ABSTRACT
Background: Chemoresistance has been considered to be a major obstacle for cancer therapy clinically. Long non-coding RNAs (LncRNAs) are asscociated with the development, prognosis and drug-resistance of non-small cell lung cancer (NSCLC). Whereas, the regulatory mechanism of lncRNA TATDN1 in the cisplatin resistance of NSCLC is still not clear.
Methods: The expression of TATDN1, miR-451 and TRIM66 in NSCLC tissues and cell lines were detected by qRT-PCR or western blot. Immunohistochemistry (IHC) assay was performed for the detection of TATDN1 expression profile. 88 patients who underwent cisplatin treatment were followed up to 60-months for the analysis of survival rate. MTT and Flow cytometry analysis were performed for the assessment of cell survival rate, proliferation and apoptosis. Bioinformatics, Dual-Luciferase reporter were employed to analyze the interaction among TATDN1, miR-451 and TRIM66. Xenograft tumor model was constructed to verify the role of TATDN1 in NSCLC treated with cisplatin (DDP) in vivo.
Results: TATDN1 and TRIM66 was significantly upregulated while miR-451 was downregulated in NSCLC tissues and cell lines, especially in DDP-resistant tumor tissues and cells. Survival rates of NSCLC patients with low TATDN1 expression were improved following DDP chemotherapy. TATDN1 upregulated TRIM66 expression via sponge for miR-451. Moreover, TATDN1 knockdown improved DDP-sensitivity in NSCLC patients by regulation of miR-451/TRIM66 axis. Finally, knockdown of TATDN1 improved the sensitivity of NSCLC to DDP in vivo.
Conclusions: TATDN1 enhanced the DDP-tolerance of NSCLC cells by upregulating TRIM66 expression via sponging miR-451, hinting a novel regulatory pathway of chemoresistance in DDP-tolerant NSCLC cells and providing a potential therapeutic target for NSCLC patients with DDP-reistance.
Keywords: lncRNA, TATDN1, miR-451, TRIM66, cisplatin resistance, DDP, NSCLC
Introduction
Worldwide, lung cancer is counted as the major contributor in cancer-induced mortality, especially in men.1 Non-small cell lung cancer (NSCLC) accounts for approximately 85% of all lung cancers with a 5-year survival rate of 17.1%.2 Generally, NSCLC is difficult to diagnose at the early stage, and the standard treatment for advanced NSCLC patients is cisplatin-based palliative chemotherapy.3 However, the emergence of acquired drug tolerance has become a significant impediment for the application of DDP in clinical.4 Thus, it is extremely urgent to develop a novel strategy for the improvement of DDP-sensitivity.
Long non-coding RNAs (LncRNAs) are defined as a novel subgroup of non-protein coding transcripts larger than 200nt in length, being able to modulate gene expression and involve in the progression of multiple cancers, as well as drug resistance.5,6 Homo sapiens TatD DNase domain containing 1 (TATDN1), known as a member of TATD family, is a highly conserved nuclease in prokaryotes and eukayotes. Reliably evidences have revealed the regulatory effect of TATDN1 in NSCLC. For instant, TATDN1 was highly expressed in NSCLC cells 95D and tumor tissues, knockdown of TATDN1 prominently inhibited the proliferation, adhesion and invasion of 95D cells via suppression of E-cadherin, HER2, β-catenin, and Ezrin expression.7 However, little is known about the function and precise mechanism of TATDN1 in DDP-resistant NSCLC.
MiRNAs, a type of endogenous and short non-coding RNA with a length of 21-23nt, regulated various biological processes via directly binding to the 3ʹ-untraslated region (3ʹ-UTR) of downstream mRNA.8 The aberrant expression of miRNAs in specific tissues may be associated with the progression and drug resistance of multiple cancers. MiR-451, function as a potential tumor suppressor during carcinogenesis, has been found downregulated in various types of cancers.9 Emerging evidences have revealed the potential role of miR-451 in NSCLC progression and prognosis, as well as in drug resistance.10,11 Recent years, many researchers have focus on the interplay between lncRNA and microRNA (miRNA), as well as the important of such interaction in tumorigenesis. A well-accepted hypothesis suggested that lncRNA could sever as a competing endogenous RNA (ceRNA) of miRNA to modulate the expression of cancer-related target gene.12,13 Ectopic expression of lncRNA led to the disorder of lncRNA/miRNA/mRNA network, ultimately involved in the process of carcinogenesis.14 However, whether TATDN1 modulates cisplatin resistance of NSCLC via interacting with miR-451 is still far from being addressed
Here, we observed a significantly elevated expression of TATDN1 and notably abated expression of miR-451 in DDP-tolerant NSCLC tumor tissues and cell lines. Furthermore, functional and mechanism analyses indicated that TATDN1 acted as a ceRNA to suppress miR-451 expression, thus leading to the release of TRIM66 in DDP-resistant NSCLC. Our research elaborated a novel regulatory pathway of TATDN1/miR-451/TRIM66 in DDP-tolerant NSCLC, which may provide a potential biomarker for the therapy of NSCLC with DDP-tolerance.
Materials and methods
Patients
A total of 30 non-small cell lung cancer (NSCLC) tumor tissues and corresponding adjacent normal tissues were collected from NSCLC patients undergoing surgical resection between 2015 and 2017. All fresh speciments were preserved in liquid nitrogen for the extraction of total RNA.
88 patients were equally divided into two groups based on the expression of TATDN1. Therewith, survival rate of all participants were calculated at the different periods (0, 20, 40, 60 month) after cisplatin treatment. This study was approved by Ethic Committee of the Second Affiliated Hospital of Zhengzhou University and written informed consents were signed by every participant prior to the surgery.
Immunohistochemistry (IHC)
Formalin-fixed and paraffin-embedded specimens were cut into 4 μm thick sections and then deparaffinized and rehydrated with a graded ethanol and xylene. The slides were incubated with 3% H2O2 to eliminate endogenous peroxidase, and blocked with 5% goat serum. After washing with PBS buffer for three times, slides were treated with specific antibody against TATDN1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4°C, followed by the incubation of biotin-labeled secondary antibody and streptavidin (Santa Cruz Biotechnology). Afterwards, protein samples were colored with diaminobenzidine (DAB, Beyotime, Shanghai, China) and counterstained with hematoxylin (Beyotime). Brown nuclear staining was considered to be positive for TATDN1 protein.
Cell culture and transfection
Human lung cancer cell lines A549 and H157 were obtained from American type culture collection (ATCC, Rockefeller, MD, USA). Sensitive cells were cultured in RPMI-1640 medium (Gibco, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS, Gibco) and 100 U/mL penicillin/streptomycin (HyClone, Logan, Utah, USA). Following that, cells were grown at 37°C in a moist atmosphere of 5% CO2. DDP-resistant lung cancer cell lines A549/DDP and H157/DDP were constructed through continue exposure of DDP.
For TATDN1 knockdown, the short hairpin RNA (shRNA) targeting TATDN1 (sh-TATDN1) and shRNA scramble control (shRNA-Control) was subcloned into pLenti6/V5-D-TOPO vector (Invitrogen) to generate TATDN1-knockdown and TATDN1-knockdown control vector sh-TATDN1 and sh-Control, respectively. TRIM66-knockdown vector (sh-TRIM66) was constructed using similar method. TATDN1-overexpression vector (OE-TATDN1) was constructed by subcloning TATDN1 gene open reading frame into the same vector, and a blank vector was used as control vector (OE-Control) for TATDN1 overexpression. Cells with positive transfection were selected using the standard puromycin selection method. MiR-451 mimic (miR-451), miRNA scramble control (miR-control), miR-451 inhibitor (anti-miR-451), miRNA inhibitor control (anti-miR-control) were obtained from GenePharma and were transfected using Lipofectamine 2000 (Invitrogen).
RNA extraction and quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from sensitive or tolerant NSCLC tissues or cultured cells using Trizol reagent (Invitrogen) according to the manufacturer’s instruction. 1 µg RNA was reversely transcribed into cDNA using High Capagity Reverse Transcription System Kit (Takara, Dalian, China) or miRNA First-Stand cDNA Synthesis Kit (GeneCopoeia, Guangzhou, China). The reverse transcription primers for miR-451 and U6 were 5ʹ-TCCAGTGCAGGGTCCGAGG TATTCGCACTG-3ʹ and 5ʹ-AACGCTTCACGAATTTGCGT-3ʹ respectively.
After that, qRT-PCR reaction was carried out using Universal SYBR Premix DimerEraser Kit (Takara, Dalian, China) on Applied Biosystems 7500 Real-time PCR Systems (Thermo Fisher Scientific, USA). The relative quantification of mRNA or miRNA expression was calculated by the 2−ΔΔCt method with GAPDH or U6 snRNA as a house-keeping gene. qRT-PCR primers were list as below. TATDN1: 5ʹ-CCACTGGAATTAGCCAATACACTAT-3ʹ (forward) and 5ʹ-AAAGATCAGGG TTATTCTTTTCAAA-3ʹ (reverse). miR-451: 5ʹ-CCGAAACCGTTACCATTAC-3ʹ (forward) and 5ʹ-GTGCAGGGTCCGAGGT-3ʹ (reverse). TRIM66: 5ʹ-GCCCTCTG TGCTACTTACTC-3ʹ (forward) and 5ʹ-GCTGGTTGTGGGTTACTCTC-3ʹ (reverse). GAPDH: 5ʹ-TATGATGATATCAAGAGGGTAGT-3ʹ (forward) and 5ʹ-TGTATCCA AACTCATTGTCATAC-3ʹ (reverse). U6: 5ʹ-CTCGCTTCGGCAGCACA-3ʹ (forward) and 5ʹ-AACGCTTCACGAATTTGCGT-3ʹ (reverse).
Cell survival and proliferation analyses
Cell survival and proliferation analysis was performed using MTT colorimetric kit (Dojindo, Tokyo, Japan) according to manufacturer’s instructions. Briefly, Transfected DDP-resistant cells were seeded at 96-well plate with a density of 5 × 103 cells/well. About 24 h post-transfection, cells were exposed to various-dose of DDP (0, 0.5, 1.0, 1.5, 2.0 µg/mL) for 48 h, and then 0.5 mg/mL of MTT reagent was added for another 4 h incubation, followed by the addition of DMSO to resolve the generated formazan. Finally, the absorbance at a wavelength of 490 nm was measured using Thermo Scientific microplate reader (Thermo Fisher Scientific) and relative survival curve was also calculated to determine the IC50 value of DDP.
Cell apoptosis assay
Transfected A549/DDP and H157/DDP cells (1 × 105 cells/well) were seeded into six-well plate for 24 h inhibition prior to treating with median lethal dose of DDP (1 µg/mL). About 48 h after incubation, cell apoptosis assay was performed using Annexin V-FITC/PI Apoptosis Detection Kit (BestBio, Shanghai) according to the manufacturer’s procedure. Briefly, after digested with trypsin (HyClone), cells were washed and resuspended with PBS buffer, then Annexin V-FITC and propidium iodide (PI) was introduced under a dark condition. After that, apoptotic cells were analyzed by flow cytometry (FCM) (BD Biosciences).
Dual-luciferase reporter assay
To further demonstrate the possible correlation among TATDN1, TRIM66 and miR-451, partial sequences of TATDN1 or TRIM66 3ʹUTR containing wile-type or mutant-type binding sites of miR-451 were amplified and cloned into psiCHECKTM-2 luciferase plasmid (Promega, Madison, WI, USA). The constructed luciferase reporter plasmids were named as wild-type TATDN1 (WT-TATDN1), mutant-type TATDN1 (MUT-TATDN1), wild-type TRIM66 3ʹUTR (WT-TRIM66-3ʹUTR) and mutant-type TRIM66 (MUT-TRIM66-3ʹUTR). DDP-tolerant lung cancer cells were maintained in 96-well plates and then co-transfected with above luciferase plasmids and miR-451, miR-control. About 48h after transfection, cells were collected and luciferase activity were determined using Dual-Luciferase Reporter assay system (Promega).
Western-blot assay
Protein samples from NSCLC tissues and cells were divided by 10% SDS-PAGE and electrotransferred onto PVDF membranes (Millipore, Billerica, MA, USA). Following blocked with 5% non-fat milk powder for 2 h, the membranes were incubated with specific antibodies against cleaved caspase-3, Bcl-2, TRIM66 and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4°C. Subsequently, the membranes were washed for three times with TBST buffer, and incubated with HRP-conjugated secondary antibody (Cell Signaling Technology, Inc, Danvers, MA, USA) for another 1 h at 37°C. At last, protein samples were analyzed using chemiluminescence assay kits (BestBio, Shanghai, China).
In vivo experiment
1 × 107 A549/DDP cells transfected with sh-TATDN1 or sh-Control were inoculated subcutaneously into the flank of female BALB/c nude mice (Six-week-old, HFK bio-Technology, Beijing, China). After 6-day of administration, mice were treated with DDP followed by the detection of tumor volume every three-day. The formula was shown as below: volume = length × width2/2. All mice were sacrificed at 21 days after tansplantation, and tumors were harvested for following weight and qRT-PCR analysis. The experiments were performed in accordance with the Guidelines for Care and Use of Laboratory Animal with approval of Ethics Committee of the Second Affiliated Hospital of Zhengzhou University.
Statistical analysis
All comparisons between two or more groups were estimated using Student’s t-test and one-way ANOVA and data were shown as means ± standard deviation (SD). P < 0.05 indicated the difference was statistically significant.
Results
TATDN1 was aberrantly upregulated in DDP-resistant NSCLC and high expression of TATDN1 was associated with the poor prognosis of NSCLC patients
To explore the role of TATDN1 in DDP-resistant NSCLC, qRT-PCR was performed to detect the expression of TATDN1 in DDP-sensitive and DDP-tolerant NSCLC tissues and cells. Results showed that TATDN1 levels were significantly higher in lung cancer tumors and cells, especially in DDP-resistant tumors and cell lines (Figure 1A, 1D and 1E). IHC assay further verified the immunoreaction positive substance of TATDN1 was obviously increased in DDP-resistant tumor slices compared with those in normal or DDP-sensitive tumor tissue (Figure 1B). Besides that, 88 patients who underwent DDP treatment were followed up to 60-mouth for the analysis of prognosis, results showed that the survival rate of patients with low TATDN1 expression was higher than those with high TATDN1 expression (Figure 1C), indicating the improvement of prognosis in NSCLC patients with low TATDN1 expression.
Figure 1.
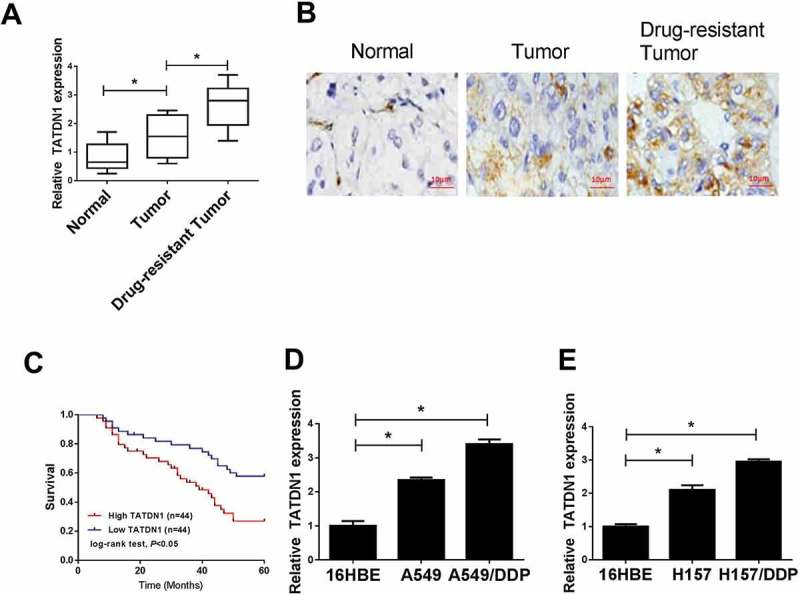
Expression of lncRNA-TATDN1 in DDP-sensitive or tolerant NSCLC tumor tissues and cell lines and the prognosis of patients after DDP-treatment. (A) qRT-PCR ananlysis of TATDN1 expression in normal tissues and DDP-sensitive or DDP-tolerant NSCLC tumor tissues. (B) TATDN1 expression profile was evaluated by immunohistochemical staining. (C) A total of 88 patients received DDP chemotherapy, followed by the statistics of survival rate in high and low TATDN1 expression groups. (D and E) TATDN1 levels in 16HBE cells and DDP-sensitive or DDP-resistant cell lines were performed by qRT-PCR. *P < 0.05.
TATDN1 knockdown enhanced DDP sensitivity in DDP-resistant NSCLC
To further explore the effects of TATDN1 on DDP resistance in lung cancer cells, TATDN1 knockdown was performed through transfection of sh-TATDN1 into A549/DDP and H157/DDP cells (Figure 2A and 2B). Afterwards, DDP-resistant lung cancer cells were treated with different concentration of DDP and transfected with sh-TATDN1 or sh-control, followed by the detection of survival rates by MTT. The results suggested that TATDN1 knockout led to a decreased survival rate of A549/DDP and H157/DDP cells (Figure 2C and 2D). To further explore the effects of TATDN1 on cell proliferation and apoptosis, MTT, FCM and western blot analyses were determined and results revealed that abated expression of TATDN1 notably suppressed cell proliferation (Figure 2E and 2F), while stimulated cell apoptosis, reflected by the increased apoptosis rate (Figure 2G and 2I) and cleaved caspase-3 level (Figure 2H and 2J), as well as the decreased expression of anti-apoptosis protein Bcl-2 (Figure 2H and 2J). Altogether, these results manifested that TATDN1 knockdown improved DDP sensitivity of DDP-tolerant NSCLC.
Figure 2.
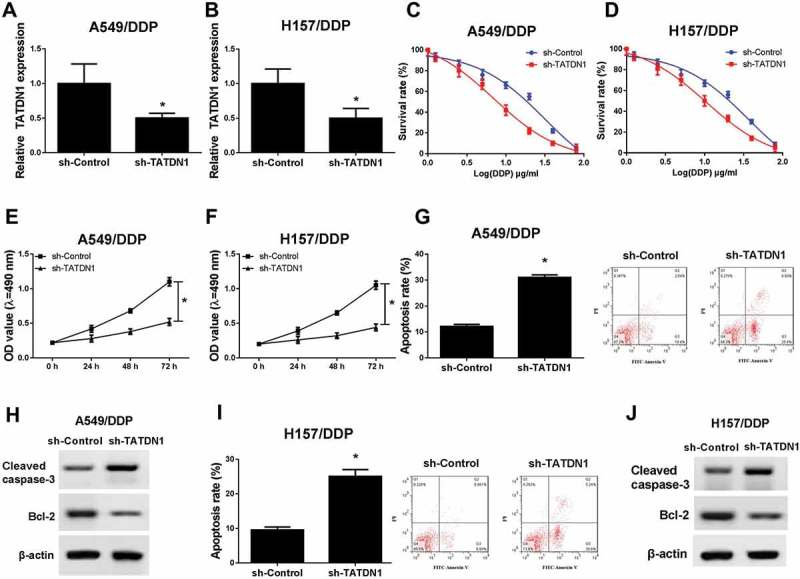
Downregulation of TATDN1 blocked the survival and proliferation while stimulated apoptosis of DDP-resistant NSCLC cells. (A and B) Expression of TATDN1 was inhibited after transfection of sh-TATDN1 into A549/DDP and H157/DDP cells. MTT analysis revealed that TATDN1 knockdown reduced survival rate (C and D) and proliferation (E and F) of A549/DDP and H157/DDP cells. (G and I) The apoptosis rates of A549/DDP and H157/DDP cells were markedly elevated following the transfection of sh-TATDN1 as measured by FCM assays. (H and J) The protein expression of cleaved caspase-3 and Bcl-2 in A549/DDP and H157/DDP cells was detected by western blot.*P < 0.05.
TATDN1 acted as a molecule sponge for mir-451
The expression of miR-451 in NSCLC tumors and cells was detected by qRT-PCR, and results exposed that miR-451 exhibited significantly lowered expression in lung cancer tumors and cells, especially in DDP-resistant tumors and cell lines (Figure 3A, 3B and 3C). It has been widely reported that lncRNA modulated mRNA expression via acting as a ceRNA to share miRNA recognition sites with mRNAs. In the following study, miRcode bioinformatics software was employed to investigate the molecule mechanism of TATDN1 in DDP tolerance of NSCLC, and results showed the existence of complementary binding sites between TATDN1 and miR-451 (Figure 3D). Afterwards, Dual-Luciferase Reporter assay and qRT-PCR were conducted to further verify the interaction of TATDN1 and miR-451. As our expected, the luciferase activity of WT-TATDN1 reporter was notably inhibited, but there was no obvious change in MUT-TATDN1 reporter after overexpression miR-451 in A549/DDP (Figure 3E) and H157/DDP (Figure 3F) cells. qRT-PCR revealed that TATDN1 silence promoted miR-451 expression, inversely, upregulation of TATDN1 repressed miR-451 expression in A549/DDP (Figure 3G) and H157/DDP (Figure 3H) cells. Overall, TATDN1 negatively modulated miR-451 expression via direct interaction.
Figure 3.
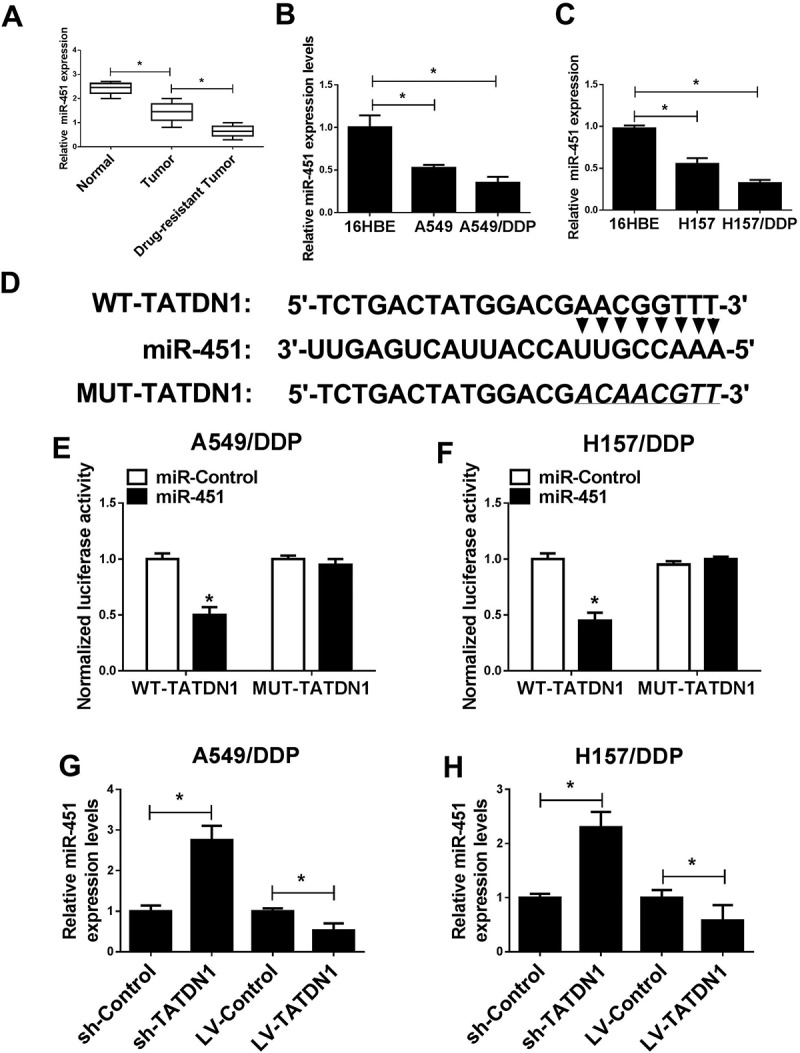
TATDN1 acted as a molecular sponge for miR-451. (A-C) Expression of miR-451 in DDP-sensitive or DDP-tolerant NSCLC tissues and cell lines. (D) The predicted binding sequences between TATDN1 and miR-451, as well as the mutation of TATDN1. (E and F) Luciferase reporter assays in A549/DDP and H157/DDP cells after the co-transfection of established WT-TATDN1 or MUT-TATDN1 reporters and miR-control or miR-451 mimics. (G and H) TATDN1 knockdown activated while TATDN1 upregulation repressed the expression of miR-451 expression in DDP-resistant NSCLC cells. *P < 0.05.
TRIM66 was a direct target gene of mir-451
In present study, TRIM66 was identified to be a candidate target gene of miR-451 (Figure 4A). Afterwards, Dual-Luciferase analysis disclosed that miR-451 upregulation suppressed the luciferase activity of WT-TRIM66 3ʹUTR compared with that of control group in A549/DDP and H157/DDP cells (Figure 4B and 4C), however, the regulatory effect of miR-451 was disappeared in MUT-TRIM66 3ʹUTR (Figure 4B and 4C). qRT-PCR and western blot assays revealed that the mRNA (Figure 4D and 4E) and protein (Figure 4F and 4G) expression of TRIM66 were significantly reduced after the transfection of miR-451 mimic, while miR-451 knockdown accelerated TRIM66 expression in DDP-tolerant cells. In sum, these results indicated the true interaction of miR-451 and TRIM66 via directly complementation binding.
Figure 4.
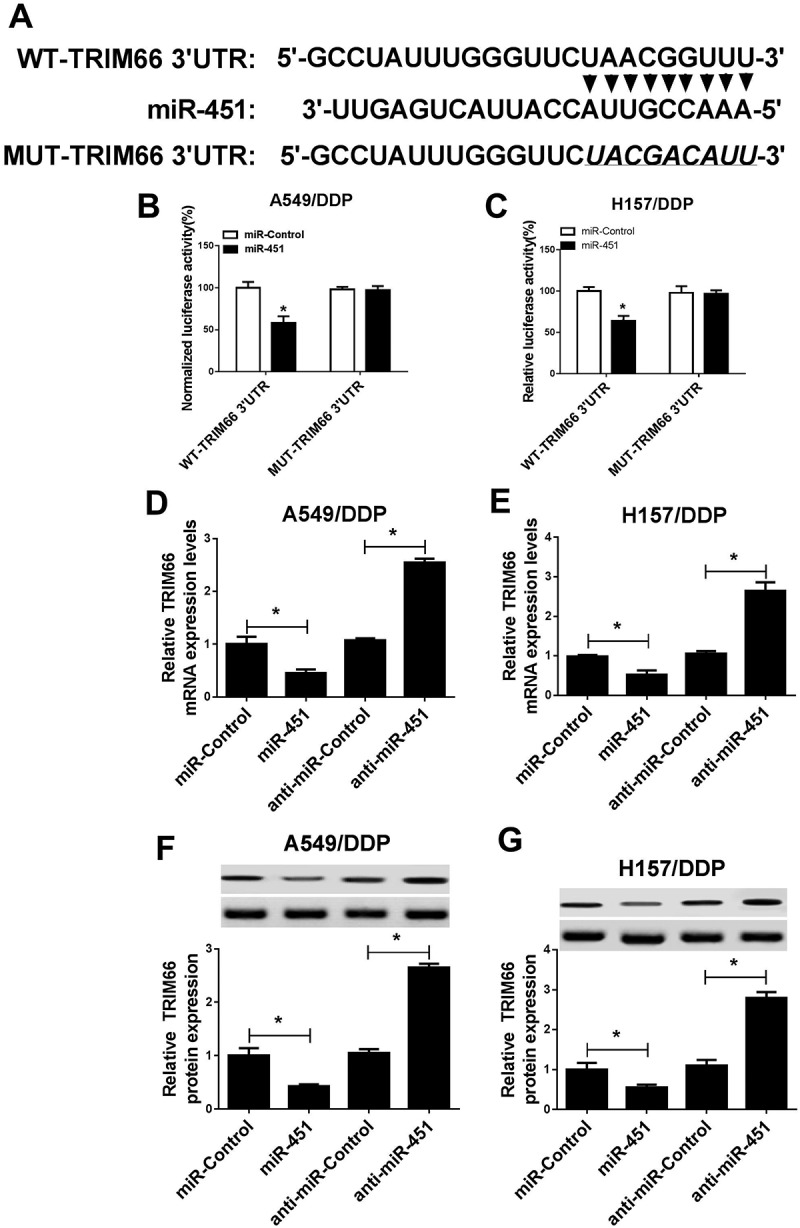
MiR-451 inhibited TRIM66 expression via binding at the 3ʹUTR fragments of TRIM66. (A) The assumed binding sites between miR-451 and TRIM66 and the generation of mutant fragments in 3ʹUTR of TRIM66. (B and C) Luciferase activity of A549/DDP and H157/DDP cells following the co-transfection of WT-TRIM66-3ʹUTR or MUT-TRIM66-3ʹUTR and miR-451 mimic or miR-control. (D and E) qRT-PCR was utilized to detect TRIM66 expression at mRNA level in A549/DDP and H157/DDP cells. (F and G) Western blot was employed to measured TRIM66 expression at protein level in A549/DDP and H157/DDP cells. *P < 0.05.
TRIM66 silence improved DDP sensitivity in DDP-resistant NSCLC
In present study, qRT-PCR and western blot analyses were first conducted for the detection of TRIM66 mRNA and protein expression, and results disclosed a markedly increased expression of TRIM66 in NSCLC tumors and cell lines compared with that in corresponding normal controls. Moreover, TRIM66 levels in DDP-resistant tumors elicited more obviously elevated than in DDP-sensitive groups (Figure 5A-5F). In following research, the functions of TRIM66 on DDP resistance were explored by MTT, FCM and western assays via knockdown of TRIM66 in A549/DDP and H157/DDP cells. After treated with multiple concentration of DDP for 48 h, survival rate of A549/DDP and H157/DDP cells was prominently decreased in sh-TRIM66 groups (Figure 5G and 5H), and OD value during a 72 h period also uncovered the inhibitory effect of sh-TRIM66 on cell proliferation (Figure 5I and 5J). On the contrary, cell apoptosis was significantly increased, reflected by the induced apoptosis rate and cleaved caspase-3 expression, as well as the downregulation of Bcl-2 (Figure 5K-5N). Overall, TRIM66 knockdown enhanced DDP sensitivity in NSCLC via repressing cell survival rate and proliferation, while inducing cell apoptosis.
Figure 5.
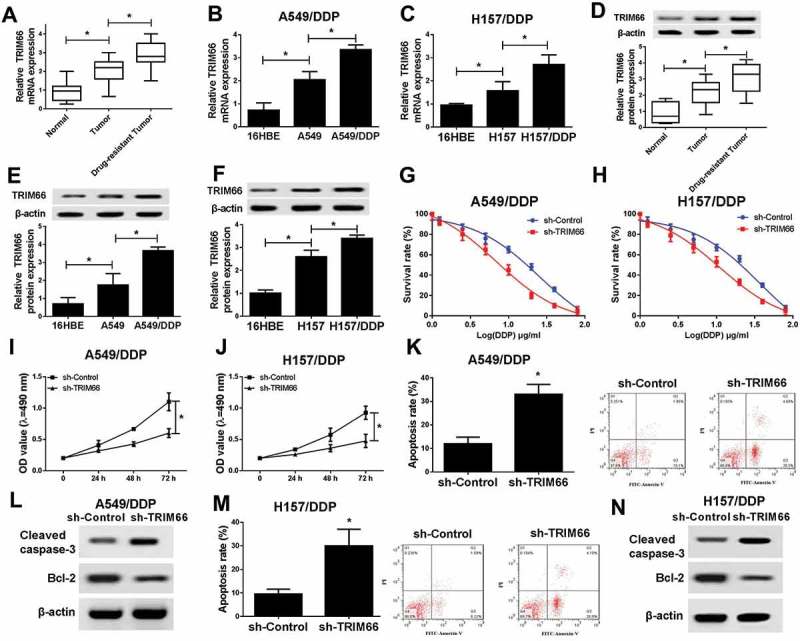
Downexpression of TATDN1 decreased the survival and proliferation while promoted apoptosis of DDP-resistant NSCLC cells. (A-C) The mRNA expression of TRIM66 in NSCLC tissues and cell lines. (D-E) The protein expression of TRIM66 in NSCLC tissues and cell lines. (G-J) Survival rates and the proliferation of A549/DDP and H157/DDP cells were notably decreased with TRIM66 knockdown. (K and M) The apoptosis rates of A549/DDP and H157/DDP cells were markedly suppressed after the transfection of sh-TATDN1. (L and N) The protein expression of cleaved caspase-3 and Bcl-2 in A549/DDP and H157/DDP cells was detected by western blot.*P < 0.05.
TATDN1 knockdown improved DDP-sensitivity of NSCLC via blocking TRIM66 expression by competitive interaction with mir-451
Subsequently, detailed mechanism of TATDN1 in the regulation of NSCLC DDP tolerance was investigated through restoration experiments. Results showed that TATDN1 overexpression weakened miR-451-reduced TRIM66 mRNA (Figure 6A and 6B) and protein expression (Figure 6C and 6D) in DDP-resistant NSCLC cells. Moreover, miR-451 knockdown reversed sh-TATDN1-lowered TRIM66 mRNA (Figure 6A and 6B) and protein expression (Figure 6C and 6D) in A549/DDP and H157/DDP cells.
Figure 6.
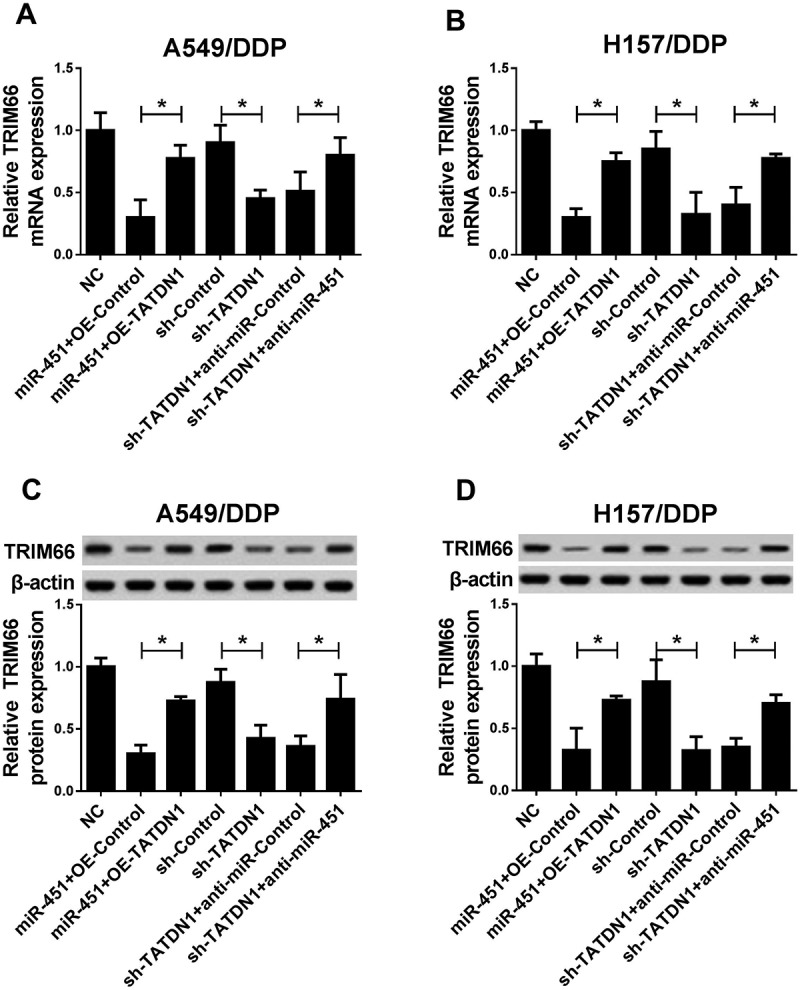
TATDN1 knockdown enhanced DDP-resistance of NSCLC through downregualtion of TRIM66 expression by sponging miR-451. (A and B) TATDN1 overexpression abolished miR-451-lowered TRIM66 mRNA expression, moreover, miR-451 knockdown reversed sh-TATDN1-repressed TRIM66 mRNA expression in A549/DDP and H157/DDP cells. (C and D) miR-451 inhibitor weakened sh-TATDN1-decreased TRIM66 protein expression in A549/DDP and H157/DDP cells. *P < 0.05.
TATDN1 knockdown improved DDP sensitivity of NSCLC in vivo
In present research, a xenograft tumor mouse model was established to confirm the impact of TATDN1 on DDP tolerance in vivo. A549/DDP cells transfected with sh-TATDN1 was subcutaneously injected into BALB/c nude mice, following by the intraperitoneal injection of sh-Control and DDP. As present in Figure 7A and 7B, TATDN1 knockdown significant prevented tumor growth, as reflected by the diminished tumor volume and weight. Subsequently, qRT-PCR analysis revealed that sh-TATDN1 introduction lowered TATDN1 expression, indicating the establishment of TATDN1 knockdown animal model (Figure 7C). The expression of miR-451 was increased (Figure 7D), while TRIM66 mRNA (Figure 7E) or protein expression (Figure 7F) was induced in tumor masses derived from sh-TATDN1-transfected NSCLC cells. Thus, it is concluded that TATDN1 knockdown enhanced DDP sensitivity of NSCLC in vivo.
Figure 7.
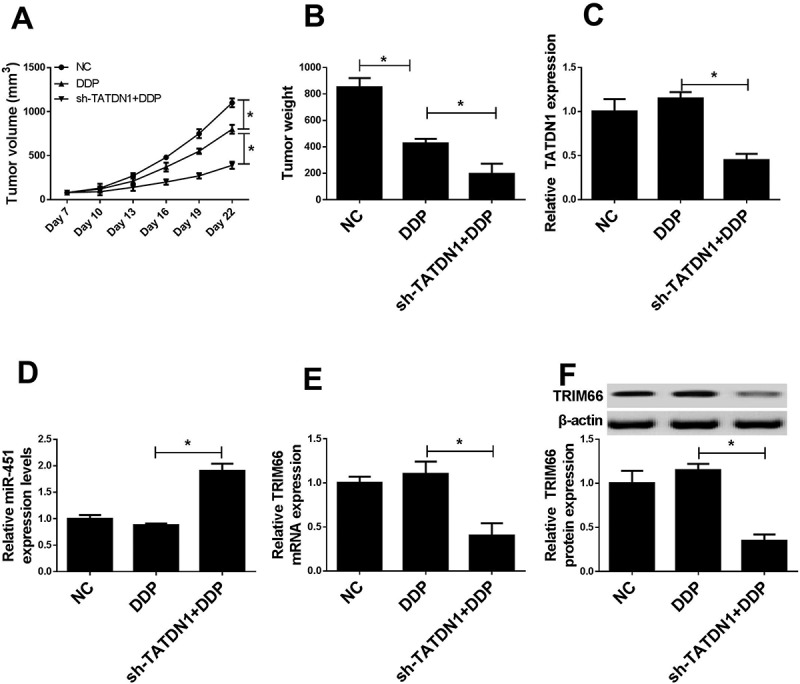
Regulatory effects of TATDN1 knockdown on tumor growth and miR-451 or TRIM66 expression in vivo. (A) Tumor volume was calculated every 3 days following DDP-treatment. (B) Tumor weights were evaluated in different groups. The expression levels of TATDN1 (C), miR-451 (D) and TRIM66 (E and F) in lumps were detected by qRT-PCR or western-blot. *P < 0.05.
Discussion
To data, the prognosis for NSCLC is still gloomy, and chemoresistance was deemed to be a major reason for treatment failure and death of NSCLC patients. Extensive published evidence has confirmed that cisplatin was an effective broad-spectrum anticancer drugs through forming different DNA-platinum adducts to interfer with DNA replication.15 Whereas, there is still an impediment to the successful therapy in cancer patients with cisplatin resistance. Thus, taking appropriate measures to improve cisplatin sensitive will contribute to the survival and prognosis of NSCLC patients in clinical.
Convincing evidence has indicated the regulatory effects of lncRNAs in drug resistance of diverse cancers, including NSCLC.16,17 For instants, lncRNA-XIST was upregulated in cisplatin-resistant NSCLC cells, and knockdown of lncRNA-XIST enhanced the chemosensitivity through suppression of autophagy via interacting with miR-17/ATG7 axis.18 Knockdown of lncRNA AK126698 activated Wnt pathway via blocking NKD2 expression and ultimately involved in the regulation of cisplatin resistance in A549 cells.19 The expression of LncRNA HOTAIR was dramatically elevated in NSCLC drug-resistant tissues and cells, and HOTAIR contributed to the drug-resistance via upregulating Kif4.20 In present study, we focused on the function and mechanism of lncRNA TATDN1 in NSCLC cisplatin resistance. As results showed that TATDN1 was obviously upregulated in DDP-tolerant NSCLC tissues and cell lines, and patients with low TATDN1 expression displayed a better long-term prognosis following DDP-treatment. In addition, knockdown TATDN1 repressed the growth and stimulated apoptosis of DDP-tolerant NSCLC cells. Xenograft model experiment also indicated the prominent inhibition of TATDN1 interference on tumor growth in BALB/c nude mice. These data indicated that TATDN1 was closely associated with the DDP-resistance of NSCLC, while the detail mechanism underlying how TATDN1 modulated drug-resistance still need to be further illuminated.
Accumulated evidences have proposed that lncRNAs were key compositions of ceRNA-mediated regulatory network and involved in tumorigenesis via interacting with miRNAs/mRNA axis.21 Thus, to better explore the inter-mechanism of TATDN1 in DDP-tolerance of NSCLC, a series of related analysis were performed, and results demonstrated that TATDN1 could act as a sponge for miR-451. MiR-451 has been demonstrated to play an critical role in the tumorigenesis and chemoresistance of multiple cancers.11 As reported by Li, miR-451 functioned as a potent suppressor of oncogenesis in T-ALLs (T cell acute lymphoblastic leukemia) bearing NOTCH1 mutations.22 Redona reported that miR-451 expression was significant lowered in serum of RCC (renal cell carcinoma) patients.23 Furthermore, miR-451 agomir induced tumor-suppression in SKBR3/PR drug-resistant xenograft model via targeting tyrosine3-monooxygenase/tryptophan5-monooxygenase activation protein zeta (YWHAZ).24 In this review, we found that miR-451 expression was upregulated in DDP-resistant NSCLC tissues and cells. Moreover, the expression of miR-451 could be negatively regulated by TATDN1.
TRIM66 has been proved to be a major oncogene in lung cancer. Abnormal high expression of TRIM66 was closely associated with cell metastasis, TNM stage and prognosis of NSCLC patients.25 In this study, TRIM66 was identified to be a target gene of miR-451, the expression of TRIM66 was dramatically elevated in DDP-tolerant NSCLC tumors and cells. Besides that, knockdown of TRIM66 elicited the inhibitory effects in the growth of drug-resistant NSCLC cells. During the last stage in the research, we revealed that TATDN1 upregulated the expression of TRIM66 by competitively binding miR-451.
In conclusion, our data indicated that TATDN1 knockdown suppressed the survival and proliferation, while accelerated apoptosis of DDP-resistant NSCLC cells by modulation of miR-451/TRIM66 axis. Therefore, our results implied that TATDN1 function as a stimulator for NSCLC DDP-resistance, hinting the possibility of TATDN1 as a potential therapy target for NSCLC patients with DDP treatment failure.
Funding Statement
This study was granted by the Scientific Research Fund of Education Bureau in Yunnan province. (No. 2014Z070).
Acknowledgments
Not applicable.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Travis WD. Pathology of lung cancer. Clin Chest Med. 2011;32:669–692. doi: 10.1016/j.ccm.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F.. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Matsuda A, Yamaoka K, Tango T. Quality of life in advanced non-small cell lung cancer patients receiving palliative chemotherapy: A meta-analysis of randomized controlled trials. Exp Ther Med. 2012;3:134. doi: 10.3892/etm.2011.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosell R, Lord RV, Taron M, Reguart N. DNA repair and cisplatin resistance in non-small-cell lung cancer. Lung Cancer. 2002;38:217. doi: 10.1016/S0169-5002(02)00224-6. [DOI] [PubMed] [Google Scholar]
- 5.Bassett AR, Akhtar A, Barlow DP, Bird AP, Brockdorff N, Duboule D, Ephrussi A, Ferguson-Smith AC, Gingeras TR, Haerty W, et al. Considerations when investigating lncRNA function in vivo. ELife. 2014;3:e03058. doi: 10.7554/eLife.03058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang G, Lu X, Yuan L. LncRNA: a link between RNA and cancer. Biochim Biophys Acta. 2014;1839:1097–1109. doi: 10.1016/j.bbagrm.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Niu Z, Zhang X, Li W, Ming Z, Zhong Y, Hou Y, Zhang Y, Meng X, Wang W, Deng W. The role and potential mechanisms of LncRNA-TATDN1 on metastasis and invasion of non-small cell lung cancer. Oncotarget. 2016;7:18219. doi: 10.18632/oncotarget.7788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilczynska A, Bushell M. The complexity of miRNA-mediated repression. Cell Death Differ. 2015;22:22–33. doi: 10.1038/cdd.2014.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riquelme I, Tapia O, Leal P, Sandoval A, Varga MG, Letelier P, Buchegger K, Bizama C, Espinoza JA, Peek RM. miR-101-2, miR-125b-2 and miR-451a act as potential tumor suppressors in gastric cancer through regulation of the PI3K/AKT/mTOR pathway. Cell Oncology. 2016;39:23–33. doi: 10.1007/s13402-015-0247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian F, Han Y, Yan X, Zhong D, Yang G, Lei J, Li X, Wang X. Upregulation of microrna‐451 increases the sensitivity of A549 cells to radiotherapy through enhancement of apoptosis. Thorac Cancer. 2016;7:226–231. doi: 10.1111/1759-7714.12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan X, Wang R, Wang ZX. The potential role of miR-451 in cancer diagnosis, prognosis, and therapy. Mol Cancer Ther. 2013;12:1153. doi: 10.1158/1535-7163.MCT-12-0802. [DOI] [PubMed] [Google Scholar]
- 12.Tay Y, Rinn J, Pandolfi PP. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014;505:344–352. doi: 10.1038/nature12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan JY, Sirey T, Honti F, Graham B, Piovesan A, Merkenschlager M, Webber C, Ponting CP, Marques AC. Extensive microRNA-mediated crosstalk between lncRNAs and mRNAs in mouse embryonic stem cells. Genome Res. 2015;25:655. doi: 10.1101/gr.181974.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Q, Guo L, Jiang F, Li L, Li Z, Chen F. Analysis of the miRNA-mRNA-lncRNA networks in ER+ and ER- breast cancer cell lines. J Cell Mol Med. 2016;19:2874–2887. doi: 10.1111/jcmm.12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen DW, Pouliot LM, Hall MD, Gottesman MM. Cisplatin resistance: a cellular self-defense mechanism resulting from multiple epigenetic and genetic changes. Pharmacol Rev. 2012;64:706. doi: 10.1124/pr.111.005637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen QN, Wei CC, Wang ZX, Sun M. Long non-coding RNAs in anti-cancer drug resistance. Oncotarget. 2016;8:1925. doi: 10.18632/oncotarget.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xue W, Li L, Tian X, Fan Z, Yue Y, Zhang C, Ding X, Song X, Ma B, Zhai Y. Integrated analysis profiles of long non-coding RNAs reveal potential biomarkers of drug resistance in lung cancer. Oncotarget. 2017;8:62868. doi: 10.18632/oncotarget.16444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun W, Zu Y, Fu X, Deng Y. Knockdown of lncRNA-XIST enhances the chemosensitivity of NSCLC cells via suppression of autophagy. Oncol Rep. 2017:3347–3354. doi: 10.3892/or.2017.6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Y, Li H, Hou S, Hu B, Liu J, Wang J. The noncoding RNA expression profile and the effect of lncRNA AK126698 on cisplatin resistance in non-small-cell lung cancer cell. PLos One. 2013;8:e65309. doi: 10.1371/journal.pone.0065309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu MY, Li XQ, Gao TH, Cui Y, Ma N, Zhou Y, Zhang GJ. Elevated HOTAIR expression associated with cisplatin resistance in non-small cell lung cancer patients. J Thorac Dis. 2016;8:3314. doi: 10.21037/jtd.2016.11.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Hou J, He D, Sun M, Zhang P, Yu Y, Chen Y. The Emerging Function and Mechanism of ceRNAs in Cancer. Trends Genet. 2016;32:211–224. doi: 10.1016/j.tig.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, Sanda T, Look AT, Novina CD, Boehmer H. Repression of tumor suppressor miR-451 is essential for NOTCH1-induced oncogenesis in T-ALL. J Exp Med. 2011;208:663–675. doi: 10.1084/jem.20102384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Redova M, Poprach A, Nekvindova J, Iliev R, Radova L, Lakomy R, Svoboda M, Vyzula R, Slaby O. Circulating miR-378 and miR-451 in serum are potential biomarkers for renal cell carcinoma. J Transl Med. 10,1(2012-03-22) 2012;10:1–8. doi: 10.1186/1479-5876-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang W, Zhang L, Wang Y, Ding Y, Chen T, Wang Y, Wang H, Li Y, Duan K, Chen S. Involvement of miR-451 in resistance to paclitaxel by regulating YWHAZ in breast cancer. Cell Death Dis. 2017;8:e3071. doi: 10.1038/cddis.2017.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma Y, Dai HY, Zhang F, Zhao D. TRIM66 expression in non-small cell lung cancer: A new predictor of prognosis. Cancer Biomark. 2017:309–315. doi: 10.3233/CBM-170207. [DOI] [PubMed] [Google Scholar]


