Abstract
Development and progression of many malignancies, including colorectal cancer, are associated with activation of multiple signaling pathways. Therefore, inhibition of these signaling pathways with noncytotoxic natural products represents a logical preventive and/or therapeutic approach for colon cancer. Curcumin and resveratrol, both of which inhibit the growth of transformed cells and colon carcinogenesis, were selected to examine whether combining them would be an effective preventive and/or therapeutic strategy for colon cancer. Indeed, the combination of curcumin and resveratrol was found to be more effective in inhibiting growth of p53-positive (wt) and p53-negative colon cancer HCT-116 cells in vitro and in vivo in SCID xenografts of colon cancer HCT-116 (wt) cells than either agent alone. Analysis by Calcusyn software showed synergism between curcumin and resveratrol. The inhibition of tumors in response to curcumin and/or resveratrol was associated with the reduction in proliferation and stimulation of apoptosis accompanied by attenuation of NF-κB activity. In vitro studies have further demonstrated that the combinatorial treatment caused a greater inhibition of constitutive activation of EGFR and its family members as well as IGF-1R. Our current data suggest that the combination of curcumin and resveratrol could be an effective preventive/therapeutic strategy for colon cancer.
INTRODUCTION
Despite recent advances in medicine, mortality from colorectal cancer, the third most common cancer worldwide, still remains unacceptably high (1). Therefore, the need to develop novel alternative preventive and therapeutic strategies remains an important goal. Recent advances in molecular pathogenesis of cancer have aided in formulating both preventive and/or therapeutic strategies.
Accumulating evidence suggests that many solid tumors show upregulation or constitutive activation of multiple signaling pathways, including EGFR and its family members ErbB-2/HER-2, ErbB-3/HER-3, and ErbB-4/HER-4 (referred to as EGFRs) as well as insulin-like growth factor-1 receptor (IGF-1R) that promote growth, angiogenesis, and metastasis (2–6). Therefore, inhibition of these signaling pathways requires a multitargeted approach. Noncytotoxic natural products (dietary ingredients) represent a logical chemopreventive and/or therapeutic approach for colorectal cancer because many of them exhibit pleiotropic properties (multitargeting agents).
Curcumin (diferuloylmethane), the major active ingredient of turmeric (curcuma longa) with no discernable toxicity, has been shown to inhibit the growth of transformed cells (7–9) and colon carcinogenesis at the initiation, promotion, and progression stages in carcinogen-induced rodent models (10–12). Curcumin has been shown to prevent the development of intestinal adenomas in Min+/− mice, a model of human familial adenomatous polyposis (13). In a Phase-I clinical trial, curcumin has been shown to inhibit the growth of a variety of tumors (14). With respect to the mechanisms of its action, it has been reported that curcumin inhibits EGFR activation in colon cancer cells (15). More recently, we have demonstrated that curcumin not only inhibits the activation of EGFR and its family members but also IGF-1R in colon cancer HCT-116 cells, leading to inhibition of the DNA binding activity of NF-κB (16). Activation of NF-κB is known to increase cell survival and causes resistance to apoptosis by many conventional chemotherapeutics (17–19). Therefore, inhibition of NF-κB activity through attenuation of EGFRs and IGF-1R signaling pathways by the combination therapy of curcumin and other nontoxic agent(s) provides a logical preventive and therapeutic approach for colorectal cancer.
Like curcumin, resveratrol (3,5,4′-trihtdroxy-trans-stilbene), a phytoalexin produced by more than 70 different plant species including grapes and peanuts, has also been shown to inhibit the processes of carcinogenesis at different stages of initiation, promotion, and progression (20,21). Resveratrol has also been found to be an effective chemopreventive agent for colorectal cancer (22). It inhibits the development of carcinogen-induced preneoplastic lesion aberrant crypt foci (ACF) in the mouse colon and suppresses intestinal tumor (adenomas) formation in Min+/− mice (22–24). In the colon, the growth inhibitory properties of resveratrol are thought to be primarily due to induction of apoptosis (20,25). However, the detailed regulatory mechanisms for resveratrol-induced inhibition of cellular growth remain to be fully delineated.
It is becoming increasingly clear that in most malignancies, multiple pathways become dysfunctional. Therefore, it is likely that the maximal and most durable preventive and/or therapeutic benefit against tumor development could be achieved with combination therapies that will affect several targets. We hypothesize that combining curcumin with resveratrol, both of which are nontoxic to humans—and they are classified as multitargeting agents—will provide a better preventive/therapeutic outcome for colorectal cancer than either agent alone. The current investigation was undertaken to test this hypothesis. Herein, we demonstrate that the combination of curcumin and resveratrol causes a greater inhibition of growth of colon cancer cells in vitro and tumor growth of colon cancer xenografts in SCID mice than either agent alone. This inhibition could partly be attributed to attenuation of EGFRs and IGF-1R signaling pathways leading to the inhibition of NF-κB activity.
METHODS AND MATERIALS
Cell Lines and Cell Cultures
Human colon cancer HCT-116 [p53+/+or HCT-116 (wt) and HT-29] cells were obtained from the American Type Culture Collection (Rockville, MD). HCT-116 (p53−/−) cells were obtained from Dr. Ping Dou at Karmanos Cancer Institute. The cells were maintained in Dulbecco’s modified Eagle medium (DMEM) in tissue culture flasks in a humidified incubator at 37°C in an atmosphere of 95% air and 5% CO2. Medium was supplemented with 10% FBS and 1% antibiotic/antimycotic. Medium was changed 3 times a week, and cells were passaged using trypsin/EDTA. Curcumin and resveratrol were purchased from Sigma Chemical Co. (St. Louis, MO). Curcumin was dissolved in 100% ethanol and subsequently diluted with the tissue culture medium. Resveratrol was dissolved in DMSO and subsequently diluted with the culture medium (final DMSO concentration for experiments was 0.05%).
Growth Inhibition Assay
Inhibition of cell growth in response to curcumin and/or resveratrol ERRP was assessed by 3-(4, 5-dimethylthiazol-2yl)-2, 5-diphenyltetrazolium bromide (MTT) assay as described previously (16,26). Briefly, cells were dispersed by trypsin-EDTA treatment and 2.5 × 104 cells/ml, resuspended in DMEM containing 10% FBS, and seeded into 96-wells culture plates with 6 replicates. After 24 h of plating, incubation was continued for another 48 h in absence (control) or presence of different testing agents as described in the legends to the figures. At the end of the 48 h incubation period, the reaction was terminated by adding 20 μl of 5 mg/ml stock of MTT to each well. The reaction was allowed to proceed for 3 to 4 h at 37°C. The culture medium was then removed. The formazan crystals were then dissolved by adding 0.1 ml of dimethyl sulfoxide (DMSO). The intensity of the color developed, which is the reflection of number of live cells, was measured at a wavelength of 570 nm. All values were compared to the corresponding controls. All assays were performed with 6 replicates.
Analysis of Interaction Between Curcumin and Resveratrol
Calcusyn software program (Biosoft, Ferguson, MO), based on the Chou–Talalay method, was employed to determine the nature of interaction between the two agents. This method utilizes a multiple drug-effect equation derived from enzyme kinetics model in which the output is represented as combination indexes (CI) and/ or isobologram analysis. Calcusyn software defines synergy as CI value less than 1. Based on CI values, the extent of synergism/antagonism may be determined. In brief, CI values between 0.9 and 0.85 would suggest a moderate synergy, whereas those in the range of 0.7 to 0.3 are indicative of clear synergistic interactions between the drugs. On the other hand, CI values in the range of 0.9 to 1.10 suggest a near additive effect. The Chou–Talalay equation is also utilized to perform isobologram analysis as represented by isobole for ED75.
Assessment of Apoptosis
Approximately 1 × 105 cells/well were plated in DMEM with 10% FBS. After 24 h of plating, the medium was changed to contain 2.5% FBS to minimize the contribution of serum-derived growth factors and subsequently treated the same way as described above for growth inhibition study. At the end of the incubation period, the cells were lysed, and the levels of apoptosis were determined using the Cell Death Detection ELISAPLUS kit from Roche Diagnostics GmbH (Penzberg, Germany), which measures the cytoplasmic histone-associated-DNA-fragments (mononucleosomes and oligonucleosomes). Western blot analysis was performed essentially according to our standard protocol(26,27). Briefly, the cells were solubilized in lysis buffer [50 mM Tris; 100 mM NaCl; 2.5 mM EDTA; 1% Triton X-100; 1% Nonidet P-40; 2.5 mM Na3VO4; 25 μg/ml aprotinin; 25 μg/ml leupeptin; 25 μg/ml pepstatin A; and 1 mM phenylmethylsulfonyl fluoride (PMSF)]). Following clarification at 10,000 g for 15 min, the supernatant was used for Western blot analysis. In all analyses, protein concentration, determined by the Bio-Rad Protein Assay kit (Bio-Rad, Hercules, CA), was standardized among the samples. Aliquots of cell lysates containing 50 μg of protein were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Following electrophoresis, proteins were transferred electrophoretically onto supported nitrocellulose membranes (Osmonics, Gloucester, MA). Membranes were incubated for 1 h at room temperature with blocking buffer, TBS-T (20 mM Tris, pH 7.6, 100 nM NaCl, 0.1% Tween-20) and 5% nonfat dry milk with gentle agitation. After washing the membranes with TBST, they were incubated overnight at 4°C in TBS-T buffer containing 5% milk and with one of the following antibodies (1:1000 dilution): phospho-EGFR (Tyr1173), phospho-ErbB-2/HER-2 (Tyr1121), phospho-ErbB-3/HER-3 (Tyr1289), IGF-1R, IGFBP-3, Akt (Ser473)) or COX-2. The membranes were washed 3 times with TBS-T and subsequently incubated with appropriate secondary antibodies (1:5000 dilutions) in TBST containing 5% milk for 2 h at room temperature with gentle agitation. The membranes were washed again with TBS-T, and the protein bands were visualized by enhanced chemiluminescence (ECL) detection system (Amersham, Buckinghamshire, England). The membranes containing the electrophoresed proteins were exposed to X-Omat film, and the signals were quantitated by densitometry using Image Quant image analysis system (Storm Optical Scanner, Molecular Dynamics, Sunnyvale, CA). Membranes were stripped (2 × for 15 min at 55°C) in stripping buffer containing 100 mM 2-mercaptoethanol, 2% sodium dodecyl sulfate, and 62.5 mM Tris-HCl pH 6.7. The membranes were then reprobed for the levels of total (nonphosphorylated) EGFR, ErbB-2, ErbB-3, Akt, or β-actin using corresponding antibodies. All Western blots were performed at least 3 times for each experiment. In experiments in which proteins were immunoprecipitated, cell lysates containing 1 mg protein were incubated for 24 h at 4°C under constant stirring with appropriate antibodies and protein-G-Sepharose beads. The beads were initially washed twice with TT buffer (50 mM Tris, pH 7.6, 0.15M NaCl, 0.5% Tween 20) and suspended in TTA buffer (TT buffer 0.1% + BSA). The incubation was conducted in a total volume of 0.7 ml TTA buffer that contained 5 μl antibody and 60 μl of the protein-G Sepharose beads. Following incubation, the immunoprecipitares were washed 6 times with TT buffer and subjected to Western blot analysis as described above. In the current investigation, the immuoprecipitates containing IGFBP-3 were subjected Western blot analysis with IGF-1. All immunoblots were scanned by HP Precision Pro3.13 (Hewlett-Packard, Palo Alto, CA). Densitometric measurements of the scanned bands were performed using the digitized scientific software program UN-SCNAT. Data were normalized to β-actin.
HCT-116 Cells Xenografts
Female homozygous ICR SCID mice, 6 wk old, were purchased from Taconic Farms (Germantown, NY). The mice were housed and maintained under sterile conditions in facilities accredited by the American Association for the Accreditation of Laboratory Animal Care and in accordance with current regulations and standards of the United States Department of Agriculture, United States Department of Health and Human Services, and the National Institute of Health. The mice were used in accordance with the Animal Care and Use Guidelines of Wayne State University under the protocol approved by the Institutional Animal Care and Use Committee. The mice received Lab Diet 5021 (Purina Mills, Inc., Richmond, IN) ad libitum throughout the experimental period. After 1 wk of acclimatization, the animals were used for our study. HCT-116 cells were harvested from subconfluent cultures after a brief exposure to 0.25% trypsin and 0.2% EDTA. Trypsinization was stopped by adding medium containing 10% FBS. The cells were washed once in serum free medium and resuspended in PBS. Only suspensions consisting of a single cell >95% viability was used for the injections. Three million cells in 100 μl serum-free RPMI media were injected subcutaneously by a 27-gauge needle into the right and left flanks of each mice. In accordance with previously published reports, we found 100% of mice to develop tumor. After the tumors were established (50–75 mm3),as determined by caliper measurements (15th day after cancer cell injection), the mice were randomized into the following 4 groups (n = 6): (a) vehicle control; (b) only curcumin—500 mg/kg body wt by gavage every day for 3 wk; (c) resveratrol—150 mg/kg body weight administered by gavage every day for 3 wk; and (d) Curcumin plus resveratrol, following a similar schedule as described for individual drug treatment. The rationale for using the current doses of curcumin and resveratrol were based on the observations by others that similar doses of these agents caused a significant reduction in tumor growth in mice (28,29). Tumor growth deduced as volume of the tumor in each group was determined by twice weekly caliper measurements. The body weight of mice in each group was also measured. All mice were euthanized one day following the last dose of treatment (3 wk) when the tumor volume in the control mice reached approximately 1500 mm3. The final body weight and tumor volume were recorded. On autopsy, the tumor was neatly excised and free of any extraneous adhering tissue. One part of the tissue was fixed in formalin for routine hemotoxylin-eosin (H&E) staining, and another part was rapidly frozen in liquid nitrogen and stored at −70°C and subsequently used for preparation of nuclear protein extracts. H&E staining confirmed the presence of tumor.
Determination of Mitotic Index (MI)
The formalin-fixed tissues were embedded into paraffin, subsequently sectioned at 4 to 5 micron, and subjected to H&E staining. Number of cells undergoing mitosis in at least 10 different areas of each slide and 5 slides from each sample were examined in a blinded manner. At least 750 cells/slide were counted using X-40 objective. The labeling index (LI) was calculated as the number of total labeled cells × 100/total cells per high power focus.
Determination of Apoptosis by TUNEL Assay
Paraffin-embedded tissues were sections as described above, and the TUNEL assay was performed to detect apoptotic cells using the in situ cell Death Detection kit from Roche Applied Science (Indianapolis, IN) according to the manufacturer’s instructions as described previously. 3-amino-9-ethylcarbazole was used as chromagen, and the sections were counterstained with hematoxylin. Apoptotic cell nuclei appeared as red stained structures against a blue-violet background. Mucosal areas of all stained tissues were measured with a 100 division eyepiece reticule and 10× objective. The apoptotic cells within the mucosa of each section were counted.
Tumor Tissue Nuclear Protein Extraction and EMSA
Randomly selected harvested tumor tissue from each group of experimental mice were homogenized using Dounce homogenizer (Kontes Co., Vineland, NJ) containing 0.3 ml of ice-cold buffer A [composed of 10 mM HEPES (pH 7.9), 10 mM KCl, 2 mM MgCI2, 1 mM DTT, 0.1 mM EDTA, and 0.1 mM PMSF]. The homogenized tissue was incubated in ice for 30 min followed by lysis with 10% NP-40 solution and centrifuging at 5,000 g at 4°C for 5 min. The crude nuclear pellet was washed in buffer A and suspended in 50 μl of ice cold nuclear extraction buffer B (50 mM HEPES, pH 7.9; 50 mM KCl; 300 mM NaCl; 1 mM DTT; 0.1 mM EDTA; 0.1 mM PMSF; 4μM leupeptidin; and glycerol 20%) and incubated on ice for 60 min with intermittent vortexing. The suspension was centrifuged at 16,000 g at 4°C for 10 min. The supernatant (nuclear proteins) was collected and kept at −70°C until use. The protein concentration was determined using the bicinchoninic acid assay kit with BSA as the standard (Pierce Chemical Co., Rockford,IL). Anti-Rb immunoblotting with nuclear proteins was done as loading control.
Statistical Analysis
Data are represented as mean ± SEM for the absolute values as indicated in the vertical axis legend of Fig. 1. The statistical significance of differential findings between experimental groups and control was determined by Student’s t-test as implemented by Excel 2000 (Microsoft Corp., Redmond, WA). P values smaller than 0.05 were considered statistically significant.
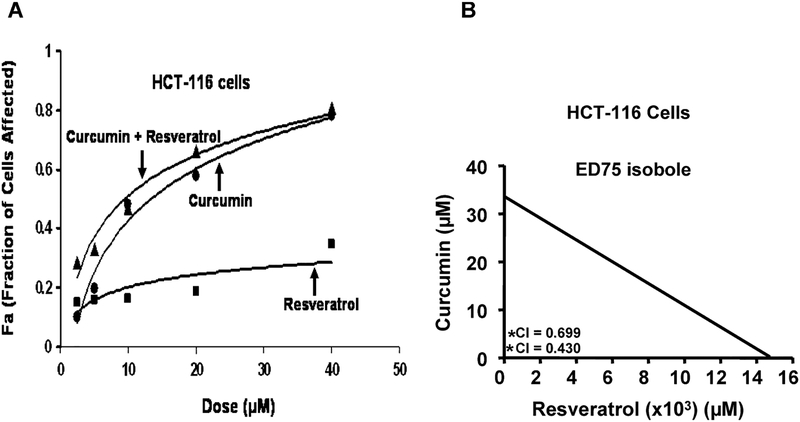
FIG. 1.
A: Typical dose-response curves produced by fixed-ratio method. Fraction of HCT-116 (wt) cells affected by different combination of curcumin and resveratrol (fixed ratio) is higher than either agent alone. Fa represents the fraction of cells that is growth inhibited in response to curcumin and/or resveratrol. This is calculated as 1 – fraction of surviving cells. Fa values for each treatment were utilized to conduct synergy analysis by Calcusyn software as described in Methods and Materials. B: The isobole for ED75, as analyzed using the Calcusyn software, shows that the combination of curcumin and resveratrol is below the line of additivity indicating synergism.
RESULTS
Curcumin together with resveratrol causes a greater inhibition of growth of colon cancer cells in vitro than either agent alone. The primary goal of this investigation was to determine whether combining curcumin with resveratrol would be a better preventive/therapeutic outcome. As a first step in accomplishing this goal, we examined the effects of incremental doses of each agent alone or in combination on the growth of colon cancer HCT-116 cells. The data obtained from this study were plotted for analysis of synergism by combination index (CI) method.
As shown in Fig. 1A, fraction of HCT-116 cells affected by curcumin and/or resveratrol increased in a dose-dependent manner. However, the fraction of cells affected by the combination of both was found to be greater than either agent alone. To elucidate whether the greater fraction of cells affected by combination therapy could be due to synergism, median effect analysis was carried out using the Calcusyn software program. The combination index (CI) for curcumin and resveratrol treatment was found to be below 1 at all the combination concentrations used in this study. This suggests that the interaction between the two agents at each concentration is synergistic (Table 1). From this analysis, it became evident that the degree of synergism is greater with concentrations of curcumin and resveratrol below 20 μM. Further, isobologram analysis shows synergism between the two agents for different effects. The isobole for ED75 shows that the combination of curcumin and resveratrol is below the line of additivity indicating synergism (Fig. 1B). All subsequent experiments were carried out using 10 μM curcumin and 10 μM of resveratrol.
TABLE 1.
Synergistic effects of curcumin with resveratrol on growth of colon cancer HCT-116 cellsa
| Fraction of Cells Affected (FA) | ||||
|---|---|---|---|---|
| Dose (μM) | Curcumin | Resveratrol | Curcumin + Resveratrol | CI |
| 2.5 | 0.10 | 0.15 | 0.29 | 0.43 |
| 5.0 | 0.20 | 0.16 | 0.33 | 0.70 |
| 10.0 | 0.48 | 0.16 | 0.47 | 0.82 |
| 20.0 | 0.58 | 0.19 | 0.66 | 0.84 |
| 40.0 | 0.78 | 0.35 | 0.81 | 0.90 |
Abbreviations are as follows: FA, fraction of cells with growth affected in response to curcumin and/or resveratrol treated vs. uncreated cells; CI, combination Index. CI < 1.0 indicates synergism.
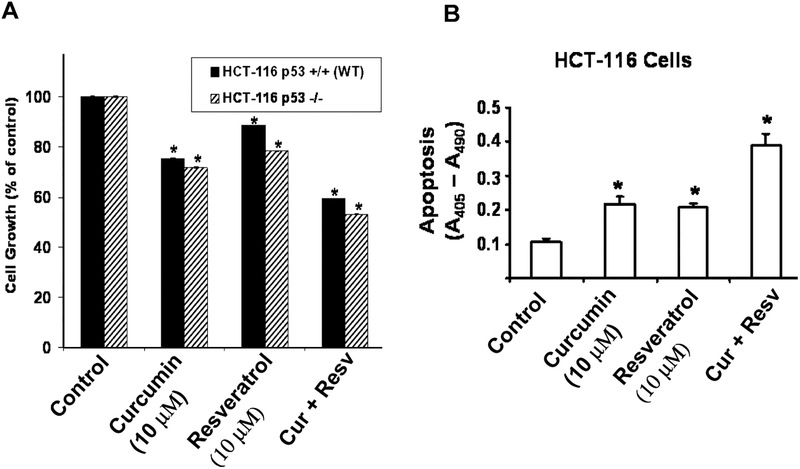
Earlier, we reported that curcumin inhibits the growth of both HCT-116 and HT-29 cells, which are p53 positive and p53 mutant, respectively, suggesting that the growth inhibitory properties of curcumin are independent of p53 status. To further determine whether and to what extent the p53 status of colon cancer cells would affect the growth inhibitory properties of curcumin and/or resveratrol, we examined the effects of these agents, each alone or in combination, on the growth of HCT-116 cells containing either p53 (p53−/−) or devoid of p53 (p53−/−). We found both agents to inhibit growth of p53 positive and p53 negative HCT116 cells (Fig. 2A). However, whereas curcumin and resveratrol, each alone, caused a 15–30% reduction in growth of both the p53-positive and p53-negative HCT-116 cells, the combination of curcumin and resveratrol produced a 40% inhibition of the same when compared with the controls (Fig. 2A). The results suggest that the growth inhibitory properties of curcumin and resveratrol are independent of p53 status. All subsequent experiments were carried out with HCT-116 (wt) cells.
FIG. 2.
A: The combination of curcumin and resveratrol causes greater inhibition of growth of colon cancer p53 positive (wild type) and the p53-negative (p53−/−) HCT-116 cells and B: also stimulates apoptosis of HCT-116 (wt) cells than either agent alone. Each value represents mean ±SEM of 4 to 6 observations. *P < 0.025 or lower compared to control. Cur, curcumin; Resv, resveratrol.
The next experiment was carried out to determine whether the comparatively greater inhibition of colon cancer cell growth in response to the combinatorial treatment of curcumin and resveratrol over the monotherapy could partly be the result of increased apoptosis. Indeed, the combination of curcumin and resveratrol produced a much higher apoptosis (300%) of HCT-116 (wt) cells than that caused a by either curcumin or resveratrol when compared with the control (Fig. 2B).
To determine whether and to what extent the cell cycle progression was affected by curcumin and/or resveratrol, flow cytometric analysis was conducted following 48 h after incubation of HCT-116 (wt) cells in the absence (control) or presence of curcumin, resveratrol, or the combination of both. Each treatment resulted in accumulation of HCT-116 cells at the S-phase of the cell cycle (Table 2). However, whereas curcumin and resveratrol, each alone, caused between 35% and 45% increase in accumulation of cells at S-phase, the combination of curcumin and resveratrol produced a 122% increase of the same with a concomitant decrease in the population of cells in the G2-M phase when compared with the controls (Table 2).
TABLE 2.
The combination of curcumin and resveratrol causes a greater arrest of S phase of the cell cycle than that caused by either agent alone
| Cell Cycle Distribution (%) | |||
|---|---|---|---|
| Treatment | G0/1 | S | G2/M |
| Control | 67.87 ± 3.62 | 22.01 ± 1.83 | 11.12 ± 1.71 |
| Curcumin (15 μM) | 48.63 ± 3.38 | 29.58 ± 1.30 | 21.79 ± 1.32 |
| Resveratrol (10 μM) | 55.31 ± 2.82 | 31.79 ± 2.45 | 12.90 ± 2.35 |
| Curcumin + resveratrol | 44.53 ± 3.45 | 48.69 ± 2.76 | 6.77 ± 0.75 |
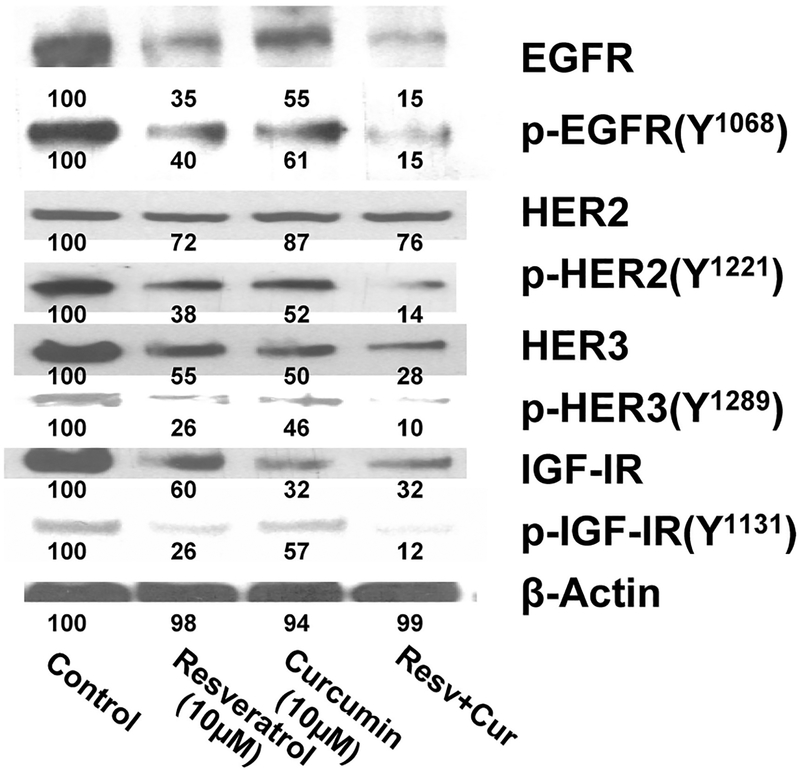
Curcumin in combination with resveratrol causes a greater inhibition of constitutive activation of EGFRs and IGF-1R in colon cancer cells in vitro. Although curcumin or resveratrol alone or in combination inhibited colon cancer cell growth, the precise regulatory mechanisms are poorly understood. Earlier, we reported that the marked inhibition of growth of colon cancer cells in vitro in response to the combination treatment of curcumin and ERRP, a pan-ErbB inhibitor, was associated with the attenuation of activation of EGFR and IGF-1R signaling (16,30). Likewise, a marked attenuation of EGFRs and IGF-1R activation in colon cancer cells was also noted in response to the combination of curcumin and FOLFOX (26). In view of these observations and the fact that multiple signaling pathways, including EGFRs and IGF-1R, are dysregulated in colorectal cancer, we examined the constitutive levels of total and the activated (tyrosine phosphorylation) forms of EGFR, HER-2, and HER-3 as well as the activation of IGF-1R in response to curcumin and/or resveratrol. We observed that although curcumin and resveratrol, each alone, inhibited the levels (13–68%) and activated forms (50–75%) of EGFR, HER-2, HER-3, and IGF-1R, the magnitude of inhibition of the levels (26–85%) and activated forms (85–90%) of the growth factor receptors were markedly greater in response to the combination of curcumin and resveratrol when compared with the controls (Fig. 3). Among the growth factor receptors, inhibition of HER-2 in response to either curcumin or resveratrol or the combination therapy was found to be much less than what was noted for EGFR, HER-3, or IGF-1R (Fig. 3).
FIG. 3.
The levels of nonphosphorylated (total) and tyrosine phosphorylated forms of EGFR, HER-2, HER-3, IGF-1R, is lower in HCT-116 (wt) cells following 48-h incubation in the presence of curcumin (10 μM), resveratrol (10 μM), or a combination of both. Cur, curcumin; Resv, resveratrol.
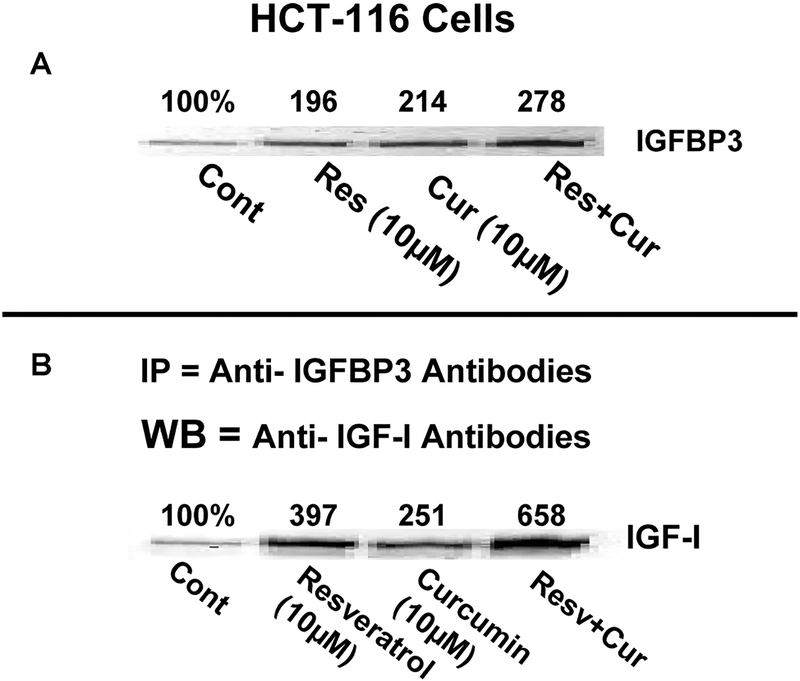
Although the precise mechanisms by which curcumin and/or resveratrol inhibit IGF-1R activation are not fully understood, we have recently reported that curcumin alone or together with FOLFOX stimulates the expression of IGFBP-3 (IGF-binding protein), which sequesters IGFs, rendering IGFs unavailable for binding to and activation of IGF-1R (26). To determine whether the same mechanism might be responsible for attenuating IGF-1R activation in response to curcumin and/or resveratrol, we initially examined the levels of IGFBP-3 in HCT-116 cells in the absence or presence of curcumin, resveratrol, or the combination of both. We observed that whereas curcumin or resveratrol produced a 96–116% increase in IGFBP-3 expression, the combinatorial treatment of curcumin and resveratrol caused 178% stimulation when compared with the controls (Fig. 4). To further determine if the increase in IGFBP-3 levels would result in increased sequestration of IGF-1 by IGFBP-3, lysates from the control and cells treated with curcumin and/or resveratrol were subjected to immunoprecipitation with IGFBP-3 antibodies followed by Western blot analysis of immunoprecipitates for IGF-1 levels. As expected, the amount of IGF-1 bound to IGFBP-3 was found to be substantially higher in response to the combinatorial treatment when compared with that achieved with either agent alone (5.5-fold for combination vs. 1.5–2-fold for monotherapy; Fig. 4).
FIG. 4.
Increases in A: IGFBP-3 expression and B: sequestration of IGF-1 by IGFBP-3 in HCT-116 (wt) in response to curcumin, resveratrol, or curcumin and resveratrol. IGFBP-3 was immunoprecipitated from cell lysates with anti-IGFBP-3 antibodies, and the immunoprecipitates were subjected to Western blot analysis for IGF-1. The numbers underneath each band represent percent of representative control. The experiment was repeated at least 3 times. Cur, curcumin; Res and Resv, resveratrol.
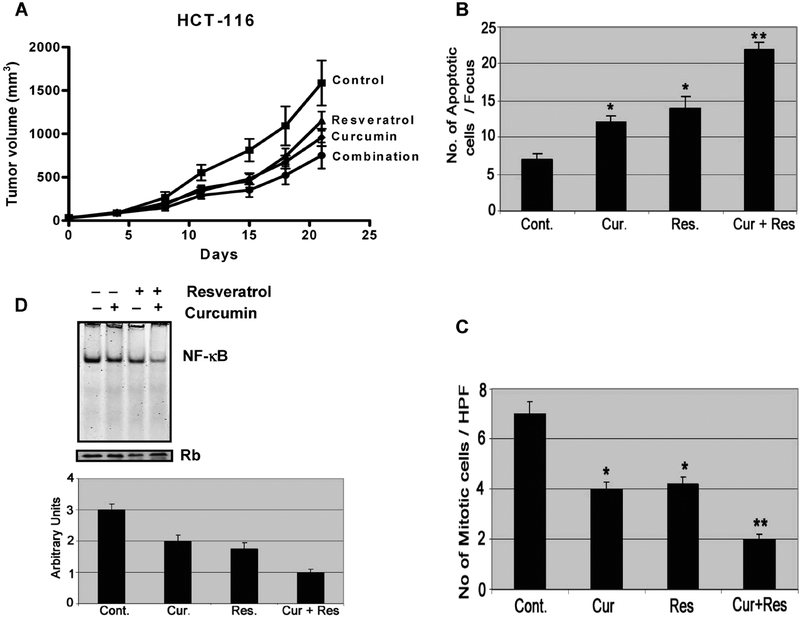
Curcumin together with resveratrol causes greater inhibition of growth of tumors of colon cancer cells in SCID mice. The above in vitro mechanistic results strongly support an efficient killing of colon cancer cells by the novel combinatorial regimen of curcumin and resveratrol. To further validate our in vitro results, we compared the effectiveness of combination therapy with monotherapy on the growth of tumors of xenograft colon cancer HCT-116 (wt) cells in SCID mice as described previously (31). We observed that curcumin (500 mg/kg body wt) and resveratrol (150 mg/kg body wt), each alone, significantly inhibited the HCT-116 tumor growth by 40% (P < 0.01) and 28% (P < 0.025), respectively, compared to the controls (Fig. 5A). However, the combination of resveratrol and curcumin produced a significant >50% inhibition (P < 0.01) in tumor growth relative to the control as well as relative to monotherapy (P < 0.05), demonstrating enhanced inhibitory effect of the combination therapy in our colon cancer experimental model (Fig. 5A). None of these treatments had any effect on body weight and diet consumption during the 3 wk of treatment. No other signs of systemic toxicity or any adverse effects as monitored by activity and posture of mice were observed, suggesting that neither curcumin nor resveratrol alone, or in combination caused any deleterious effects under the present experimental conditions.
FIG. 5.
Curcumin in combination with resveratrol causes A: a greater inhibition of HCT-116 (wt) colon cancer xenograft growth in SCID mice, B: is associated with decreased proliferation (mitotic index), C: increased apoptosis [TUNEL assay], and D: decreased NF-κB DNA binding activity. *P < 0.01 compared to control. Data in panel (A) for control and curcumin are similar to those recently published by our laboratory (39).
To determine whether curcumin and/or resveratrol induced inhibition of tumor growth could in part be attributed to inhibition of proliferation and stimulation of apoptosis, remnants of tumors harvested at the end of the experimental period were examined for mitosis (mitotic index; MI) and apoptosis. As expected, although daily gavage of curcumin or resveratrol caused a 40–45% inhibition of proliferation, as evidenced by inhibition of mitotic index, the combination therapy produced a marked 70% inhibition of the same when compared with the controls (Fig. 5B). In contrast, number of cells undergoing apoptosis was increased by 215% in response to the combination therapy as opposed to 110% increase following curcumin or resveratrol treatment when compared with the controls (Fig. 5C).
Further, to unravel the molecular mechanism of therapeutic benefit observed by the combinatorial regimen in potentiating the antitumor effect, we performed EMSA to examine the status of the transcription factor NF-κB using harvested tumor tissue extracts. The results revealed that in response to the combination therapy of curcumin and resveratrol, the DNA binding activity of NF-κB was decreased by 67% as opposed to 30–35% in response to either curcumin or resveratrol when compared with the controls (Fig. 5D). Our results suggest that the inactivation of NF-κB is, at least, one of the molecular events by which the combination of curcumin and resveratrol potentiates antitumor growth in our experimental model.
DISCUSSION
Accumulating evidence indicates that the development and progression of many malignancies, including colorectal cancer, are associated with multiple signaling pathways that promote proliferation, inhibit apoptosis, and induce metastasis (32). Recent evidence indicates a crucial role for the EGF-receptor (EGFR) and/or some of its family members—specifically, ErbB-2/HER-2 and ErbB-3/HER-3—in regulating a number of pathways that affect tumor cell survival, angiogenesis, motility, and invasiveness (2–6). In addition, a growing number of studies have implicated the role of insulin-like growth factor (IGF)/IGF-receptor-1 (IGF-1R system in the development and progression of colorectal cancer (5,6). Since multiple signal transduction pathways become dysregulated in most malignancies, including colorectal cancer, it is likely that the maximal and most durable preventive and/or therapeutic benefit against tumor growth could be achieved with combination therapies that will affect several targets. Thus, agent(s)/regimen(s) that target EGFRs and IGF-1R should be more effective than narrowly focused therapies, as they are likely to impact several aspects of tumor progression.
Earlier, we reported that curcumin by itself was able to inhibit activation of EGFR, HER-2, and HER-3 (referred to as EGFRs) as well as IGF-1R in colon cancer HCT-116 cells (16,26). Moreover, in combination with either ERRP, a pan-ErbB inhibitor (30), or FOLFOX, curcumin caused a greater inhibition of activation of EGFRs as well as IGF-1R in colon cancer HCT-116 cells than either agent alone (16,26). Our current data from in vitro studies further demonstrate, for the first time, that like curcumin, resveratrol also inhibits EGFR and IGF-1R in colon cancer cells and that together they are more effective in attenuating the activation of EGFRs and IGF-1R than either agent alone. At least for inhibition of IGF-1R activation in response to the current treatment, this could partly be attributed to enhanced expression of IGFBP-3, a protein that sequesters IGFs, rendering IGFs unavailable for binding to and activation of IGF-1R. A similar observation was also noted in colon cancer cells in response to the combination treatment of curcumin and FOLFOX (26). Earlier epidemiologic studies have demonstrated that an increase in circulating IGF-1 and decreased IGFBP-3 levels are frequently associated with the development of several types of epithelial cancers including colorectal cancer (33). Furthermore, it has been demonstrated that blockade of the IGF/IGF-1R axis by a soluble inhibitor of IGF-1R attenuates IGF-1-induced Akt activation and inhibits growth of human colon cancer xenografts in mice (34). Additionally, several chemopreventive agents, particularly green tea polyphenols, have been shown to stimulate the expression of IGFBP-3 in TRAMP model of prostate cancer (35).
Our current data also demonstrate that curcumin or resveratrol alone or in combination causes inhibition of growth of p53 positive colon cancer HCT-116 as well as p53 mutant HT-29 cells, suggesting that the growth inhibitory properties of these agents are not cell specific and independent of p53 status. The current observation with curcumin is similar to what we noted earlier in colon cancer HCT-116 and HT-29 cells (16,26). Moreover, our current observation that resveratrol also inhibits the growth of p53 positive (wild type) and p53 negative colon cancer HCT-116 cells suggests that like curcumin, the growth inhibitory properties of resveratrol are also independent of p53 status.
Consistent with our in vitro observations, curcumin together with resveratrol produced a significantly greater inhibition of colon cancer cell growth in vivo in the SCID mice xenograft model than either agent alone. This could be partly attributed to decreased proliferation as evidenced by reduced number of mitototic cells and increased apoptosis in the tumors of curcumin, resveratrol, or a combination of both.
A plethora of evidence suggests that the chemopreventive effect of curcumin is mainly due to inhibition of COX-2, which leads to inhibition of prostaglandin E2 and subsequent colon cancer growth (36,37). Curcumin has also shown to be an effective inhibitor of cell growth in prostaglandin-synthesis deficient cancer cells (HCT-15), suggesting that it may also act via prostaglandin independent pathways (7). Nevertheless, numerous studies have suggested that the curcumin-induced COX-2 inhibition of growth of colon and other epithelial cancer cells is associated with attenuation of activation of the transcription factor NF-κB, which stimulates transcription of genes (such as COX-2) that are critically involved in inducing cell survival (17–19,38). Earlier, we reported that the inhibition of growth of colon cancer cells in vitro in response to either curcumin or curcumin together with ERRP is associated with a concomitant inhibition of NF-NF-κB activity (16). Data from our in vivo study with SCID mice xenograft model demonstrate, for the first time, that the combination therapy of curcumin and resveratrol causes a significantly greater inhibition of growth of tumors than either agent alone. This, together with the observation that inhibition of NF-κB activity in tumors is associated with a concomitant stimulation of apoptosis in the tumors, further indicates a role for NF-κB in regulating colon cancer tumor growth.
In summary, our data show that the combination therapy of curcumin and resveratrol is highly effective in inhibiting the growth of colon cancer cells in vitro and in vivo, which could be attributed to inhibition of proliferation and stimulation of apoptosis resulting from attenuation of NF-κB activity. Results of the in vitro studies suggest that curcumin or resveratrol also attenuates the constitutive activation of EGFR and its family members as well as IGF-1R, and that together, they cause a greater inhibition in activation of the growth factor receptors than that observed with either agent alone. Inhibition of IGF-1R activation in response to either mono or combinatorial treatment could be attributed to enhanced expression of IGFBP-3 leading to increased sequestration of IGF-1 by IGFBP-3, rendering the growth factor unavailable for binding to and activation of IGF-1R.
ACKNOWLEDGMENTS
The work was supported by grants from the National Institutes of Health/National Institute on Aging (APNM; R01 AG014343), Department of Veterans Affairs.
Contributor Information
Adhip P. N. Majumdar, John D. Dingell VA Medical Center, Karmanos Cancer Center, and Wayne State University, Detroit, Michigan, USA
Bhaumik B. Patel, John D. Dingell VA Medical Center and Karmanos Cancer Center, Detroit, Michigan, USA
Althea A. Elliott, John D. Dingell VA Medical Center, Detroit, Michigan, USA
Edi Levi, John D. Dingell VA Medical Center and Wayne State University, Detroit, Michigan, USA.
Fazlul H. Sarkar, Karmanos Cancer Center and Wayne State University, Detroit, Michigan, USA
REFERENCES
- 1.Bond JH: Colorectal cancer update: prevention, screening, treatment, and surveillance for high- risk groups. Med Clin North Am 84, 1163–1182, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Salomon OS, Brandt R, Ciardiello F, et al. : Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol 19, 183–232, 1995. [DOI] [PubMed] [Google Scholar]
- 3.Messa C, Russo F, Caruso MG, et al. : EGF, TGF-a and EGF-R in human colorectal adenocarcinoma. Acta Oncol 37, 285–289, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Harari PM and Huang S-M: Modulation of molecular targets to enhance radiation. Clin Cancer Res 6, 323–325, 2000. [PubMed] [Google Scholar]
- 5.Hakam A, Yeatman TJ, Liu L, Mora L, Marcet G, et al. : Expression of insulin-like growth factor-1 receptor in human colorectal cancer. Hum Pathol 30, 1128–1133, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Adachi Y, Lee CT, Coffee K, Yamagata N, Ohm JE, et al. : Effects of genetic blockade of insulin-like growth factor receptor in human colon cancer cell lines. Gastroenterology 123, 1191–1204, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Hanif R, Qiao L, Shift SJ, and Rigas B: Curcumin, a natural plant phenolic food additive, inhibits cell proliferation and induces cell cycle changes in colon adenocarcinoma cell lines bye prostaglandin-independent pathway. J Lab Clin Med 130, 576–584, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Chauhan OP: Chemotherapeutic potential of curcumin for colorectal cancer. Curr Pharm Des 8, 1695–1706, 2002, [DOI] [PubMed] [Google Scholar]
- 9.Shishodia S, Chaturvedi MM, and Aggarwal BB: Role of curcumin in cancer therapy. Curr Prob Cancer 31, 243–305, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Huang MI, Wang ZY, Georgiadis CA, Laskin JO, and Conney AH: Inhibitory effects of curcumin on tumor initiation by benzo[a]pyrene and 7,12-dimethylbenz[a anthracene. Carcinogenesis 13, 2183–2186, 1992. [DOI] [PubMed] [Google Scholar]
- 11.Rao CV, Simi B, and Reddy BS: Inhibition by dietary curcumin of azoxymethane-induced ornithine decarboxylase, tyrosine protein kinase, arachidonic acid metabolism and aberrant crypt foci formation in the rat colon. Carcinogenesis 14, 2219–2225, 1993. [DOI] [PubMed] [Google Scholar]
- 12.Rao CV, Rivenson A, Simi B, and Reddy BS: Chemoprevention of colon carcinogenesis by dietary curcumin, a naturally occurring plant phenolic compound. Cancer Res 55, 259–266, 1995. [PubMed] [Google Scholar]
- 13.Perkins S, Verschoyle RD, Hill K, Parveen I, Threadgill MO, et al. : Chemo-preventive efficacy and pharmacokinetics of curcumin in the Min/+mouse, a model of familial adenomatous polyposis. Cancer Epidemiol Biomarkers Prev 11, 535–540, 2002. [PubMed] [Google Scholar]
- 14.Sharma RA, Euden SA, Platton SL, Cooke ON, Shafayat A, et al. : Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res 10, 6847–6854, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Chen A and Xu J: Activation of PPARy by curcumin inhibits Moser cell growth and mediates suppression of gene expression of cyclin Dl and EGFR. Am J Physiol Gastrointest Liver Physiol 288, 0447–G56, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Reddy S, Rishi AK, Xu H, Levi E, Sarkar FH, et al. : Mechanisms of curcumin and EGF-receptor related protein (ERRP)-dependent growth inhibition of colon cancer cells. Nutr Cancer 55, 185–194, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Li Y and Sarkar FH: Inhibition of nuclear factor KB activation in PC3 cells by genistein is mediated via Akt signaling pathway. Clin Cancer Res 8, 2369–2377, 2002. [PubMed] [Google Scholar]
- 18.Das KC and White CW: Activation of NF-KB by antineoplastic agents: role of protein kinase. J Biol Chem 272, 14914–14920, 1977. [DOI] [PubMed] [Google Scholar]
- 19.Chuang SE, Yeh PY, Lu VS, et al. : Basal levels and patterns of anticancer drug-induced activation of nuclear factor KB (NE-KB), and its attention by tamoxifen, dexamethsone and curcumin in carcinoma cells. Biochem Pharmacol 63, 1709–1716,2002. [DOI] [PubMed] [Google Scholar]
- 20.Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, et al. : Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res 24, 3–59, 2004. [PubMed] [Google Scholar]
- 21.Jang M, Cai L, Udeani GO, Slowing Ky, Thomas CF, et al. : Cancer prevention activity of resveratrol, a natural product derived from grapes. Science 275, 218–220, 1997. [DOI] [PubMed] [Google Scholar]
- 22.Sengottuvelan M, Viswanathan P, and Nalini N: Chemopreventive effect of trans–resveratrol—a phytoalexin against colonic aberrant crypt foci and cell proliferation in 1,2-dimethylhydrazine induced colon carcinogenesis. Carcinogenesis 27, 1038–1046, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Schneider V, Duranton B, Gosse F, Schleiffer R, Seiler N, et al. : Resveratrol inhibits intestinal turnorigenesis and modulates host-defense-related gene expression in an animal model of human familial adenomatous polyposis. Nutr Cancer 39, 102–107, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Sengottuvelan M and Nalini N: Dietary supplementation of resveratrol suppresses colonic tumour incidence in 1 2-dimethylhydrazine-treated rats by modulating biotransforming enzymes and aberrant crypt foci development. Br J Nutr 96, 145–153, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Delmas D, Rebes C, Lacour 5, Filomenko R, Athias A, et al. : Resveratrol-induced apoptosis is associated with Fas redistribution in the rafts and the formation of a death-inducing signaling complex in colon cancer cells. J Biol Chem 278, 41482–41490, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Patel BB, Sengupta R, Qazi S, Vachhani, Yingjie Yu, et al. : Curcumin enhances the effects of 5-fluorouracil and oxaliplatin in mediating growth inhibition of colon cancer cells by modulating EGFR and IGF-1R. Int J Cancer 122, 267–273, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Schmelz EM, Xu H, Sengupta R, Du H, Banerjee S, et al. : Regression of early and intermediate stages of colon cancer by targeting multiple members of the EGFR family with EGF-receptor related protein. Cancer Res 67, 5389–5396, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Athar M, Back JH, Tang X, Kim KH, Kopelovich L, et al. : Resveratrol: a review of pre-clinical studies for human cancer prevention. Toxicol Appl Pharmacol 224, 274–283, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin YG, Kunnumakkara AB, Aggarwal BB, and Sood AK: Curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor-κB pathway. Clin Cancer Res 13, 3423–3430, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Xu H, Yu Y, Marciniak DJ, Rishi A, Sarkar FH, et al. : EGFR-Related Protein (ERRP) Inhibits multiple members of EGFR family in colon and breast cancer cells. Mol Cancer Ther 4, 435–442, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Marciniak D, Moragoda L, Mohammad RM, Yu Y, Nagothu KK, et al. : Epidermal growth factor receptor-related protein: a potential therapeutic agent for colorectal cancer. Gastroenterology 124, 1337–1347, 2003. [DOI] [PubMed] [Google Scholar]
- 32.McCarty MF: Targeting multiple signaling pathways as a strategy for managing prostate cancer: multifocal signal modulation therapy. Integr Cancer Ther 3, 349–380, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Rajaram S, Baylink DJ, and Mohan S: Insulin-like growth factor binding proteins in serum and other biological fluids: regulation and functions. Endocr Rev 18, 801–831, 1997. [DOI] [PubMed] [Google Scholar]
- 34.Adachi Y, Lee CT, Coffee K, Yamagata N, Ohm JE, et al. : Effects of genetic blockade of insulin-like growth factor receptor in human colon cancer cell lines. Gastroenterology 123, 1191–1204, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Adhami VS, Siddiqui IA, Ahmad N, Gupta S, and Mukhtar H: Oral consumption of green tea polyphenols inhibits insulin-like growth factor-I-induced signaling in an autochthonous mouse model of prostate cancer Cancer Res 64, 8715–8722, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Zhang F, Altorki NK, Mestre JR, Subbaramaiah K, and Dannenberg AJ: Curcumin inhibits cyclooxygenase-2 transcription in bile acid- and phorbol ester-treated human gastrointestinal epithelial cells. Carcinogenesis 20, 445–451, 1999. [DOI] [PubMed] [Google Scholar]
- 37.Goel A, Boland CR, and Chauhan DP: Specific inhibition of cyclooxygenase-2 (COX-2) expression by dietary curcumin in HT-29 human colon cancer cells. Cancer Lett 172, 111–118, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Aggarwal BB, Ichikawa H, Garodia P, Weerasinghe P, Sethi G, et al. : From traditional medicine to modern medicine: identification of therapeutic targets for suppression of inflammation and cancer. Expert Opin Targets 10, 87–118, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Milacic V, Banerjee S, Landis-Pinowar KR, Sarkar FH, Majumdar AP. Curcumin inhibits the proteosome activity in human colon cancer cells in vitro and in vivo. Cancer Res 68, 7283–7292, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]