in men and 11% in women (1). It is the second most common cause of death from cancer in the United States and other developed countries. Surgery and subsequent chemotherapy can cure over 75% of colon cancer patients, but more than 30% of these patients develop new neoplastic polyps, and 10% progress to frank second malignancy (2–4). The risk of second malignancy is higher for microsatelite instable tumor (5). Metastatic colorectal cancer has poor prognosis, with 5-yr survival of less than 10% (1). As a result of great efforts being made on improving chemotherapeutic interventions for metastatic colon cancer, the median survival has improved to over 20 mo in this group of patients (6). However, this comes at a cost of additional toxicities, some of which are even fatal. The validation of a nontoxic agent that could improve on the current chemotherapeutic regimen would therefore be highly desirable. Curcumin (diferuloylmethane), the major active ingredient of turmeric, derived from the dried roots of Curcuma longa with no discernable toxicity, could be one such agent. This review summarizes some of the most relevant information on utilization curcumin with and without current therapeutics in the treatment of colorectal cancer.
CURCUMIN IN COLORECTAL CANCER
In its pure crystalline state, curcumin is a diferuloylmethane existing in a stable enol form under alkaline conditions. Its chemical formula is I,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione. Besides curcumin, turmeric powder contains other chemical constituents known as the curcuminoids, composed of demethoxycurcumin, bisdemethoxycurcumin, and cyclocurcumin (7,8). Curcumin, which is used as a coloring and flavoring additive in many South Asian cuisines, has been shown to inhibit the growth of transformed cells (9,10) and colon carcinogenesis at the initiation, promotion, and progression stages in carcinogen-induced rodent models (11–13). Curcumin has been found to inhibit chemically induced carcinogenesis in the skin, fore stomach, and colon when administered during initiation and/or postinitiation phases (14–17). Development of azoxymethane induced preneoplastic and neoplastic lesions of the colon are also inhibited in experimental animals fed a diet containing 0.2–1.6% curcumin (18,19). In addition, curcumin has been reported to prevent adenoma development in the intestinal tract of Min/+ mice, a model of human familial adenomatous polyposis (20). In a Phase I clinical trial, curcumin was shown to be effective in inhibiting tumor growth (21).
In addition to the possible direct/indirect effects of curcumin at the preinitiation and initiation stages (22–24), curcumin has been shown to affect the postinitiation and progression stages of colon carcinogenesis. Several in vivo and in vitro studies, including our own, have demonstrated that curcumin treatment inhibits cyclooxygenase-2 (COX-2) expression and activity, leading to a reduction in prostaglandin synthesis and loss of cancer cell growth (25–27). However, curcumin was also shown to be an effective inhibitor of cell growth in prostaglandin-synthesis deficient cancer cells (HCT-15), suggesting that curcumin may act via prostaglandin independent pathways (28).
Since metabolism and absorption of dietary agents can significantly impact their biological efficacy, studies have been performed to determine the biological availability of curcumin. Most preclinical and clinical studies have demonstrated a poor oral bioavailability of curcumin. On oral administered curcumin undergoes extensive first pass metabolism in the liver by conjugation to glucuronide and sulphate. Curcumin and its metabolites were measured by HPLC in the tissues, plasma, and feces in Min+/− mice after long-term ingestion of dietary curcumin (0.2%–0.5%) in doses that reduced adenoma multiplicity by about 40% (20,29). Most of the ingested curcumin was present in the feces as authentic curcumin sulfate, 20–25% was measured in the colonic mucosa, and 5–10% was present in the mucosa of small intestine. In mice fed 0.2% curcumin, 100 pm/g of curcumin was found in the liver, approximately 0.001% of that measured in the intestinal mucosa (20). After termination of dietary curcumin intake, tissue curcumin levels declined rapidly to unquantifiable amounts (within 3–6 h), whereas fecal levels declined more slowly (with a 23-h half-life) (20). In contrast, intraperitoneal (i.p.) injection of a bolus dose of 14C-curcumin produced a 10-fold higher concentration in the intestinal mucosa than in the plasma; the half-life of ip administered curcumin was also highest in the intestinal mucosa (approximately 8 h) compared to all other organs (2–4 h). These studies have suggested that orally administered curcumin may exert its inhibitory effects primarily via luminal and/or intramucosal routes (although negligible levels were absorbed into the circulation via this route) (29). Pilot studies in colon cancer patients (30,31) and results from experiments in rodents (20,32,33) also suggest that the systemic availability of curcumin is poor as a result of dietary ingestion. In spite of poor absorption into the circulation, intake of dietary curcumin reduces the growth of tumors remote from the site of absorption (34; for review see Ref. 35), and corrects biochemical defects in diseases such as cystic fibrosis (36).
The concentrations of curcumin required to elicit biochemical changes germane to chemoprevention in experiments in vitro are in the 5 to 50 μmole/l range. Hence, for the development of curcumin as a potential colorectal cancer chemopreventive agent, it is of paramount importance to establish whether intestinal levels of curcumin in this concentration range are achievable in humans who receive oral curcumin, thus potentially eliciting pharmacologic changes, which, when maintained over prolonged periods of time, might elicit chemoprevention. Concentration of curcumin in human colorectum after daily consumption of 1.8 or 3.6 g are of an order of magnitude shown to elicit pharmacologic activity in cells in vitro (37–40).
The poor systemic availability of curcumin has raised concerns about its use for the chemoprevention or treatment of malignancies remote from the site of absorption (20). However, this would not preclude its use in prevention/treatment of gastrointestinal malignancies (20), as curcumin distribution in the gastrointestinal tract is, to a great extent, independent of systemic availability.
CURCUMIN AND CHEMOTHERAPEUTICS IN COLORECTAL CANCER
Despite the use of surgical resection and aggressive chemotherapy, nearly 50% of patients with colorectal carcinoma develop recurrent disease, highlighting the need for improved therapies (1). Suffice it to mention that chemotherapy has limited efficacy in treatment of advanced gastrointestinal malignancies, which comes at a cost of significant toxicities. Resistance to chemotherapy has been partly attributed to its activation of NF-κB transcription factor leading to upregulation of various antiapoptotic genes (41–43). Therefore, improving efficacy of chemotherapeutic agents by addition of nontoxic agents such as curcumin is highly desirable. Curcumin has been shown to be synergistic with chemotherapy in inhibiting colorectal and pancreatic cancer cell growth. This synergistic effect appears to be partly due to inhibition of NF-κB and growth factor receptors.
Gemcitabine, a pyrimidine analogue, is routinely used in the treatment of pancreatic cancer. In advanced pancreatic cancer, gemcitabine produces a very modest response rate in single digits with prolongation of survival by only a few weeks. Addition of various chemotherapies to gemcitabine has only resulted in increased toxicity with limited improvement in efficacy (44). Resistance of pancreatic cancer to gemcitabine has been attributed to activation of NF-κB, leading to increased expression of cyclin D1 and vascular endothelial growth factor (VEGF) (45,46). Addition of curcumin to gemcitabine has been shown to suppress NF-κB activation in pancreatic cells, resulting in downregulation of the NF-κB-regulated gene products that inhibit apoptosis such as Bcl-2, Bcl-xL, X-linked inhibitor of apoptosis protein, and cellular inhibitor of apoptosis protein-1 (cIAP-1)] and stimulate proliferation (e.g. COX-2, cyclin D1, and c-myc), angiogenesis (VEGF and interleukin-8), and invasion [matrix metalloproteinase-9 (MMP-9)] (47). Curcumin inhibits the growth of various pancreatic cancer cells at a dose that can be physiologically achieved when administered orally in various Phase I studies (48). This growth inhibition is found to be synergistic when combined with gemcitabine (47,48). In 1 of the experiments, groups of mice were randomized to receive vehicle only, curcumin (1 g/kg) orally daily, gemcitabine (25 mg/kg) ip twice weekly, and combination of curcumin and gemcitabine. They were sacrificed on Day 35, and tumor volume was assessed. The tumor volume in the combination of curcumin and gemcitabine group was found to be significantly lower than that caused by gemcitabine alone or those treated with vehicle (control group; P < 0.05 vs. gemcitabine; P < 0.001 vs. control) (47).This was accompanied by the concomitant inhibition of COX-2, MMP-9, and ICAM-1 expression (47). In view of these observations, it is reasonable to speculate that addition of curcumin to gemcitabine could be an effective therapeutic strategy for pancreatic cancer and should be further tested in clinical trials. Additionally, constitutive activation of NF-κB has been shown to be responsible for maintaining resistance to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in various human cancer cells (49). TRAIL ligand is currently being evaluated clinical trials for treatment of variety of cancers including gastrointestinal cancers due to its selectivity for inducing apoptosis in cancer cells (50). In addition to inhibition of NF-κB, curcumin is also shown to upregulate DR-5, a receptor for TRAIL, in various cancer cells including colorectal and hepatocellular cancers (51). Thus, curcumin further sensitized TRAIL sensitive cells and reverses TRAIL resistance in various GI cancer cells. Therefore, curcumin should be explored in combination with TRAIL in clinical trials.
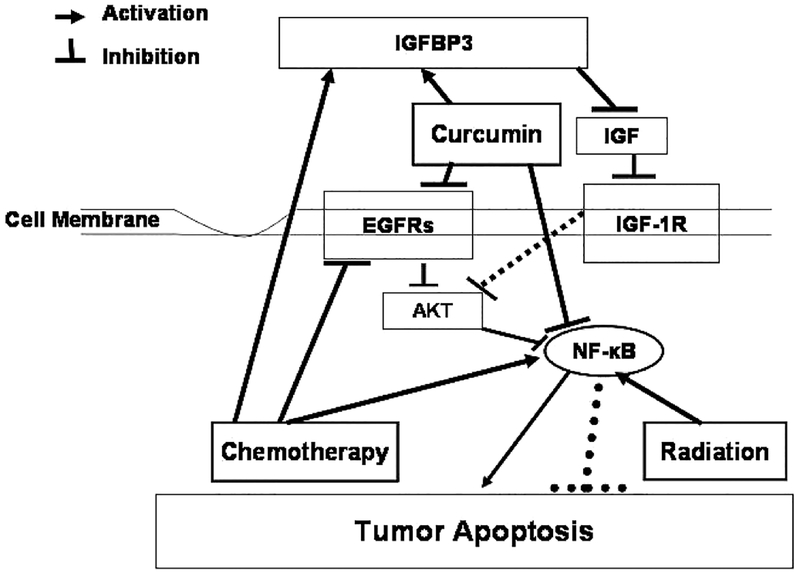
Combination of 5-fluorouracil and oxaliplatin (FOLFOX) forms the backbone of colorectal cancer chemotherapeutics. One of the important mechanisms of chemotherapy induced growth inhibition of colon cancer growth is its ability to down-regulate the expression and activation of growth factor receptors, specifically EGF-receptor (EGFR) and its family members (referred to as EGFRs) as well as insulin like-growth factor (IGF)-1-receptor (IGF-1R). It is becoming increasingly evident that the development and progression of many malignancies, including colorectal cancer, are associated with constitutive activation of multiple signaling pathways, induced by many growth factor receptors, specifically, EGFRs and IGF-1R that promote proliferation, inhibit apoptosis, and induce metastasis (3–5). Therefore, it is likely that the maximal and most durable therapeutic benefit against tumor growth will be achieved with combination therapies that affect several targets. Thus, agent(s)/regimen(s) that target EGFRs and IGF-1R should be more effective than narrowly focused therapies, as they are likely to impact several aspects of tumor progression. This hypothesis was tested in an in vitro study in which addition of curcumin to FOLFOX resulted in a significantly greater inhibition of growth HCT-116 and HT-29 colon cancer cells that that observed following treatment with curcumin, curcumin plus 5-FU, or FOLFOX when compared with the untreated controls (52). A part of the growth inhibition due to combination of curcumin and FOLFOX could be attributed to inhibition of expression and activation of EGFR, HER-2, and HER-3 as well as IGF-1R (52). Curcumin-induced downregulation of EGFR was found to be due to inhibition of EGFR promoter activity, whereas the effect of FOLFOX on EGFR expression was thought to be posttranslational (52). Inhibition of IGF-1R activation by curcumin and FOLFOX was attributed to increased sequestration of IGFs by IGFBP-3, whose levels were greatly increased by the combination of curcumin and FOLFOX (52). Curcumin in combination with 5-FU has also found been to cause a greater inhibition of growth of gastric cancer cells growth via G2/M arrest (53). Additionally, we reported that the combination of curcumin and ERRP, a universal inhibitor of EGFR and its family members (54), causes a significantly greater inhibition of growth of colon cancer cells in vitro than either agent alone (25).This has been partly attributed to attenuation of NF-κB activity (25). More recently, we have observed that a combination of curcumin and dasatinib (BMS-354825; Bristol-Myers Squibb)—a newly developed, highly potent, ATP-competitive Src and Abl kinase inhibitor—causes a greater inhibition of growth of colon cancer cells accompanied by inhibition of EGFRs and IGF-1R activation and attenuation of NF-κB activity (unpublished observation). Hence, addition of nontoxic curcumin can improve the efficacy of gastrointestinal cancer chemotherapy without any additional toxicity. Since curcumin accumulates in the gastrointestinal mucosa after oral administration, it could serve as a radiosensitizer and could also improve the efficacy of chemoradiotherapy for rectal and/or gastroesophageal cancers (55). A schematic representation of the mechanisms of how curcumin may exert its synergistic effect on current therapies for colorectal cancer is shown in Fig. 1. Herein, we propose that radiation and chemotherapy by themselves can stimulate NF-κB activity, which may lead to increased cell survival (Fig. 1). However, curcumin and chemotherapy can both inhibit the activation of EGFR and its family members, resulting in inhibition of NF-κB leading to increased apoptosis. Curcumin can also inhibit the activation of IGF-1R by stimulating the expression of IGFBP-3, which in turn leads to increased sequestration of IGFs rendering the ligand(s) unavailable for activation of IGF-1R (Fig. 1). Further experiments are undoubtedly needed to fully establish the therapeutic effectiveness of curcumin either alone or in combination with chemotherapeutics in gastrointestinal malignancies, with particular reference to colorectal cancer.
FIG. 1.
Schematics of mechanism of synergism between curcumin and current therapeutics in colorectal cancer.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, et al. : Cancer statistics, 2008. CA Cancer J Clin 58, 71–96, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Andre T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, et al. : Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 350, 2343–2351, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Neugut AI, Lautenbach E, Abi-Rached B, and Forde KA.: Incidence of adenomas after curative resection for colorectal cancer. Am J Gastroenterol 91, 2096–2098, 1996. [PubMed] [Google Scholar]
- 4.O’dwyer ST, Renehan AG, Zwahlen M, and Egger M.: Risk of second primary colorectal cancer with particular reference to age at diagnosis. Colorectal Dis 9, 814, 2006. [Google Scholar]
- 5.Shitoh K, Konishi F, Miyakura Y, Togashi K, Okamolo T, et al. : Microsatellite instability as a marker in predicting metachronous multiple colorectal carcinomas after surgery: a cohort-like study. Dis Colon Rectum 45, 329–333, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, et al. : Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350, 2335–2342, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Srinivasan KR: A chromatographic study of the curcuminoids in Curcuma longa, L. J Pharm Pharmacol 5, 448–457, 1953. [DOI] [PubMed] [Google Scholar]
- 8.Kiuchi F, Goto Y, Sugimoto N, Akao N, Kondo K, et al. Nematocidal activity of turmeric: synergistic action of curcuminoids. Chem Pharm Bull (Tokyo) 41, 1640–1643, 1993. [DOI] [PubMed] [Google Scholar]
- 9.Hanif R, Qiao L, Shiff SJ, and Rigas B: Curcumin, a natural plant phenolic food additive, inhibits cell proliferation and induces cell cycle changes in colon adenocarcinoma cell lines by a prostaglandin-independent pathway. J Lab Clin Med 130, 576–584, 1997. [DOI] [PubMed] [Google Scholar]
- 10.Chauhan DP: Chemotherapeutic potential of curcumin for colorectal cancer. Curr Pharm Des 8, 1695–1706, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Huang MT, Wang ZY, Georgiadis CA, Laskin JD, and Conney AH: Inhibitory effects of curcumin on tumor initiation by benzo[a]pyrene and 7,12-dimethylbenz[a]anthracene. Carcinogenesis 13, 2183–2186, 1992. [DOI] [PubMed] [Google Scholar]
- 12.Rao CV, Simi B, and Reddy BS: Inhibition by dietary curcumin of azoxymethane-induced ornithine decarboxylase, tyrosine protein kinase, arachidonic acid metabolism and aberrant crypt foci formation in the rat colon. Carcinogenesis 14, 2219–2225, 1993. [DOI] [PubMed] [Google Scholar]
- 13.Rao CV, Rivenson, Simi B, and Reddy BS: Chemoprevention of colon carcinogenesis by dietary curcumin, a naturally occurring plant phenolic compound. Cancer Res 55, 259–266, 1995. [PubMed] [Google Scholar]
- 14.Huang MT, Wang ZY, Georgiadis CA, Laskin JD, and Conney AH: Inhibitory effects of curcumin on tumor initiation by benzo[a]pyrene and 7,12-dimethylbenz[a]anthracene. Carcinogenesis 13, 2183–2186, 1992. [DOI] [PubMed] [Google Scholar]
- 15.Huang MT, Smart RC, Wong CQ, and Conney AH: Inhibitory effect of curcumin, chlorogenic acid, caffeic acid, and ferulic acid on tumor promotion in mouse skin by 12-o-tetradecanoylphorbol-13-acetate. Cancer Res 48, 5941–5946, 1988. [PubMed] [Google Scholar]
- 16.Huang MT, Lou Yr, Ma W, Newmark HL, Reuhl KR, et al. : Inhibitory effects of dietary curcumin on forestomach, duodenal, and colon carcinogenesis in mice. Cancer Res 54, 5841–5847, 1994. [PubMed] [Google Scholar]
- 17.Huang MT, Ma W, Yen P, Xie JG, Han J, et al. : Inhibitory effects of topical application of low doses of curcumin on 12-O-tetradecanoylphorbol-13-acetate-induced tumor promotion and oxidized DNA bases in mouse epidermis. Carcinogenesis 18, 83–88, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Rao CV, Simi B, and Reddy BS: Inhibition by dietary curcumin of azoxymethane-induced ornithine decarboxylase, tyrosine protein kinase, arachidonic acid metabolism and aberrant crypt foci formation in the rat colon. Carcinogenesis 14, 2219–2225, 1993. [DOI] [PubMed] [Google Scholar]
- 19.Rao CV, Rivenson, Simi B, and Reddy BS: Chemoprevention of colon carcinogenesis by dietary curcumin, a naturally occurring plant phenolic compound. Cancer Res 55, 259–266, 1995. [PubMed] [Google Scholar]
- 20.Perkins S, Verschoyle RD, Hill K, Parveen I, Threadgill MD, et al. : Chemo-preventive efficacy and pharmacokinetics of curcumin in the Min/+ mouse, a model of familial adenomatous polyposis. Cancer Epidem Biomark Prev 11, 535–540, 2002. [PubMed] [Google Scholar]
- 21.Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, et al. : Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res 10, 6847–6854, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Owen RW: Faecal steroids and colorectal carcinogenesis. Scand J Gastroenterol Suppl 222, 76–82, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Devasena T, Rajasekaran KN, Gunasekaran G, Viswariathan P, and Menon VP: Anticarcinogenic effect of bis-1,7-(2-hydroxyphenyl)-hepta-1,6-diene-3,5-dione a curcumin analog on DMH-induced colon cancer model. Pharmacol Res 47, 133–140, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Rao CV, Simi B, Wynn TT, Garr K, and Reddy BS. Modulating effect of amount and types of dietary fat on colonic mucosal phospholipase A2, phosphatidylinositol-specific phospholipase C activities, and cyclooxygenase metabolite formation during different stages of colon tumor promotion in male F344 rats. Cancer Res 56, 532–537, 1996. [PubMed] [Google Scholar]
- 25.Reddy S, Rishi AK, Xu H, Levi E, Sarkar FH, and Majumdar APN: Mechanisms of curcumin and EGF-receptor related protein (ERRP)-dependent growth inhibition of colon cancer cells. Nutr Cancer 55, 185–194, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Zhang F, Altorki NK, Mestre JR, Subbaramaiah K, and Dannenberg AJ: Curcumin inhibits cyclooxygenase-2 transcription in bile acid- and phorbol ester-treated human gastrointestinal epithelial cells. Carcinogenesis 20, 445–451, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Goel A, Boland CR, and Chauhan DP: Specific inhibition of cyclooxygenase-2 (COX-2) expression by dietary curcumin in HT-29 human colon cancer cells. Cancer Lett 172, 111–118, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Hanif R, Qiao L, Shiff SJ, and Rigas B: Curcumin, a natural plant phenolic food additive, inhibits cell proliferation and induces cell cycle changes in colon adenocarcinoma cell lines by a prostaglandin-independent pathway. J Lab Clin Med 130, 576–584, 1997. [DOI] [PubMed] [Google Scholar]
- 29.Barnes CJ and Lee M: Chemoprevention of spontaneous intestinal adenomas in the adenomatous polyposis coli Min mouse model with aspirin. Gastroenterology 114, 873–877, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, et al. : Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res 21, 2895–2900, 2001. [PubMed] [Google Scholar]
- 31.Sharma RA, McLefland HR, Hill KA, Ireson CR, Euden SA, et al. : Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin Cancer Res 7, 1894–1900, 2001. [PubMed] [Google Scholar]
- 32.Ravindranath V and Chandrasekhara N: Absorption and tissue distribution of curcumin in rats. Toxicology 16, 259–265, 1980. [DOI] [PubMed] [Google Scholar]
- 33.Parveen I and Threadgill MD: Labeled compounds of interest as anti-tumour agents-VII1. [3H]- and [14C]-curcumin. J Labeled Compd Radiopharm 43, 883–889, 2000. [Google Scholar]
- 34.Pereira MA, Grubbs CJ, Barnes LH, Li H, Olson GR, et al. : Effects of the phytochemicals, curcumin and quercetin, upon azoxymethane-induced colon cancer and 7,12-dimethylbenz[a]anthracene-induced mammary cancer in rats. Carcinogenesis 17, 1305–1311, 1996. [DOI] [PubMed] [Google Scholar]
- 35.Shishodia S, Chaturvedi MM, and Aggarwal BB: Role of curcumin in cancer therapy. Curr Prob Cancer 31, 243–305, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Egan ME, Pearson M, Weiner SA, Rajendran V, Rubin D, et al. : Curcumin, a major constituent of turmeric, corrects cystic fibrosis defects. Science 304, 600–602, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Kuo ML, Huang TS, and Lin JK: Curcumin, an antioxidant and antitumour promoter induces apoptosis in human leukemia cells. Biochem Biophys Acta 1317, 965–1000, 1996. [DOI] [PubMed] [Google Scholar]
- 38.Kawamori T, Lubet R, Steele VE, Kelloff GJ, Kaskey RB, et al. : Chemopreventive effect of curcumin, a naturally occurring anti-inflammatory agent, during the promotion/progression stages of cancer. Cancer Res 559, 597–601, 1999. [PubMed] [Google Scholar]
- 39.Arbiser JL, Klauber N, Rohan R, van Leeuwen R, Huang MT, et al. : Curcumin is an in vivo inhibitor of angiogenesis. Mol Med 4, 376–483, 1998. [PMC free article] [PubMed] [Google Scholar]
- 40.Garcea G, Berry DP, Jones DJ, Singh R, Dennison AR, et al. : Consumption of the putative chemopreventive agent curcumin by cancer patients: assessment of curcumin levels in the colorectum and their phrmacodynamic consequences. Cancer Epidemiol Biomarkers Prev 14, 120–125, 2005. [PubMed] [Google Scholar]
- 41.Li Y and Sarkar FH: Inhibition of nuclear factor κB activation in PC3 cells by genistein is mediated via Akt signaling pathway. Clin Cancer Res 8, 2369–2377, 2002. [PubMed] [Google Scholar]
- 42.Das KC and White CW: Activation of NF-κB by antineoplastic agents. Role of protein kinase C. J Biol Chem 272, 14914–14920, 1977. [DOI] [PubMed] [Google Scholar]
- 43.Chuang SE, Yeh PY, Lu YS, et al. : Basal levels and patterns of anticancer drug-induced activation of nuclear factor κB (NF-κB), and its attention by tamoxifen, dexamethsone and curcumin in carcinoma cells. Biochem Pharmacol 63, 1709–1716, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Heinemann V, Boeck S, Hinke A, Labianca R, and Louvet C: Meta-analysis of randomized trials: evaluation of benefit from gemcitabine-based combination chemotherapy applied in advanced pancreatic cancer. BMC Cancer 8, 82–91, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fujioka S, Sclabas GM, Schmidt C, et al. : Function of nuclear factor B in pancreatic cancer metastasis. Clin Cancer Res 9, 346–354, 2003. [PubMed] [Google Scholar]
- 46.Kornmann M, Ishiwata T, Itakura J, Tangvoranuntakul P, Beger HG, et al. : Increased cyclin D1 in human pancreatic cancer is associated with decreased postoperative survival. Oncology 55, 363–369, 1998. [DOI] [PubMed] [Google Scholar]
- 47.Kunnumakkara AB, Guha S, Krishnan S, Diagaradjane P, Gelovani J, et al. : Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angio-genesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res 67, 3853–3861, 2007. [DOI] [PubMed] [Google Scholar]
- 48.Lev-Ari S, Vexler A, Starr A, Ashkenazy-Voghera M, Greif J, et al. : Curcumin augments gemcitabine cytotoxic effect on pancreatic adenocarcinoma cell lines. Cancer Invest 25, 411–418, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Khanbolooki S, Nawrocki ST, Arumugam T, Andtbacka R, Pino MS, et al. : Nuclear factor-kappa B maintains TRAIL resistance in human pancreatic cancer cells. Mol Cancer Ther 5, 2251–2260, 2006. [DOI] [PubMed] [Google Scholar]
- 50.Ashkenazi A: Targeting death and decoy receptors of the tumor-necrosis factor superfamily. Nat Rev Cancer 2, 420–430, 2002. [DOI] [PubMed] [Google Scholar]
- 51.Jung EM, Park JW, Choi KS, Park JW, Lee HI, et al. : Curcumin sensitizes tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis through CHOP-independent DR5 upregulation. Carcinogenesis 27, 2008–2017, 2006. [DOI] [PubMed] [Google Scholar]
- 52.Patel BB, Sengupta R, Qazi S, Vachhani H, Yu Y, et al. : Curcumin enhances the effects of 5-fluorouracil and oxaliplatin in mediating growth inhibition of colon cancer cells by modulating EGFR and IGF-1R. Int J Cancer 122, 267–273, 2008. [DOI] [PubMed] [Google Scholar]
- 53.Koo JY, Kim HJ, Jung KO, and Park KY: Curcumin inhibits the growth of AGS human gastric carcinoma cells in vitro and shows synergism with 5-fluorouracil. J Med Food 7, 117–721, 2004. [DOI] [PubMed] [Google Scholar]
- 54.Xu H, Yu Y, Marciniak DJ, Rishi A, Sarkar FH, et al. : EGFR-related protein (ERRP) Inhibits multiple members of EGFR family in colon and breast cancer Cells. Mol Cancer Ther 4, 435–442, 2005. [DOI] [PubMed] [Google Scholar]
- 55.Kunnumakkara AB, Diagaradjane P, Guha S, Deorukhkar A, Shentu S, et al. : Curcumin sensitizes human colorectal cancer xenografts in nude mice to gamma-radiation by targeting nuclear factor-kappaB-regulated gene products. Clin Cancer Res Apr 14, 2128–2136, 2004. [DOI] [PubMed] [Google Scholar]