Abstract
Objective.
Restoration of motor function in paralyzed limbs using functional electrical stimulation (FES) is undermined by rapid fatigue associated with artificial stimulation. Typically, single electrodes are used to activate muscles with FES. However, due to the highly distributed branching of muscle nerves, a single electrode may not be able to activate the entire array of motor axons supplying a muscle. Therefore, stimulating muscle with multiple electrodes might enable access to a larger volume of muscle and thereby reduce fatigue.
Approach.
Accordingly, we compared the endurance times that ankle dorsiflexion could be sustained at 20% maximum voluntary force using feedback controlled stimulation (25 Hz) of human tibialis anterior (TA) using one or four percutaneous intramuscular electrodes. In addition, we measured endurance times in response to direct stimulation of the nerve supplying TA and during voluntary contraction. In all sessions involving electrical stimulation, an anesthetic nerve block proximal to the site of stimulation was used to isolate the effects of stimulation and alleviate discomfort.
Main results.
Endurance time associated with stimuli delivered by a single intramuscular electrode (84 ± 19 s) was significantly smaller than that elicited by four intramuscular electrodes (232 ± 123 s). Moreover, endurance time in response to nerve stimulation (787 ± 201 s) was not significantly different that that produced during voluntary contraction (896 ± 272 s).
Significance.
Therefore, excessive fatigue associated with FES is probably due to the inability of conventional FES systems to enlist the full complement of motor axons innervating muscle and can be mitigated using multiple electrodes or nerve-based electrodes.
Keywords: functional electrical stimulation, fatigue, paralysis, skeletal muscle, spinal cord injury
Introduction
Functional electrical stimulation (FES) is a rehabilitative technology that serves to restore motor function in paralyzed individuals. FES takes advantage of the retained excitability of motor axons that innervate most paretic skeletal muscles. This enables induction of muscle contraction through artificial electrical stimulation delivered by surface electrodes, intramuscular electrodes, or by electrodes that encircle peripheral nerves supplying muscles. The utility of FES, however, is undermined because of the rapid muscle fatigue that occurs during FES (Bhadra and Peckham 1997, Mizrahi 1997, Kesar et al 2008, Doucet et al 2012, Guiraud et al 2014, Ibitoye et al 2016, Barss et al 2018). While a component of this accelerated fatigue is due to peripheral adaptations that occur in chronically paralyzed muscle (Grimby et al 1976, Martin et al 1992, Stein et al 1992, Shields 1995, Butler and Thomas 2003, Thomas et al 2003), FES-induced contractions also fatigue rapidly in able-bodied subjects (Naess and Storm-Mathisen 1955, Binder-Macleod and Snyder-Mackler 1993, Karu et al 1995).
One reason proposed to account for rapid fatigue with FES is that the normal recruitment order of motor units, from weakest and most fatigue resistant toward the strongest and most fatigable, is disrupted. This is thought to occur because extracellular stimulation favors activation of the larger diameter axons (Blair and Erlanger 1933, McNeal 1976, Rattay 1986, Fang and Mortimer 1991, Grill and Mortimer 1995) that innervate strong, fatigable motor units (Wuerker et al 1965, Jami and Petit 1975, Zajac and Faden 1985). In addition, everything else being equal, axons closest to the stimulating electrode are those most readily activated by electrical stimulation (Mortimer 1981, Grill and Mortimer 1995). Because axons of varying diameters appear to be intermingled within motor nerves and muscle, there would be no particular spatial bias favoring activation of one type of motor unit over another (Thomas et al 2002). As a consequence, investigators have suggested that electrical stimulation tends either to invert the normal recruitment order (Parker et al 1986, Kubiak et al 1987, Sinacore et al 1990, Trimble and Enoka 1991, Binder-Macleod and Snyder-Mackler 1993, Yoshida and Horch 1993, Heyters et al 1994, Mizrahi 1997, McDonnall et al 2004, Navarro et al 2005, Sheffler and Chae 2007, Malešević et al 2010) or to activate motor units in a relatively random way (Knaflitz et al 1990, Binder-Macleod et al 1995, Feiereisen et al 1997, Bickel et al 2011, Barss et al 2018). It should be noted, however, that some studies have shown little disruption in normal recruitment order with electrical stimulation (Thomas et al 2002, Farina et al 2004).
A second reason often cited as a possible cause for rapid fatigue with FES is related to the synchronized discharge of motor units induced by peripheral electrical stimulation (Binder-Macleod and Snyder-Mackler 1993, Karu et al 1995, Mizrahi 1997, Chou et al 2008, Popović and Malešević 2009, Malešević et al 2010, Rohm et al 2013, Sayenko et al 2014, Downey et al 2015, Lou et al 2017, Barss et al 2018, Zheng and Hu 2018). Such synchronization can lead to marked fluctuations in evoked force, which in turn, can itself provoke fatigue because of the additional work required by the contractile apparatus repeatedly shortening against series elastic elements in muscle (Garland et al 1988, Sandercock 2006). To minimize force fluctuations (which also compromises force control), stimulus frequencies can be increased. Yet excessively high stimulus frequencies can also promote rapid fatigue (Naess and Storm-Mathisen 1955, Jones et al 1979, Metzger and Fitts 1986, Jones 1996, McDonnall et al 2004). Therefore, some FES investigators have turned to asynchronous stimulation (Lind and Petrofsky 1978, Yoshida and Horch 1993, Wise et al 2001, McDonnall et al 2004, Malešević et al 2010, Nguyen et al 2011, Maneski et al 2013, Sayenko et al 2014, Downey et al 2015, Bergquist et al 2016, 2017, Laubacher et al 2017, Lou et al 2017), an approach originally described by Rack and Westbury (1969), wherein different sets of motor units are activated sequentially at relatively low rates using multiple electrodes. Such asynchronous (or interleaved stimulation) can produce reasonably smooth muscle force despite low stimulus rates delivered to each set of motor units that, on their own, would cause markedly unfused contractions (Rack and Westbury 1969, Wise et al 2001, Sandercock 2006).
What is puzzling, however, is that the advantage of asynchronous over synchronous stimulation practically disappears for stimulus rates above ~10 Hz (Rack and Westbury 1969, Sandercock 2006). And while those studies involved cat soleus, the average contraction time of cat soleus (76 ms, Nelson (1969)) is briefer (and hence, the fusion frequency higher) than that found in many lower limb muscles of humans (e.g. 81 ms for tibialis anterior, Marsh et al (1981), 87 ms for quadriceps, Bergstrom and Hultman (1990), 104 ms for triceps surae, Marsden and Meadows (1970)). Yet, many interleaved FES protocols involve frequencies ⩾10 Hz (Malešević et al 2010, Nguyen et al 2011, Maneski et al 2013, Sayenko et al 2014, Bergquist et al 2016, 2017, Lou et al 2017). Indeed, we have recently shown that there was no difference in the degree of fatigue induced with interleaved versus synchronous stimulation when delivered to two different locations in a muscle using stimulus frequencies >15 Hz at each electrode (Buckmire et al 2018). Therefore, it seems possible that the documented improvement in fatigue resistance using interleaved stimulation compared to single site stimulation (Malešević et al 2010, Nguyen et al 2011, Sayenko et al 2014, Downey et al 2015, Bergquist et al 2016, Laubacher et al 2017, Lou et al 2017) was not primarily because of the asynchronous activation per se. Rather, given the widespread distribution of motor nerve branches within human muscle (Amirali et al 2007, Mu and Sanders 2010, Won et al 2011, Yu et al 2016), multi-site stimulation may simply enable access to more of the muscle fibers within a muscle (Buckmire et al 2018).
To test this possibility, here we compared the duration that submaximal isometric contractions of human tibialis anterior could be sustained when feedback-controlled electrical stimulation was delivered through a single intramuscular electrode to that delivered synchronously through multiple electrodes. In addition, in separate sessions we also measured contraction duration evoked by direct stimulation of the peripheral nerve proximal to its entry into tibialis anterior (i.e. at a site where motor axons are spatially constrained) and during voluntary contractions.
We found that multi-electrode stimulation markedly extended the endurance time of submaximal contractions over single-electrode stimulation. Moreover, the duration of contractions induced by electrical stimulation delivered directly to the peripheral nerve was no different, and in some cases longer, than that achieved during voluntary contractions. These findings indicate that the rapid fatigue associated with conventional FES is unlikely to be primarily caused by synchronized discharge or disrupted recruitment order of motor units but rather because only a fraction of the motor units can be readily enlisted using single electrodes placed in or over muscle.
Methods
Subjects and muscle
Five healthy human subjects (one female, four male), ages 20 to 58 were included in this study in accordance with human subjects guidelines and approved by the University of Arizona institutional review board. Each subject participated in four experimental sessions (separated by ⩾2 d) involving sustained isometric contraction of the tibialis anterior muscle. The tibialis anterior (TA) was selected for this study because it is readily accessible for intramuscular stimulation and it generates the preponderance of the dorsiflexion torque at the ankle. In addition, the nerve supplying the TA (deep peroneal neve) is reasonably accessible for stimulation while the major nerve (common peroneal nerve) giving rise to the deep peroneal nerve can be anesthetically blocked several centimeters proximal to the deep peroneal nerve, thereby isolating the TA for study.
Force and EMG measurements
Subjects were seated in a dental chair with their knee extended and their right foot secured to a custom-built footplate instrumented with a transducer to measure isometric force during dorsiflexion. The footplate rotated freely about an axis aligned approximately co-linear with the talocrural joint axis of the ankle. Once the foot was secured with Velcro straps, the footplate was rotated such that it held the ankle in a plantar-flexed position. An isometric force transducer (Grass FT-10, Warwick, RI, USA using custom-built heavy-duty springs inserted into the housing of the transducer) was then attached to the distal end of the footplate (22.5 cm from the axis of rotation of the footplate) that resisted ankle dorsiflexion. The knee was held in an extended position with a wide strap that ran over the anterior surface of the distal thigh and was tightened and secured to the chair. Bipolar surface electrodes (4 mm diameter, ~5 cm inter-electrode separation) were placed on the skin over the TA and over the triceps surae to record electromyographic (EMG) activity. EMG signals were amplified (×1000, band-pass filtered 30 to 1000 Hz., Grass Technologies Product Group, Astro-Med Inc.; West Warwick Rhode Island). Force and EMG signals were digitally sampled (1000 and 4000 samples s−1, respectively) by a computer-controlled data acquisition system (Power 1401, Spike2, Cambridge Electronic Design, Cambridge England).
Electrical stimulation
Current-regulated stimuli (0.25 ms duration, rectangular, monophasic, cathodic pulses) were delivered to the TA or deep peroneal nerve through percutaneous tungsten microelectrodes (250 μm shaft diameter, 1–5 μm tip diameter, 2–4 mm of insulation removed from the tip, 30 mm total length, Frederick Haer, Bowdoin Maine, USA) using a programmable multi-channel stimulator (STG4008 MultiChannel Systems, Reutlinger, Germany). Surface electrodes (Covidien/Kendall, Pediatric cloth ECG Hydrogel Electrodes H59P, Medtronic, Dublin, Ireland) placed over the tibia or the lateral malleolus of the fibula served as common return electrodes for electrical stimulation. Current pulses delivered by the stimulator were digitally sampled (12 kHz) by measuring the voltage drop across an in-series resistance (~150 Ω).
Anesthetic block
Strong electrical stimulation can be painful. Such painful stimuli can trigger spinal reflexes and descending activity that interferes with measures of force from the target muscle. Furthermore, some subjects may not tolerate the high stimulus intensities delivered over prolonged periods needed for tests of muscle endurance. Therefore, we used an anesthetic block of the common peroneal nerve supplying the TA to largely eliminate sensory feedback associated with the stimulation and to fully paralyze the TA.
Under ultrasound guidance, 10–15 ml of 1.5% Mepivacaine was administered to the peri-neural space surrounding the common peroneal nerve at a site ~8–10 cm proximal to the head of the fibula. Complete anesthetic block was confirmed by the subject’s inability to voluntarily generate detectable dorsiflexion force. This occurred within ~20 min of the injection in all cases but one. In that one case where a complete nerve block was not achieved, the experimental session was terminated and the subject returned on a different day during which the nerve block was successful. As a precaution, prior to the nerve block, an intravenous line was placed into a peripheral vein in the upper extremity to administer fluids or medications in the unlikely event of anesthetic toxicity. No such events occurred in any of the subjects tested. Following experiments involving the nerve block, subjects wore a plastic ankle cast to prevent foot drop for a period of about 4–5 h until the paralysis resolved.
Procedure
Subjects participated in four experimental sessions in random order, one session involving sustained voluntary contraction of the TA and three sessions involving sustained stimulation of the TA with a nerve block present. For sessions using electrical stimulation, in one session a single intramuscular electrode was used to deliver stimuli, in a second session four intramuscular electrodes distributed throughout TA and each controlled by a separate stimulus channel were used to deliver stimuli, and in a third session stimuli were delivered by a single electrode placed adjacent to the deep peroneal nerve just distal to the fibular head.
In each session, subjects first performed three brief (~2 s duration) maximum voluntary contractions (MVC) of ankle dorsiflexion with about 60 s between trials. The largest force exerted among the three trials was deemed the MVC force. For the voluntary fatigue task, subjects observed a target force of 20% MVC displayed on a computer screen and matched that force by isometric dorsiflexion of the ankle. To keep subjects motivated, subjects were verbally encouraged throughout the contraction to sustain the target force for as long as possible (e.g. Fuglevand and Keen (2003)). The task was terminated when the force continuously remained below the target force for a period of 5–10 s.
For sessions involving intramuscular stimulation, sterilized microelectrodes were inserted through alcohol cleansed skin and into the TA following induction of paralysis by the nerve block. For experiments involving a single intramuscular electrode, the initial electrode placement was at a proximal site ~1/3 of the length of the muscle and ~2 cm lateral to the tibial ridge. This site was selected based on nerve dissection (Watt et al 2013) and surface EMG-array studies (Barbero et al 2012) indicating that this site approximates the location where major branches of the deep peroneal nerve typically penetrate the TA. For experiments involving four electrodes, one electrode was inserted at this proximal site, two electrodes were placed at ~50% of the length of the TA with one located ~1 cm lateral to the tibial ridge and the other ~3 cm lateral to the ridge, and the fourth electrode was inserted at a distal site ~2/3 of the length of muscle and inserted into the midline of the TA.
Each electrode was initially inserted to a depth of about 5–8 mm below the skin. Brief (1 s) trains of stimuli (5 mA, 25 Hz) were delivered and the evoked force recorded. The electrode was then advanced in ~2 mm steps to a maximum depth of ~30 mm with stimulation repeated at each step. Electrode depth was estimated at each stimulation site by measuring the length of the electrode extending above the skin surface. The insertion site was marked with ink and then the electrode was removed and reinserted at sites ~1–2 cm proximal, distal, medial, and lateral to the original insertion site and the process repeated. Following this survey, the electrode was then reinserted at the site that evoked the largest force in response to the stimulus train. Stimulation was repeated at this site to confirm similar levels of evoked force as detected originally. If needed, small adjustments to the electrode depth were made to ensure robust force responses were evoked. In cases involving four electrodes, this process was repeated for each electrode. The time needed to place all four electrodes was usually about 1 h. Because of concerns that the anesthetic might begin to wear off, we did not carry out additional procedures to assess the degree of independence of each electrode by stimulating each electrode separately and in various combinations with other electrodes and measuring the degree of force summation.
For sessions involving nerve stimulation, a single tungsten microelectrode was inserted at an oblique angle to the skin immediately distal to the head of the fibula in order to approach the deep peroneal nerve. The electrode position was manually adjusted until strong dorsiflexion forces were elicited in response to 1 s trains of 1 mA pulses delivered at 25 Hz. We often also observed toe extension during stimulation of the nerve indicating activation of extensor hallucis longus and extensor digitorum longus. This was largely unavoidable because axons to those muscles are also carried in the deep peroneal nerve.
Once electrodes were in place, single stimulus pulses were delivered to each electrode separately and the associated twitch forces were recorded with amplitude incremented in 1 mA steps from 1 mA to 32 mA and with a 2 s delay between pulses. The associated current—twitch force relationships were evaluated immediately to identify the operating range of currents for each electrode for the upcoming fatigue task.
Prior to the fatigue test, a few 1 s trains of stimuli (25 Hz) were delivered to identify the stimulus pulse amplitudes needed initially to elicit the 20% MVC target force. For the case involving four electrodes, stimulus amplitudes were identified separately for each electrode that evoked ~5% MVC force (assuming that the forces would sum near linearly). A custom-designed Matlab (Mathworks, Natick MA) program was then used to provide on-line feedback control of stimulus pulse amplitude during the fatigue task.
The inputs to the program included the target force (20% MVC), the starting current levels (based on those identified with 1 s trains), the upper current levels (based on the current—twitch force relationship), the force exerted by the subject sampled in real time, and a gain factor. Force was sampled in parallel by two data acquisition systems: one dedicated exclusively for feedback control (120 samples s−1, USB 6001, National Instruments, Austin, TX) and one for general data acquisition and storage that was used for off-line analyses (1000 samples s−1, CED Spike2). The gain factor in this simple proportional feedback system was used to transform the detected error between target and a six-point (50 ms) moving average of the actual force into a current adjustment scaled to the operating range of the electrode. We used a nominal gain value of 0.25 indicating that 25% of the full current range would be added to the ongoing current in the case of an error representing 100% of the full force range. In brief tests before the fatigue run, if overt force oscillations developed, we reduced the magnitude of the gain. If, on the other hand, evoked force was slow to approach the target, we increased the gain. For the majority of cases tested, however, a gain of 0.25 worked reasonably well.
The commanded adjustments in current amplitude were then dispatched every ~240 ms to the MultiChannel Systems stimulator that delivered continuous 25 Hz (0.25 ms pulse duration) stimuli to the electrode(s). A stimulus frequency of 25 Hz was selected because it evokes fused force responses and is within the upper range of motor unit firing rates of TA recorded during voluntary contractions (Connelly et al 1999, De Luca and Hostage 2010). In the case of multielectrode stimulation, the timing of the pulses were offset by 1 ms across electrodes to help prevent summation of otherwise subthreshold electric fields (i.e. ‘subliminal fringe’) at sites relatively distant from the electrodes (Mortimer 1981, Branner et al 2001). For example, we found in some experiments that precisely synchronized stimulation (i.e. without the 1 ms delay) could lead to overt plantar flexion that was not evoked by any electrode alone when stimulating using maximal intensities. Feedback-controlled stimulation was maintained until the evoked force was clearly below the target force by at least 10% for ~5–10 s despite escalating current intensities.
Data analysis
For each fatigue trial, we used a custom-written program (Spike2) to measure the endurance time as the duration from when the force initially came within 10% of the target force until the time when force fell 10% below the target for more than 5 s. A one-way repeated-measures analysis of variance (ANOVA) was performed to determine whether endurance time varied significantly with different fatigue protocols. Mann–Whitney rank sum post-hoc test was used to evaluate differences in endurance times between fatigue protocols with Bonferroni correction for multiple comparisons. The level of statistical significance was set at P < 0.05 and data are reported as means ± one standard deviation (SD).
Results
Current—twitch force relation
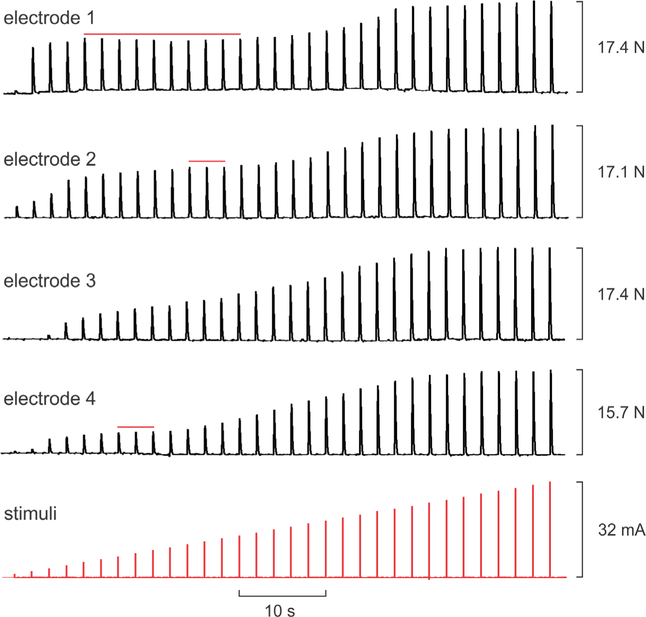
Figure 1 shows example twitch force responses to escalating current-amplitude stimuli delivered during separate trials to each of four intramuscular electrodes placed in different locations in TA in one subject during a single session. In this example, a small twitch was detected for the lowest current delivered (1 mA) on electrodes 1, 2, and 4, while 3 mA of current was needed to elicit a detectable twitch on electrode 3. Peak twitch forces of 17.4, 17.1, 17.4, and 15.7 N were attained at 31, 32, 29, and 30 mA for electrodes 1–4, respectively. The difference in peak forces between the electrode that evoked the largest twitch and that which evoked the smallest was 9.8%.
Figure 1.
Example dorsiflexion twitch force responses (upper four traces) to increasing stimulus current pulses (bottom trace) delivered through four intramuscular electrodes placed in different locations within tibialis anterior in one subject. Stimulation through each electrode was performed in separate trials but traces have been aligned for compact display. Red horizontal lines indicate intermediate plateaus wherein force saturated across a range of increasing stimulus intensities.
Across all subjects and all cases involving one or four intramuscular electrodes, the average threshold current for evoking a detectable twitch response was 1.6 ± 1.0 mA while the average current associated with peak twitch force was 27.4 ± 5.7 mA (range 9–32 mA). For the case of four intramuscular electrodes, the average percent difference across electrodes evoking the largest and smallest peak forces was 25.6% ± 18.1% (range 9.8%–51.6%). During nerve stimulation we did not get clear measures of the threshold currents due to the large increments (1 mA) used for the current—twitch force assessment. In all subjects during nerve stimulation, 1 mA (the smallest value tested) always evoked a strong twitch (>50% of the peak twitch force) while the current associated with peak twitch force was ⩽5 mA.
Intermediate plateaus
While twitch forces tended to progressively increase with current above threshold up to the current associated with peak force, there were often intermediate plateaus wherein evoked force saturated across a range of increasing currents. Such plateaus are highlighted with red horizontal lines in the examples shown in figure 1. Force responses to stimuli delivered by electrode 1, for example, saturated across a nearly three-fold increase in stimulus intensities (from 5–14 mA). We quantified the prevalence of such intermediate plateaus using a method we described previously (Buckmire et al 2018). Namely, we calculated the percentage change in force associated with each 1 mA increment in current for all of the current—twitch force sequences involving intramuscular electrodes. We then identified the number of cases for which the change in force fell below 5% for two or more consecutive steps in current and which was then followed by increases in force above 5%. For all 25 intramuscular electrode sequences tested (20 from 4-electrode experiments, five from 1-electrode experiments), 80% exhibited one or more intermediate plateaus. The average span of currents over which twitches saturated during these intermediate plateaus was 4.7 ± 3.4 mA (range 2–14 mA).
Fatigue
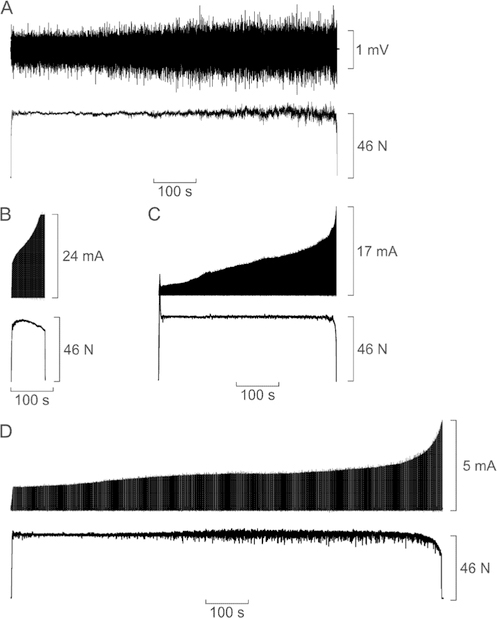
The target force for all fatigue trials was set at 20% of the MVC obtained in each session. Across all sessions and subjects, the average MVC force was 218.8 ± 32.2 N (49.2 ± 7.2 N • m of torque). There was little variation in MVC force across sessions for individual subjects (average coefficient of variation =5.6% ± 3.9%). Figure 2 shows example force responses obtained in a single subject during sustained voluntary effort (figure 2(A)), stimulation of the TA with a single intramuscular electrode (figure 2(B)), stimulation of the TA with four intramuscular electrodes (figure 2(C)), and stimulation of the deep peroneal nerve supplying TA (figure 2(D)). The TA surface EMG is shown in figure 2(A), whereas the feedback controlled stimulus currents are shown in figures 2(B)–(D). For clarity, only one of the four stimulus-current signals is shown in figure 2(C). All four panels in figure 2 are depicted using the same time base so their durations can be directly compared.
Figure 2.
Example force responses (bottom traces) recorded in a single subject during four sessions involving (A) voluntary contraction, (B) intramuscular stimulation of the tibialis anterior (TA) with a single electrode, (C) intramuscular stimulation of the TA with four electrodes, (D) stimulation of deep peroneal nerve. In (A), the top trace shows the surface EMG signal recorded from the TA. In (B)–(D), the top trace indicates the feedback controlled stimulus current delivered to the electrodes. In (C), only one of the four stimulus current signals is depicted for clarity. All four stimulus current signals associated with the trial shown in (C) increased exactly in parallel but the absolute values were different.
During the voluntary contraction (figure 2(A)), EMG activity progressively increased reflecting increased motor unit recruitment and rate coding needed to compensate for diminishing force capacities of the active muscle fibers. Eventually, however, the increased drive to the muscle was insufficient to maintain the target force and the contraction was halted. Across all five subjects, the average value of the rectified TA EMG signal measured over the last 10 s of the trial was 71.6% ± 34.5% (range 47.8%–132.1%) larger than that measured over the initial 10 s. The endurance time for the voluntary contraction shown in figure 2(A) was 740 s. In the session involving stimulation with a single intramuscular electrode (figure 2(B)), ~10 mA of current was needed at the outset to achieve the target force. The stimulus current then increased rapidly up to its assigned upper limit of 24 mA under feedback control in order to maintain the target force. Despite the increasing current, force decayed slowly over much of the trial and stimulation was halted when the force was <90% of the target level. The endurance time for this trial was 80 s. When four intramuscular electrodes were used to deliver stimuli (figure 2(C)), force was maintained five times longer (endurance time 448 s) compared to stimulation with a single electrode. At the outset, the evoked force overshot the target but with feedback control, the stimulus intensities delivered to the four electrodes were rapidly adjusted and the evoked force was then stably maintained at the target force. Stimulus current then increased almost linearly over much of the trial except near the end when current amplitude rapidly accelerated in an attempt to maintain the target force in the face of weakening output of the active muscle fibers. Despite this increase in stimulus intensity, additional force was not generated and the trial was halted when force dropped below 90% of the target even though current had not reached the upper limit on any of the four stimulus channels.
The most remarkable trial was that associated with nerve stimulation (figure 2(D)). In this case, the endurance time (993 s) far surpassed (by 34%) that of the voluntary contraction (figure 2(A)). The stimulus currents involved were much lower than that used for intramuscular stimulation and the amplitude only gradually increased over much of the trial except a sharp escalation near the end. In one other subject, endurance time of nerve stimulation exceeded that of the voluntary contraction by 42%. As can be seen in figure 2(D), force fluctuations were evident throughout the trial but grew in intensity during the latter two-thirds of the trial. These force fluctuations were seen in all subjects and were dominated by a 4.2 Hz oscillation. It was only discovered later that these oscillations were due to an inadvertent lengthening of every 5th interpulse interval (occurring at cycle period of ~240 ms), presumably due to buffering delays in the computer-stimulator interface. As such, the average stimulus rate was ~22 Hz rather than 25 Hz for all subjects.
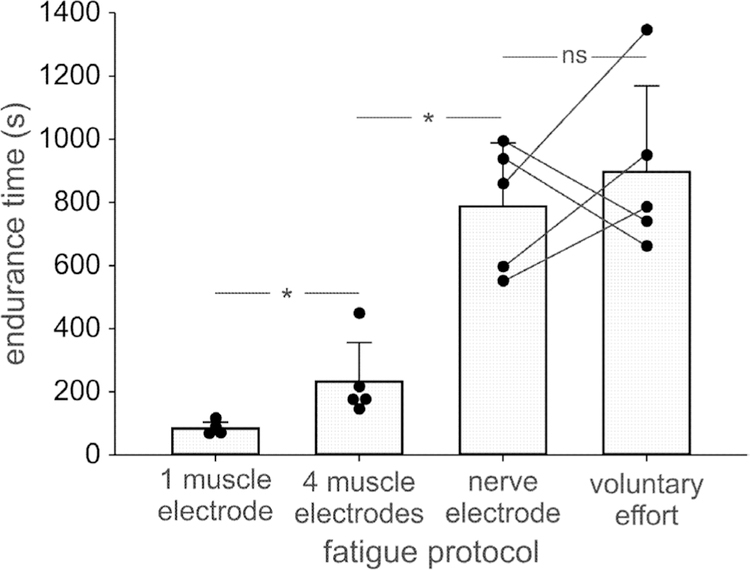
The mean (SD) endurance times for the five subjects across the four fatigue tasks is shown in figure 3. ANOVA indicated a significant effect of fatigue task on endurance time (P < 0.001). Post hoc analysis indicated no significant difference (P = 0.46) in the mean endurance times between voluntary (896 ± 272 s) and nerve stimulation tasks (787 ± 201 s). The mean endurance time associated with stimuli delivered by a single intramuscular electrode (84 ± 19 s) was significantly (P < 0.01) smaller than that elicited by four intramuscular electrodes (232 ± 123 s). All other between-task comparisons were significant (P < 0.01).
Figure 3.
Mean (SD) and individual values (dots) of endurance times of ankle dorsiflexion at a 20% MVC force target in response to feedback controlled stimulation of tibialis anterior with one or four intramuscular electrodes, feedback controlled stimulation of the deep peroneal nerve, or during voluntary contraction. ns—non-significant difference (P = 0.46). *—significant difference (P < 0.01).
Discussion
Here we have shown that the rapid fatigue associated with electrical stimulation of muscle can be partially mitigated by increasing the number of intramuscular electrodes used to activate muscle. Furthermore, stimulating the nerve proximal to where it enters muscle produced a target force that could be sustained as long as, and in some cases longer, than that produced during voluntary contraction. We conclude, therefore, that the excessive fatigue associated with FES must primarily be due to the inability of conventional FES systems to enlist the full complement of motor units within muscle. Moreover, these results indicate that neither altered motor unit recruitment order nor synchronized motor unit activity can account for much of the fatigue seen with FES as both of these factors presumably were in play in the present experiments.
A likely explanation as to why a single stimulating electrode (as has been the convention in many FES systems) is unable to activate all the motor units in a muscle is because of the widely distributed arrangement of nerve branches within muscle. A long-held view is that nerves typically enter muscle at a single location to innervate muscle fibers along a constrained central region referred to as the innervation zone (Coërs and Telerman-Toppet 1977, Lee et al 2012, Behringer et al 2014, Jahanmiri-Nezhad et al 2015). Yet, many anatomical studies have clearly shown extensive and complex ramification of nerve branches prior to entry into (Sunderland and Hughes 1946) and throughout large expanses of skeletal muscle (Amirali et al 2007, Mu and Sanders 2010, Won et al 2011, Yu et al 2016). Because of the steep decay in the electric field with distance from a stimulating electrode (McIntyre and Grill 2002, Rattay 2004), it may be challenging in practice to deliver sufficient current to excite all of the widely dispersed nerve branches, particularly in large muscles such as the tibialis anterior. As such, using more electrodes situated in different regions of a muscle should enable electrical access to a larger subset of the nerve branches. In support of this idea, we have recently shown that the maximum force that could be evoked using intramuscular electrodes was always greater when using multiple compared to a single electrode (Buckmire et al 2018).
Indeed, the prevalence of intermediate plateaus in evoked force responses to increasing stimulus intensities observed here (figure 1) and previously (Crago et al 1980, Cameron et al 1998, Buckmire et al 2018) probably reflects the presence of widely separated nerve branches within muscle. Namely, the initial increase in force with increased stimulus intensity likely arises due to progressive activation of more motor axons contained within nerve branches in the vicinity of the electrode. Eventually, however, most motor axons in such nearby branches might be recruited and thereafter, no additional force would be elicited over a range of increased stimulus strengths (Cameron et al 1998). At some point, however, sufficient current could be delivered such that other distant nerve branches begin to be activated, leading to an additional rise in evoked forces with increasing current. In the present experiment, there was no way to be sure that additional intermediate plateaus might have been detected had we delivered currents higher than the 32 mA maximum allowed by our stimulator.
It is possible that secondary increases in evoked force after a plateau might have been due to activation of neigh-boring synergist muscles, such as extensor hallucis longus or extensor digitorum longus, both of which contribute to ankle dorsiflexion. Yet, we only rarely detected toe extension during intramuscular stimulation of tibialis anterior, which would have been indicative of activating those synergists. Furthermore, such intermediate plateaus were observed in response to intramuscular stimulation of cat hindlimb muscle when no muscles, other than the target muscle, were attached to the force transducer (Crago et al 1980, Cameron et al 1998).
In the context of fatigue resistance, the ability to engage more motor units with multiple electrodes is beneficial. A larger reserve of motor units that can be called upon (by increasing stimulus strength) as force declines in earlier activated motor units will enable a given target force to be maintained for a longer duration. Indeed, when we stimulated the deep peroneal nerve (at a site where most of the motor axons supplying tibialis anterior are bundled together), no evidence of excessive fatigue was found. Moreover, in two of the five subjects tested, the duration over which the target force could be sustained with such nerve stimulation exceeded that associated with voluntary effort. Although some previous studies have shown that the extent of fatigue to be similar during sustained maximum voluntary contractions and maximal nerve stimulation (Merton 1954, Bigland-Ritchie et al 1979, Jones et al 1979, Marsden et al 1983), we are unaware of previous cases for which fatigue resistance of electrically evoked contractions surpassed that of voluntary effort.
While caution against over-interpretation of these two cases is certainly warranted, some consideration as to why such supra-endurance arose in these cases seems worthwhile. First, it is possible that the two subjects simply did not exert themselves fully to sustain the voluntary contraction for as long as possible. Indeed, those two subjects had the briefest endurance times associated with the voluntary contraction (see figure 3). Yet, both subjects showed substantial increases in TA EMG during the voluntary fatigue task indicative of increasing exertion. Indeed, the subject who showed the greatest increase in endurance time with nerve stimulation compared to voluntary contraction also exhibited the greatest increase (>100%) in TA EMG during the voluntary task.
Thus, if taken at face value, it is then important to ask why such supra-endurance has not been observed previously. Perhaps one reason is that few other studies have used feedback control during electrical stimulation to determine the duration over which a given target force can be maintained. Rather, most fatigue studies involving electrical stimulation measure the change in force in response to a fixed stimulus intensity applied over a set duration (e.g. Yoshida and Horch (1993), Thomas et al (2002), Lou et al (2017) and Buckmire et al (2018)). Because it is difficult to ‘clamp’ the intensity of voluntary drive during fatiguing contractions, it is not possible to directly compare such electrically evoked contractions to that produced voluntarily. On the other hand, voluntary contractions naturally lend themselves to visual feedback control of a displayed target force and as such, can be compared to that produced by electrical stimulation under force-feedback control, as was done here. It should be said that under open-loop stimulation involving fixed stimulus intensities, the same set of motor units would be activated throughout a stimulation bout. In this case, the tendency of extracellular stimulation to favor activation of higher threshold, fatigable motor units would indeed contribute to more rapid loss in force than that associated with activation of motor units that mimics that which occurs naturally.
A second possible reason for the absence of such observations previously is that few such studies have used anesthetic nerve blocks as we used here to isolate the effects of electrical stimulation. Intense electrical stimulation delivered to muscle not only activates motor axons but also engages an array of sensory axons including nociceptors. The associated sensory signals can provoke spinal reflexes and perhaps even descending inputs leading to unregulated contraction of agonists and antagonists, which in turn contaminates the force signals meant to detect the effect of electrical stimulation alone (Lagerquist et al 2009). Furthermore, subjects may not readily tolerate the pain associated with prolonged intense stimulation and investigators may avoid imposing such discomfort on human volunteers.
An additional possible reason relates to the fortuitous selection of the TA as the target muscle in the present study. The deep peroneal nerve supplying the TA arises as one of two main branches (the other being the superficial peroneal nerve) of the common peroneal nerve. From this bifurcation point, there typically is about a one centimeter span of the deep peroneal nerve before it gives rise to the first of multiple branches destined for the TA along ~20 cm of length of the nerve (Sunderland and Hughes 1946). This span of the nerve was targeted for stimulation in the present experiments. What is advantageous about this site is that it also carries axons supplying the two other ankle dorsiflexors, extensor hallucis longus and extensor digitorum longus. Therefore, stimulation at this site engaged all of the ankle dorsiflexors (as evidenced by toe extension as well as ankle dorsiflexion during nerve stimulation), just as would likely occur during voluntary dorsiflexion. Consequently, the total muscle mass involved in voluntary and nerve stimulation experiments was reasonably similar.
It should be noted, however, that the branching patterns of the peroneal nerves are highly variable across human cadaver specimens (Sunderland and Hughes 1946, Aigner et al 2004). For example, in six of 20 specimens, the first branch to the TA arose from the common peroneal nerve above its bifurcation to the deep and superficial nerves (Sunderland and Hughes 1946). Therefore, a portion of the TA would not have been activated with nerve stimulation in the present study in subjects with such a branching arrangement. In addition, the superficial peroneal nerve runs almost adjacent to the deep peroneal nerve in the region targeted for stimulation such that the distance between the centers of the two nerves may be as small as 5 mm (Aigner et al 2004). It is possible, therefore, that with increasing stimulus intensity during the fatigue task, some portion of the superficial peroneal nerve supplying the peroneus muscles, may have been activated. Indeed, in some subjects, we visually observed contraction of the peroneus muscles during the later stages of the fatigue protocol involving nerve stimulation. Because the peroneus muscles contribute to ankle plantarflexion, their activation would tend to curtail dorsiflexion endurance time. Therefore, these two factors (possibility of not activating all nerve branches to TA and possibility of activating antagonists), may have limited the measured endurance time in response to nerve stimulation in some of the subjects.
The results of the nerve stimulation experiments also bear on fundamental questions related to the contribution of the central nervous system (CNS) to voluntary muscle fatigue. The observation that endurance time in some cases was longer with electrical stimulation than during voluntary contraction strongly suggests some degree of failure of the CNS to fully engage muscle during prolonged activity in those cases. There is a significant body of work that supports this contention and numerous mechanisms have been proposed to account for such fatigue-related impairment of CNS drive (see reviews by Gandevia (2001) and Taylor et al (2016)).
Finally, based on the findings of the present study, it would seem appropriate to consider using multiple stimulating electrodes (particularly for large muscles), and where possible, to stimulate the peripheral nerves supplying muscle for FES applications in paralyzed individuals. Of course, this must be weighed against the increased complexity of the control system and associated hardware, and added surgical challenges for implanted systems (Memberg et al 2014). For therapeutic interventions involving surface electrodes, using more than one active electrode would also seem beneficial. Indeed, the efficacy of interleaved stimulation among multiple surface electrodes suggests this to be the case (Malešević et al 2010, Nguyen et al 2011, Maneski et al 2013, Sayenko et al 2014, Downey et al 2015, Bergquist et al 2016, 2017, Laubacher et al 2017, Lou et al 2017). The results reported here and previously (Buckmire et al 2018) indicate that the improved fatigue resistance associated with interleaved stimulation is most likely related to the use of multiple electrodes (providing access to a greater volume of muscle) rather than the asynchronous activation induced by the interleaved protocol. Additional studies will need to be performed to determine the degree of improved fatigue resistance using multiple electrodes or nerve stimulation for a variety of tasks including intermittent contractions and different target forces. Likewise, it will be especially important to evaluate the degree of improved fatigue resistance in individuals with spinal cord injuries given the changes in fiber type composition and atrophy that often occur with paralysis. Nevertheless, we expect such an approach to increase both the strength and endurance of electrically evoked contractions and thereby enhance the capability of FES to restore movement in paralyzed individuals.
Acknowledgments
We are grateful to Brady Hasse and Daniel Macias for their assistance with this project.
Funding: National Institutes of Health Grant R01 NS102259.
References
- Aigner F, Longato S, Gardetto A, Deibl M, Fritsch H and Piza-Katzer H 2004. Anatomic survey of the common fibular nerve and its branching pattern with regard to the intermuscular septa of the leg Clin. Anat 17 503–12 [DOI] [PubMed] [Google Scholar]
- Amirali A, Mu L, Gracies J-M and Simpson DM 2007. Anatomical localization of motor endplate bands in the human biceps brachii J. Clin. Neuromuscul. Dis 9 306–12 [DOI] [PubMed] [Google Scholar]
- Barbero M, Merletti R and Rainoldi A 2012. Atlas of Muscle Innervation Zones (Berlin: Springer; ) [Google Scholar]
- Barss TS, Ainsley EN, Claveria-Gonzalez FC, Luu MJ, Miller DJ, Wiest MJ and Collins DF 2018. Utilizing physiological principles of motor unit recruitment to reduce fatigability of electrically-evoked contractions: a narrative review Arch. Phys. Med. Rehabil 99 779–91 [DOI] [PubMed] [Google Scholar]
- Behringer M, Franz A, McCourt M and Mester J 2014. Motor point map of upper body muscles Eur. J. Appl. Physiol 114 1605–17 [DOI] [PubMed] [Google Scholar]
- Bergquist AJ, Babbar V, Ali S, Popovic MR and Masani K 2016. Fatigue reduction during aggregated and distributed sequential stimulation Muscle Nerve 56 271–81 [DOI] [PubMed] [Google Scholar]
- Bergquist AJ, Wiest MJ, Okuma Y and Collins DF 2017. Interleaved neuromuscular electrical stimulation after spinal cord injury Muscle Nerve 56 989–93 [DOI] [PubMed] [Google Scholar]
- Bergstrom M and Hultman E 1990. Contraction characteristics of the human quadriceps muscle during percutaneous electrical stimulation Pflugers Arch 417 136–41 [DOI] [PubMed] [Google Scholar]
- Bhadra N and Peckham PH 1997. Peripheral nerve stimulation for restoration of motor function J. Clin. Neurophysiol 14 378–93 [DOI] [PubMed] [Google Scholar]
- Bickel CS, Gregory CM and Dean JC 2011. Motor unit recruitment during neuromuscular electrical stimulation: a critical appraisal Eur. J. Appl. Physiol 111 2399–407 [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Jones DA and Woods JJ 1979. Excitation frequency and muscle fatigue: electrical responses during human voluntary and stimulated contractions Exp. Neurol 64 414–27 [DOI] [PubMed] [Google Scholar]
- Binder-Macleod SA and Snyder-Mackler L 1993. Muscle fatigue: clinical implications for fatigue assessment and neuromuscular electrical stimulation Phys. Ther 73 902–10 [DOI] [PubMed] [Google Scholar]
- Binder-Macleod SA, Halden EE and Jungles KA 1995. Effects of stimulation intensity on the physiological responses of human motor units Med. Sci. Sports Exerc 27 556–65 [PubMed] [Google Scholar]
- Blair EA and Erlanger J 1933. A comparison of the characteristics of axons through their individual electrical responses Am. J. Physiol 106 524–64 [Google Scholar]
- Branner A, Stein RB and Normann RA 2001. Selective stimulation of cat sciatic nerve using an array of varying-length microelectrodes J. Neurophysiol 85 1585–94 [DOI] [PubMed] [Google Scholar]
- Buckmire AJ, Lockwood DR, Doane CJ and Fuglevand AJ 2018. Distributed stimulation increases force elicited with functional electrical stimulation J. Neural Eng 15 026001–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JE and Thomas CK 2003. Effects of sustained stimulation on the excitability of motoneurons innervating paralyzed and control muscles J. Appl. Physiol 94 567–75 [DOI] [PubMed] [Google Scholar]
- Cameron T, Richmond FJ and Loeb GE 1998. Effects of regional stimulation using a miniature stimulator implanted in feline posterior biceps femoris IEEE Trans. Biomed. Eng 45 1036–43 [DOI] [PubMed] [Google Scholar]
- Chou L-W, Lee SC, Johnston TE and Binder-Macleod SA 2008. The effectiveness of progressively increasing stimulation frequency and intensity to maintain paralyzed muscle force during repetitive activation in persons with spinal cord injury Arch. Phys. Med. Rehabil 89 856–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coërs C and Telerman-Toppet N 1977. Morphological changes of motor units in Duchenne’s muscular dystrophy Arch. Neurol 34 396–402 [DOI] [PubMed] [Google Scholar]
- Connelly DM, Rice CL, Roos MR and Vandervoort AA 1999. Motor unit firing rates and contractile properties in tibialis anterior of young and old men J. Appl. Physiol 87 843–52 [DOI] [PubMed] [Google Scholar]
- Crago PE, Peckham PH and Thrope GB 1980. Modulation of muscle force by recruitment during intramuscular stimulation IEEE Trans. Biomed. Eng 27 679–84 [DOI] [PubMed] [Google Scholar]
- De Luca CJ and Hostage EC 2010. Relationship between firing rate and recruitment threshold of motoneurons in voluntary isometric contractions J. Neurophysiol 104 1034–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet BM, Lam A and Griffin L 2012. Neuromuscular electrical stimulation for skeletal muscle function Yale J. Biol. Med 85 201–15 [PMC free article] [PubMed] [Google Scholar]
- Downey RJ, Bellman MJ, Kawai H, Gregory CM and Dixon WE 2015. Comparing the induced muscle fatigue between asynchronous and synchronous electrical stimulation in able-bodied and spinal cord injured populations IEEE Trans. Neural Syst. Rehabil. Eng 23 964–72 [DOI] [PubMed] [Google Scholar]
- Fang ZP and Mortimer JT 1991. Selective activation of small motor axons by quasitrapezoidal current pulses IEEE Trans. Biomed. Eng 38 168–74 [DOI] [PubMed] [Google Scholar]
- Farina D, Blanchietti A, Pozzo M and Merletti R 2004. M-wave properties during progressive motor unit activation by transcutaneous stimulation J. Appl. Physiol 97 545–55 [DOI] [PubMed] [Google Scholar]
- Feiereisen P, Duchateau J and Hainaut K 1997. Motor unit recruitment order during voluntary and electrically induced contractions in the tibialis anterior Exp. Brain Res 114 117–23 [DOI] [PubMed] [Google Scholar]
- Fuglevand AJ and Keen DA 2003. Re-evaluation of muscle wisdom in the human adductor pollicis using physiological rates of stimulation J. Physiol 549 865–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC 2001. Spinal and supraspinal factors in human muscle fatigue Physiol. Rev 81 1725–89 [DOI] [PubMed] [Google Scholar]
- Garland SJ, Garner SH and McComas AJ 1988. Relationship between numbers and frequencies of stimuli in human muscle fatigue J. Appl. Physiol 65 89–93 [DOI] [PubMed] [Google Scholar]
- Grill WM and Mortimer JT 1995. Stimulus waveforms for selective neural stimulation IEEE Eng. Med. Biol. Mag 14 375–85 [Google Scholar]
- Grimby G, Broberg C, Krotkiewska I and Krotkiewski M 1976. Muscle fiber composition in patients with traumatic cord lesion Scand. J. Rehabil. Med 8 37–42 [PubMed] [Google Scholar]
- Guiraud D, Azevedo-Coste C, Benoussaad M and Fattal C 2014. Implanted functional electrical stimulation: case report of a paraplegic patient with complete SCI after 9 years J. Neuroeng. Rehabil 11 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyters M, Carpentier A, Duchateau J and Hainaut K 1994. Twitch analysis as an approach to motor unit activation during electrical stimulation Can. J. Appl. Physiol 19 451–61 [DOI] [PubMed] [Google Scholar]
- Ibitoye MO, Hamzaid NA, Hasnan N, Abdul Wahab AK and Davis GM 2016. Strategies for rapid muscle fatigue reduction during fes exercise in individuals with spinal cord injury: a systematic review PLoS One 11 e0149024–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanmiri-Nezhad F, Barkhaus PE, Rymer WZ and Zhou P 2015. Innervation zones of fasciculating motor units: observations by a linear electrode array Frontiers Hum. Neurosci 9 287–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jami L and Petit J 1975. Correlation between axonal conduction velocity and tetanic tension of motor units in four muscles of the cat hind limb Brain Res 96 114–8 [DOI] [PubMed] [Google Scholar]
- Jones DA 1996. High-and low-frequency fatigue revisited Acta Physiol 156 265–70 [DOI] [PubMed] [Google Scholar]
- Jones DA, Bigland-Ritchie B and Edwards RH 1979. Excitation frequency and muscle fatigue: mechanical responses during voluntary and stimulated contractions Exp. Neurol 64 401–13 [DOI] [PubMed] [Google Scholar]
- Karu ZZ, Durfee WK and Barzilai AM 1995. Reducing muscle fatigue in FES applications by stimulating with N-let pulse trains IEEE Trans. Biomed. Eng 42 809–17 [DOI] [PubMed] [Google Scholar]
- Kesar T, Chou L-W and Binder-Macleod SA 2008. Effects of stimulation frequency versus pulse duration modulation on muscle fatigue J. Electromyogr. Kinesiol 18 662–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaflitz M, Merletti R and De Luca CJ 1990. Inference of motor unit recruitment order in voluntary and electrically elicited contractions J. Appl. Physiol 68 1657–67 [DOI] [PubMed] [Google Scholar]
- Kubiak RJ, Whitman KM and Johnston RM 1987. Changes in quadriceps femoris muscle strength using isometric exercise versus electrical stimulation J. Orthop. Sports Phys. Ther 8 537–41 [DOI] [PubMed] [Google Scholar]
- Lagerquist O, Walsh LD, Blouin J-S, Collins DF and Gandevia SC 2009. Effect of a peripheral nerve block on torque produced by repetitive electrical stimulation J. Appl. Physiol 107 161–7 [DOI] [PubMed] [Google Scholar]
- Laubacher M, Aksöz AE, Riener R, Binder-Macleod S and Hunt KJ 2017. Power output and fatigue properties using spatially distributed sequential stimulation in a dynamic knee extension task Eur. J. Appl. Physiol 117 1787–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DR, You JH, Yi C-H and Jeon H-S 2012. Motor point location index using regression equations for the tibialis anterior muscle NeuroRehabilitation 30 307–13 [DOI] [PubMed] [Google Scholar]
- Lind AR and Petrofsky JS 1978. Isometric tension from rotary stimulation of fast and slow cat muscles Muscle Nerve 1 213–8 [DOI] [PubMed] [Google Scholar]
- Lou JWH, Bergquist AJ, Aldayel A, Czitron J and Collins DF 2017. Interleaved neuromuscular electrical stimulation reduces muscle fatigue Muscle Nerve 55 179–89 [DOI] [PubMed] [Google Scholar]
- Malešević NM, Popović LZ, Schwirtlich L and Popović DB 2010. Distributed low-frequency functional electrical stimulation delays muscle fatigue compared to conventional stimulation Muscle Nerve 42 556–62 [DOI] [PubMed] [Google Scholar]
- Maneski LZP, Malešević NM, Savić AM, Keller T and Popović DB 2013. Surface-distributed low-frequency asynchronous stimulation delays fatigue of stimulated muscles Muscle Nerve 48 930–7 [DOI] [PubMed] [Google Scholar]
- Marsden CD and Meadows JC 1970. The effect of adrenaline on the contraction of human muscle J. Physiol 207 429–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden CD, Meadows JC and Merton PA 1983. ‘Muscular wisdom’ that minimizes fatigue during prolonged effort in man: peak rates of motoneuron discharge and slowing of discharge during fatigue Adv. Neurol 39 169–211 [PubMed] [Google Scholar]
- Marsh E, Sale D, McComas AJ and Quinlan J 1981. Influence of joint position on ankle dorsiflexion in humans J. Appl. Physiol 51 160–7 [DOI] [PubMed] [Google Scholar]
- Martin TP, Stein RB, Hoeppner PH and Reid DC 1992. Influence of electrical stimulation on the morphological and metabolic properties of paralyzed muscle J. Appl. Physiol 72 1401–6 [DOI] [PubMed] [Google Scholar]
- McDonnall D, Clark GA and Normann RA 2004. Interleaved, multisite electrical stimulation of cat sciatic nerve produces fatigue-resistant, ripple-free motor responses IEEE Trans. Neural Syst. Rehabil. Eng 12 208–15 [DOI] [PubMed] [Google Scholar]
- McIntyre CC and Grill WM 2002. Extracellular stimulation of central neurons: influence of stimulus waveform and frequency on neuronal output J. Neurophysiol 88 1592–604 [DOI] [PubMed] [Google Scholar]
- McNeal DR 1976. Analysis of a model for excitation of myelinated nerve IEEE Trans. Biomed. Eng 23 329–37 [DOI] [PubMed] [Google Scholar]
- Memberg WD, Polasek KH, Hart RL, Bryden AM, Kilgore KL, Nemunaitis GA, Hoyen HA, Keith MW and Kirsch RF 2014. Implanted neuroprosthesis for restoring arm and hand function in people with high level tetraplegia Arch. Phys. Med. Rehabil 95 1201–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merton PA 1954. Voluntary strength and fatigue J. Physiol 123 553–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger JM and Fitts RH 1986. Fatigue from high- and low-frequency muscle stimulation: role of sarcolemma action potentials Exp. Neurol 93 320–33 [DOI] [PubMed] [Google Scholar]
- Mizrahi J 1997. Fatigue in muscles activated by functional electrical stimulation Crit. Rev. Phys. Rehabil. Med 9 93–129 [Google Scholar]
- Mortimer JT 1981. Motor prostheses Handbook of Physiology: the Nervous System II ed Brooks VB (Bethesda, MD: American Physiological Society; ) pp 155–87 [Google Scholar]
- Mu L and Sanders I 2010. Sihler’s whole mount nerve staining technique: a review Biotechnol. Histochem 85 19–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naess K and Storm-Mathisen A 1955. Fatique of sustained tetanic contractions Acta Physiol 34 351–66 [DOI] [PubMed] [Google Scholar]
- Navarro X, Krueger TB, Lago N, Micera S, Stieglitz T and Dario P 2005. A critical review of interfaces with the peripheral nervous system for the control of neuroprostheses and hybrid bionic systems J. Peripher. Nerv. Syst 10 229–58 [DOI] [PubMed] [Google Scholar]
- Nelson PG 1969. Functional consequences of tenotomy in hind limb muscles of the cat J. Physiol 201 321–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen R, Masani K, Micera S, Morari M and Popovic MR 2011. Spatially distributed sequential stimulation reduces fatigue in paralyzed triceps surae muscles: a case study Artif. Organs 35 1174–80 [DOI] [PubMed] [Google Scholar]
- Parker MG, Berhold M, Brown R, Hunter S, Smith MR and Runhling RO 1986. Fatigue response in human quadriceps femoris muscle during high frequency electrical stimulation J. Orthop. Sports Phys. Ther 7 145–53 [DOI] [PubMed] [Google Scholar]
- Popović LZ and Malešević NM 2009. Muscle fatigue of quadriceps in paraplegics: comparison between single versus multi-pad electrode surface stimulation Eng. Med. Biol. Soc pp 6785–8 [DOI] [PubMed] [Google Scholar]
- Rack PMH and Westbury DR 1969. The effects of length and stimulus rate on tension in the isometric cat soleus muscle J. Physiol 204 443–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattay F 1986. Analysis of models for external stimulation of axons IEEE Trans. Biomed. Eng 33 974–7 [DOI] [PubMed] [Google Scholar]
- Rattay F 2004. Central nervous system stimulation Neuroprosthetics: Theory and Practice ed Horch K and Dhillon G (Singapore: World Scientific; ) pp 429–47 [Google Scholar]
- Rohm M, Schneiders M, Müller C, Kreilinger A, Kaiser V, Müller-Putz GR and Rupp R 2013. Hybrid brain–computer interfaces and hybrid neuroprostheses for restoration of upper limb functions in individuals with high-level spinal cord injury Artif. Intell. Med 59 133–42 [DOI] [PubMed] [Google Scholar]
- Sandercock TG 2006. Extra force from asynchronous stimulation of cat soleus muscle results from minimizing the stretch of the common elastic elements J. Neurophysiol 96 1401–05 [DOI] [PubMed] [Google Scholar]
- Sayenko DG, Nguyen R, Popovic MR and Masani K 2014. Reducing muscle fatigue during transcutaneous neuromuscular electrical stimulation by spatially and sequentially distributing electrical stimulation sources Eur. J. Appl. Physiol 114 793–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffler LR and Chae J 2007. Neuromuscular electrical stimulation in neurorehabilitation Muscle Nerve 35 562–90 [DOI] [PubMed] [Google Scholar]
- Shields RK 1995. Fatigability, relaxation properties, and electromyographic responses of the human paralyzed soleus muscle J. Neurophysiol 73 2195–206 [DOI] [PubMed] [Google Scholar]
- Sinacore DR, Delitto A, King DS and Rose SJ 1990. Type II fiber activation with electrical stimulation: a preliminary report Phys. Ther 70 416–22 [DOI] [PubMed] [Google Scholar]
- Stein RB, Gordon T, Jefferson J, Sharfenberger A, Yang JF, de Zepetnek JT and Belanger M 1992. Optimal stimulation of paralyzed muscle after human spinal cord injury J. Appl. Physiol 72 1393–400 [DOI] [PubMed] [Google Scholar]
- Sunderland S and Hughes ESR 1946. Metrical and non‐metrical features of the muscular branches of the sciatic nerve and its medial and lateral popliteal divisions J. Comp. Neurol 85 205–22 [DOI] [PubMed] [Google Scholar]
- Taylor JL, Amann M, Duchateau J, Meeusen R and Rice CL 2016. Neural contributions to muscle fatigue Med. Sci. Sports Exerc 48 2294–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CK, Griffin L, Godfrey S, Ribot-Ciscar E and Butler JE 2003. Fatigue of paralyzed and control thenar muscles induced by variable or constant frequency stimulation J. Neurophysiol 89 2055–64 [DOI] [PubMed] [Google Scholar]
- Thomas CK, Nelson G, Than L and Zijdewind I 2002. Motor unit activation order during electrically evoked contractions of paralyzed or partially paralyzed muscles Muscle Nerve 25 797–804 [DOI] [PubMed] [Google Scholar]
- Trimble MH and Enoka RM 1991. Mechanisms underlying the training effects associated with neuromuscular electrical stimulation Phys. Ther 71 273–80 [DOI] [PubMed] [Google Scholar]
- Watt T, Hariharan AR, Brzezinski DW, Caird MS and Zeller JL 2013. Branching patterns and localization of the common fibular (peroneal) nerve: an anatomical basis for planning safe surgical approaches Surg. Radiol. Anat 36 821–8 [DOI] [PubMed] [Google Scholar]
- Wise AK, Morgan DL, Gregory JE and Proske U 2001. Fatigue in mammalian skeletal muscle stimulated under computer control J. Appl. Physiol 90 189–97 [DOI] [PubMed] [Google Scholar]
- Won S-Y, Kim D-H, Yang H-M, Park J-T, Kwak H-H, Hu K-S and Kim H-J 2011. Clinical and anatomical approach using Sihler’s staining technique (whole mount nerve stain) Anat. Cell Biol 44 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuerker RB, McPhedran AM and Henneman E 1965. Properties of motor units in a heterogeneous pale muscle (m. gastrocnemius) of the cat J. Neurophysiol 28 85–99 [DOI] [PubMed] [Google Scholar]
- Yoshida K and Horch K 1993. Reduced fatigue in electrically stimulated muscle using dual channel intrafascicular electrodes with interleaved stimulation Ann. Biomed. Eng 21 709–14 [DOI] [PubMed] [Google Scholar]
- Yu D, Yin H, Han T, Jiang H and Cao X 2016. Intramuscular innervations of lower leg skeletal muscles: applications in their clinical use in functional muscular transfer Surg. Radiol. Anat 38 675–85 [DOI] [PubMed] [Google Scholar]
- Zajac FE and Faden JS 1985. Relationship among recruitment order, axonal conduction velocity, and muscle-unit properties of type-identified motor units in cat plantaris muscle J. Neurophysiol 53 1303–22 [DOI] [PubMed] [Google Scholar]
- Zheng Y and Hu X 2018. Improved muscle activation using proximal nerve stimulation with subthreshold current pulses at kilohertz-frequency J. Neural Eng 15 046001 [DOI] [PubMed] [Google Scholar]