ABSTRACT
β-catenin has roles in cell-cell adhesion and Wnt signaling. We recently showed that β-catenin protein abundance is decreased at higher intracellular pH (pHi), mediated by pH-sensitive interaction with the beta-transducin repeat containing E3 ubiquitin protein ligase (β-TrCP). Increased pHi facilitates β-TrCP binding and degradation of β-catenin. β-catenin mutations that abrogate the pH-sensitive interaction induce significant tumors not seen with other β-catenin stabilizing mutants.
Keywords: β-catenin, intracellular pH, Wnt signaling, ubiquitination, proteasome, β-TrCP, tumorigenesis
Transient increases in intracellular pH (pHi) have been shown to be either necessary or sufficient for diverse cellular processes such as directed cell migration,1 cell cycle progression,2 and differentiation.3 Dysregulated pHi dynamics are a hallmark of diseases such as cancer, where increased pHi enables various cancer cell behaviors.4,5 These cell behaviors are mediated by proteins termed pH sensors whose activity, binding, or localization are sensitive to physiological changes in pHi.6 We recently reported that CTTNB1 (catenin beta 1, best known as β-catenin) is a pH sensor, with decreased stability at increased pHi.7 At high pHi, β-catenin association with beta-transducin repeat containing E3 protein ligase (β-TrCP) is increased, leading to lower levels of β-catenin at junctions, in the nucleus, and in whole cell lysates. We identified a single histidine (His) residue in β-catenin that mediates this pH sensitive function. When that evolutionarily-conserved histidine (His36 in Human β-catenin) is mutated to a non-titratable residue, pH-sensitive binding to β-TrCP is abrogated. Moreover, this His is mutated in human cancers to arginine (Arg), and when we expressed the analogous mutation (His42Arg) in Drosophila eyes, we observed Wnt signaling activation as well as formation of ectopic tumors not seen with other stabilized β-catenin mutants. Our results suggest that pHi dynamics regulate Wnt signaling by modulating β-catenin stability, and that cancer-associated mutations circumvent physiological mechanisms that decrease Wnt signaling at increased pHi.
After determining that increased pHi produced a strong dysplasia phenotype in the Drosophila eye,8 we performed a genetic screen to identify pH sensitive proteins. We identified armadillo (arm, Drosophila β-catenin) in this screen and found that overexpression of arm suppressed the dysplasia phenotype. We confirmed that Arm was decreased at cell-cell junctions in the Drosophila eye as well as in whole head lysates with increased pHi. Importantly, we saw no change in total levels of other adherens junction proteins such as Shotgun (Drosophila E-cadherin) or N-cadherin. We confirmed these results in mammalian epithelial cells, and demonstrated that both junctional and nuclear β-catenin levels were decreased at higher pHi. These data suggested that increased pHi was leading to decreased β-catenin abundance, and we confirmed that β-catenin degradation was increased at high pHi using a metabolic pulse-chase assay.
β-catenin levels are regulated primarily by ubiquitination and proteasome-mediated degradation. Degradation requires obligate phosphorylation of N-terminal residues of β-catenin by the kinases casein kinase 1 (CK1) and glycogen synthase kinase-3 beta (GSK3-β) for recognition by the E3-ligase β-TrCP. We first tested whether high pHi was increasing phosphorylation of β-catenin by these kinases, leading to increased degradation at high pHi. However, we found no difference in phosphorylation of β-catenin by these kinases either in vitro or in cells. Our next hypothesis was that β-catenin binding to the E3-ligase β-TrCP was sensitive to changes in pH. β-TrCP binds to an evolutionarily-conserved destruction motif (DSGIHS) in β-catenin (Figure 1). Since histidines can titrate within the physiological range and can function as molecular switches, we predicted that pH-sensitive binding of β-catenin to β-TrCP might be mediated by the conserved histidine residue in the destruction motif of β-catenin. Based on the published crystal structure of β-catenin complexed with β-TrCP,9 we predict that at lower pHi, a protonated histidine will repel the positively charged Lysine 365 on β-TrCP to reduce binding (Figure 1, left panel). At increased pHi, we predict that deprotonation of this histidine will promote binding to β-TrCP, leading to the observed decreased in total protein levels (Figure 1, center panel). Consistent with this model, we report that β-catenin binds to β-TrCP with higher affinity at high pH. Supporting our hypothesis, when we mutated that histidine residue to a non-titratable arginine (His36Arg, Figure 1, right panel) or alanine residue, we lost pH sensitive binding.
Figure 1.
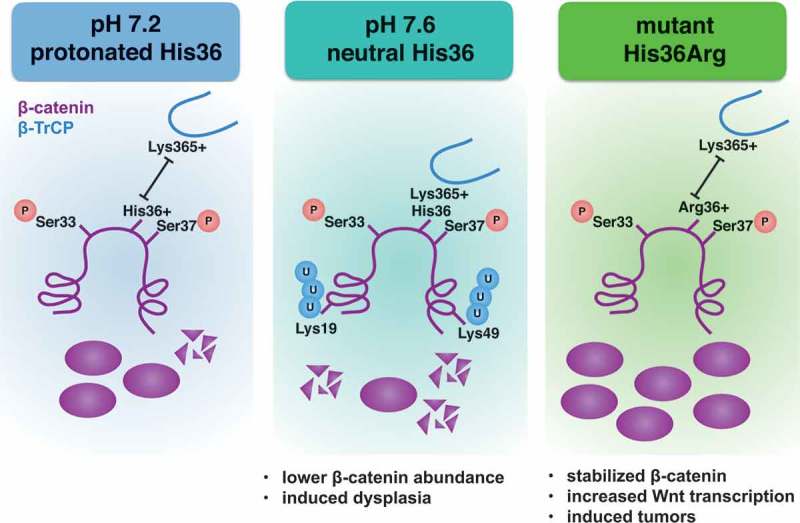
Potential pH-sensing mechanism of β-catenin. Obligate phosphorylation is unchanged with changes in intracellular pH (pHi), but titration of a single histidine residue with changes in pHi is sufficient to alter binding of β-catenin (purple) to beta-transducin repeat containing E3 protein ligase (β-TrCP, blue). We predict interactions between His36 and Lys365 in β-TrCP mediate the pH sensitive binding. At high pHi, β-catenin interaction with β-TrCP is increased, leading to decreased protein abundance and dysplasia. When the cancer-associated arginine mutation is present, pH sensing is abrogated, β-catenin is stabilized, Wnt signaling is increased, and ectopic tumors are formed in the fly eye.
After determining that His36 is the residue mediating pH-sensitive β-catenin abundance, we asked whether this residue is mutated in disease. We found that His36Arg is a recurrent mutation in the Catalog of Somatic Mutations in Cancer, associated with liver and biliary tract tumors (COSMIC Mutation ID: COSM27378). When we mutated the analogous residue (His42Arg-Armadillo) and expressed it in the developing Drosophila eye, we observed increased Wnt signaling resulting in large, protruding tumors. This phenotype is very different than the Drosophila eye phenotype that results from other stabilized β-catenin mutants, which produce reduced, rough eyes and no protruding tumors. These data are, to our knowledge, the first studies of this cancer-associated mutation, and support a mechanism whereby His36Arg increases transcription of Wnt target genes and uniquely has tumorigenic phenotypes that are not seen with other β-catenin mutations that alter phosphorylation or ubiquitination.
Our work introduces a novel effect on β-catenin whereby increased pHi functions in coincidence with obligate phosphorylation to regulate proteasome-mediated β-catenin degradation. Given that increased pHi is an early event in cancer development,10 we predict that pH-sensitive loss of β-catenin from cell-cell junctions may be one mechanism mediating dysplasia initiation and early metastasis. Additionally, mutations that stabilize β-catenin protein by altering phosphorylation or ubiquitination may show stabilized β-catenin levels even at the higher pHi found in cancer cells, bypassing this pH-regulatory step and elevating Wnt signaling, which is associated with breast, colon, and liver carcinomas.11
References
- 1.Putney LK, Barber DL.. Na-H exchange-dependent increase in intracellular pH times G2/M entry and transition. J Biol Chem. 2003;278:44645–44649. [DOI] [PubMed] [Google Scholar]
- 2.Denker SP, Barber DL. Cell migration requires both ion translocation and cytoskeletal anchoring by the Na-H exchanger NHE1. J Cell Biol. 2002;159:1087–1096. doi: 10.1083/jcb.200208050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ulmschneider B, .Grillo-Hill BK, Benitez M, Azimova DR, Barber DL, Nystul TG Increased intracellular pH is necessary for adult epithelial and embryonic stem cell differentiation. J Cell Biol. 2016;215:345–355. doi: 10.1083/jcb.201606042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Webb BA, Chimenti M, Jacobson MP, Barber DL. Dysregulated pH: a perfect storm for cancer progression. Nat Rev Cancer. 2011;11:671–677. doi: 10.1038/nrc3110. [DOI] [PubMed] [Google Scholar]
- 5.White KA, Grillo-Hill BK, Barber DL. Cancer cell behaviors mediated by dysregulated pH dynamics at a glance. J Cell Sci. 2017;130:663–669. doi: 10.1242/jcs.195297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schönichen A, Webb BA, Jacobson MP, Barber DL. Considering protonation as a posttranslational modification regulating protein structure and function. Annu Rev Biophys. 2013;42:289–314. doi: 10.1146/annurev-biophys-050511-102349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White KA, Grillo-Hill BK, Esquivel M, Peralta J, Bui VN, Chire I, Barber DL. β-catenin is a pH sensor with decreased stability at higher intracellular pH. J Cell Biol. 2018;217. doi: 10.1083/jcb.201712041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grillo-Hill BK, Choi C, Jimenez-Vidal M, Barber DL. Increased H+ efflux is sufficient to induce dysplasia and necessary for viability with oncogene expression. Elife. 2015;4:e03270. doi: 10.7554/eLife.06416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu G, Xu G, Schulman BA, Jeffrey PD, Harper JW, Pavletich NP. Structure of a beta-TrCP1-Skp1-beta-catenin complex: destruction motif binding and lysine specificity of the SCF(beta-TrCP1) ubiquitin ligase. Mol Cell. 2003;11(6):1445–1456. [DOI] [PubMed] [Google Scholar]
- 10.Reshkin SJ, Bellizzi A, Caldeira S, Albarani V, Malanchi I, Poignee M, Alunni-Fabbroni M, Casavola V, Tommasino M. Na+/H+ exchanger-dependent intracellular alkalinization is an early event in malignant transformation and plays an essential role in the development of subsequent transformation-associated phenotypes. FASEB J. 2000;14:2185–2197. doi: 10.1096/fj.00-0029com. [DOI] [PubMed] [Google Scholar]
- 11.Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387–398. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]


