ABSTRACT
Modeling renal cancer in the mouse has been challenging. We recently showed that upregulation of mechanistic target of rapamycin complex 1 (mTORC1) in a restricted segment of the renal tubule leads to downregulation of the tricarboxylic acid (TCA) cycle enzyme fumarate hydratase, to accumulation of the oncometabolite fumarate, and gradual transformation from benign cysts into cystadenomas and papillary carcinomas.
Keywords: Renal carcinoma, oncometabolite, fumarate hydratase, mTOR, fumarate
Text
In a recent study, we described a novel animal model faithfully and robustly recapitulating a papillary type II Renal Cell Carcinoma (PRCC).1 Of interest, this was obtained by inactivation of a single tumor suppressor gene, Tuberous Sclerosis Complex 1 Tsc1, a master negative regulator of the mTORC1 complex. We found that inactivation of the Tsc1 gene in a renal tubular segment-restricted Cre mouse line (Cadherin 16-Cre, also called Kidney Specific Cre, Ksp-Cre) results in a mild renal cystogenesis.2 The late onset and slow progression of cyst formation likely allowed sufficient time for the cystic lesions to transform into papillae, initially layered by a monostratified epithelium and subsequently acquiring multistratification. These initial steps are followed by formation of cystadenomas and ultimately an overt carcinoma phenotype that was shown positive for specific markers of papillary type II renal cell carcinoma, providing the first animal model of type II PRCC generated to date.1 Importantly, the different type of lesions are progressive and occur with full-penetrance at different time points after birth of the animals.1 Mechanistic studies on these tissues revealed an unexpected and previously unsuspected link between two important tumorigenic cascades in the kidney: that of the mTORC13 and the tumor suppressor Fumarate Hydratase (FH) whose impairment leads to accumulation of the oncometabolite fumarate.4
There are several relevant novel aspects related to the above study. First, modelling renal cancer in the mouse has been a difficult task since inactivation in the mouse kidney of classic oncosuppressors typically mutated in human Renal Cell Carcinoma (RCC) such as Von Hipple Lindau (VHL), Birt-Hogg Dubè Syndrome (BHD), TSC1 and Fumarate Hydratase (FH) all resulted in aggressive and fulminant polycystic kidney disease. As a consequence animals died due to renal failure, rather than progressing into cancer.5,6 In contrast, simultaneous inactivation of multiple tumor suppressors in the kidney was recently reported to be required for oncogenesis to occur in animal models.7,8
Several possible reasons might explain the recent study showing that inactivation of Tsc1 was sufficient to drive tumorigenesis. First, the mild renal phenotype, likely due to the tubular restricted inactivation of the gene might have allowed for the hyper-proliferative epithelia to accumulate mutations ultimately leading to the malignant phenotype observed. A secondpossibility is that this specific Cre line might be targeting a tubular subpopulation responsible for the phenotype observed. Indeed, recent work using single cell sequencing has uncovered that the cellular complexity within the murine nephron is even higher than previously appreciated, and novel cell types could be observed.9
One important feature of the animal model described is the progressive transformation from benign, cystic lesions, to the formation of cystadenomas and carcinomas.1 This important feature allowed for the dissection of metabolic alterations in the pre-malignant versus malignant lesions. Indeed, untargeted metabolomics revealed metabolic changes in the cancerous versus pre-cancerous mutant kidneys, some of which were expected based on previous work in non-renal cancers, such as glycolysis, pentose phosphate pathway, purine biosynthesis and fatty acids biosynthesis. However, the studies also uncovered a previously unrecognized role for mTORC1 in regulating the levels of fumarate. This metabolite has been shown to accumulate in a subtype of renal carcinomas, i.e. the papillary type II RCC caused by mutation of FH, an enzyme deputed to convert fumarate into malate in the TCA cycle. Our study demonstrates that the fumarate hydratase enzyme is downregulated in response to mTORC1 chronic upregulation causing a gradual accumulation of fumarate, which correlates with, and likely causes the transforming phenotype.
In recent years the concept of onco-metabolites arose, proposing a role for metabolic reprogramming that goes beyond the energetic demands and adaptation of cells per se. Indeed, large accumulation of few key metabolites was shown to drive profound epigenetic alterations. In particular, storage of fumarate was shown to cause epigenetic changes resulting in epithelial to mesenchymal transition (EMT).10 Thus, one possibility is that the combined upregulation of mTORC1 signaling and of fumarate accumulation might lead on the one hand to metabolic reprogramming actively contributing to cell proliferation, while on the other hand fumarate storage might lead to EMT in the cells that accumulate higher levels. Together, these two activities might be driving the progressive transformation of the cystic benign epithelial lesions into more aggressive carcinomas (Figure 1).
Figure 1.
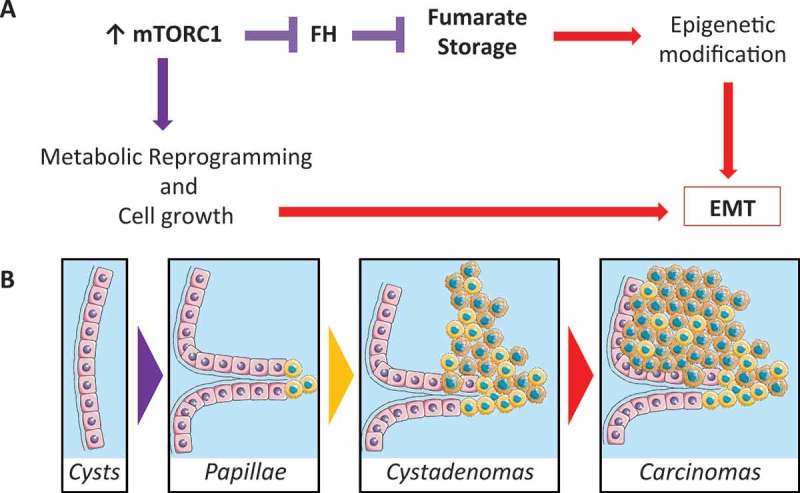
Schematic overview of the step-wise transformation of renal cysts into cystadenomas and carcinomas, driven by the mTORC1-Fumarate axis. B. Schematic representation of the progressive transformation of cysts into papillae which gradually become cystadenomas and carcinomas. A. The proposed series of events that occurs downstream of the chronic activation of mechanistic target of rapamycin complex 1 (mTORC1). Downregulation of the enzyme fumarate hydratase (FH) leads to the accumulation of the oncometabolite fumarate, previously described to drive a program of epithelial to mesenchymal transition (EMT) though epigenetic regulation. Thus, fumarate-driven EMT along with the metabolic reprogramming and proliferation mediated by the mTORC1 cascade, are likely responsible for the transforming phenotype observed. Part of the images were taken from Servier Medical Art Images (http://smart.servier.com/).
Finally, we provide evidence that the novel link between mTORC1 and the FH enzyme is conserved in humans given that clear cell carcinoma specimens (ccRCCs) carrying mTORC1 upregulation were found to manifest a strong correlation with FH downregulation and Nuclear factor (erythroid-derived 2)-like 2 (NFE2L2 also known as Nrf2) upregulation. Future studies are needed to test whether this mTORC1/fumarate axis can be exploited to better classify cancers and/or for therapeutic purposes. For instance, the work suggests that renal cell carcinomas accumulating fumarate (which could in principle be detected in the urine) are expected to respond better to the use of rapalogues in the clinic.
In conclusion, we demonstrated that chronic activation of mTORC1 in a restricted segment of the renal tubule is sufficient to drive a progressive transformation of benign lesions into cancers in the mouse. Mechanistically, we found that mTORC1 activation drives down-regulation of Fh1 and subsequent accumulation of the oncometabolite fumarate, ultimately leading to transformation. Our data provide a causal link between metabolic derangement downstream of mTORC1 and carcinogenesis in the kidney.
Funding Statement
This work was supported by the Associazione Italiana per la Ricerca sul Cancro [IG18706 and IG14382].
Acknowledgments
The authors are grateful to other members of the Boletta lab for helpful discussion. The work of the authors is supported by the Italian Association of Research on Cancer (AIRC, grants IG14382 and IG18706 to A.B).
References
- 1.Drusian L, Nigro EA, Mannella V, Pagliarini R, Pema M, Costa ASH, Benigni F, Larcher A, Chiaravalli M, Gaude E, et al. mTORC1 Upregulation Leads to Accumulation of the Oncometabolite Fumarate in a Mouse Model of Renal Cell Carcinoma. Cell Rep. 2018;24(1093–1104):e1096. doi: 10.1016/j.celrep.2018.06.106. [DOI] [PubMed] [Google Scholar]
- 2.Pema M, Drusian L, Chiaravalli M, Castelli M, Yao Q, Ricciardi S, Somlo S, Qian F, Biffo S, Boletta A.. 2016. mTORC1-mediated inhibition of polycystin-1 expression drives renal cyst formation in tuberous sclerosis complex. Nat Commun. 7:10786. doi: 10.1038/ncomms10786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yecies JL, Manning BD.. 2011. mTOR links oncogenic signaling to tumor cell metabolism. J Mol Med (Berl). 89:221–228. doi: 10.1007/s00109-011-0726-6. [DOI] [PubMed] [Google Scholar]
- 4.Tomlinson IP, Alam NA, Rowan AJ, Barclay E, Jaeger EE, Kelsell D, Leigh I, Gorman P, Lamlum H, Rahman S, et al. 2002. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet. 30:406–410. doi: 10.1038/ng849. [DOI] [PubMed] [Google Scholar]
- 5.Traykova-Brauch M, Schonig K, Greiner O, Miloud T, Jauch A, Bode M, Felsher DW, Glick AB, Kwiatkowski DJ, Bujard H, et al. 2008. An efficient and versatile system for acute and chronic modulation of renal tubular function in transgenic mice. Nat Med. 14:979–984. doi: 10.1038/nm.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adam J, Hatipoglu E, O’Flaherty L, Ternette N, Sahgal N, Lockstone H, Baban D, Nye E, Stamp GW, Wolhuter K, et al. 2011. Renal cyst formation in Fh1-deficient mice is independent of the Hif/Phd pathway: roles for fumarate in KEAP1 succination and Nrf2 signaling. Cancer Cell. 20:524–537. doi: 10.1016/j.ccr.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gu YF, Cohn S, Christie A, McKenzie T, Wolff N, Do QN, Madhuranthakam AJ, Pedrosa I, Wang T, Dey A, et al. 2017. Modeling Renal Cell Carcinoma in Mice: bap1 and Pbrm1 Inactivation Drive Tumor Grade. Cancer Discov. 7:900–917. doi: 10.1158/2159-8290.CD-17-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bailey ST, Smith AM, Kardos J, Wobker SE, Wilson HL, Krishnan B, Saito R, Lee HJ, Zhang J, Eaton SC, et al. 2017. MYC activation cooperates with Vhl and Ink4a/Arf loss to induce clear cell renal cell carcinoma. Nat Commun. 8:15770. doi: 10.1038/ncomms15770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park J, Shrestha R, Qiu C, Kondo A, Huang S, Werth M, Li M, Barasch J, Susztak K. 2018. Single-cell transcriptomics of the mouse kidney reveals potential cellular targets of kidney disease. Science (80- ). 360:758–763. doi: 10.1126/science.aar2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sciacovelli M, Goncalves E, Johnson TI, Zecchini VR, Da Costa AS, Gaude E, Drubbel AV, Theobald SJ, Abbo SR, Tran MG, et al. 2016. Fumarate is an epigenetic modifier that elicits epithelial-to-mesenchymal transition. Nature. 537:544–547. doi: 10.1038/nature19353. [DOI] [PMC free article] [PubMed] [Google Scholar]


