ABSTRACT
As a novel vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor (VEGFR2-TKI), apatinib has a certain anti-tumor effect for a variety of solid tumors. The present study evaluates its efficacy and safety in advanced hepatocellular carcinoma (HCC). In this study, 47 patients with advanced HCC were included. TACE monotherapy group included 22 patients that responded to TACE, while the group that received TACE and apatinib included 25 patients that progressed on TACE and were able to receive apatinib off label. Median overall survival (OS) was significantly improved in the apatinib plus TACE group compared with the TACE group. Similarly, apatinib in combination with TACE significantly prolonged median progression-free survival (PFS) compared with TACE monotherapy. Furthermore, there was a significant difference between combination therapy and monotherapy in both Barcelona clinic liver cancer (BCLC) B and BCLC C group. The combination therapy had a dramatic effect on OS and PFS for patients at both BCLC B and BCLC C level. The most common clinically adverse events of apatinib plus TACE group were fatigue, weight loss, hypertension, hand-foot syndrome and anorexia, which were manageable and tolerable. The efficacy analysis showed that there was no significant association of survival benefit with age, gender, Eastern Cooperative Oncology Group (ECOG) performance status, hypertension and hand-foot syndrome. Patients with macrovascular invasion and extrahepatic invasion showed worse survival benefits. In conclusion, apatinib combined with TACE revealed certain survival benefits for HCC patients who experienced progression following TACE, which can provide a promising strategy for HCC treatment.
Keywords: hepatocellular carcinoma; apatinib; transarterial chemoembolization; targeted therapy; combination therapy, vascular endothelial growth factor receptor-2; angiogenesis
Introduction
Hepatocellular carcinoma (HCC) is the second leading cause of cancer-related death in men worldwide, and it is generally considered resistant to chemotherapy.1 HCC is often diagnosed at advanced stage due to its insidious onset and nonspecific nature of the symptoms.2 For patients with advanced and unresectable HCC, transarterial chemoembolization (TACE) and tyrosine kinase inhibitors are considered as standard therapeutic methods.3 As a widely accepted treatment strategy for HCC, TACE could effectively inhibit tumor progression.1,4 However, TACE has been shown to induce hypoxia and elevate the level of proangiogenic factor vascular endothelial growth factor (VEGF) in the residual surviving HCC tissues, resulting in significant neoangiogenetic reactions and relapses after treatment.5-7 Since there is no ideal treatment for HCC patients who experienced disease progression following TACE, it is of vital importance to develop novel therapeutic methods for these patients.
Angiogenesis facilitate the supply of oxygen and nutrients to tumor cells, and therefore plays a critical role in tumor growth, development and metastasis.8 Tyrosine kinase inhibitors (TKIs) such as sorafenib and regorafenib suppress vessel growth, and they have been shown to exert some survival benefit for HCC patients.9,10 Therefore, to evaluate the antineoplastic effect of anti-angiogenic therapy is critical in HCC treatment.11 Recently, apatinib, a novel TKI has shown promising therapeutic potential in various types of cancers with tolerable level of toxicity.12 The combination of angiogenesis inhibitor apatinib and TACE can effectively inhibit peripheral angiogenesis of tumors as well as delay tumor progression.12 The evidence above reveal the significant therapeutic potential of TACE combined with apatinib in HCC. Thus, in the present study, we conducted a retrospective evaluation of the therapeutic effect of TACE combined with apatinib on patients with advanced HCC who experienced progression after TACE treatment.
Results
Patient characteristics
Forty-seven patients with advanced hepatocellular carcinoma between September 2016 and August 2017 were included. TACE monotherapy group included twenty-two patients that responded to TACE treatment alone (control group), while the group that received TACE and apatinib included twenty-five patients that progressed on TACE and were able to receive apatinib off label (experimental group) (Figure 1). Clinic pathological characteristics at the initiation of treatment were shown in Table 1. Baseline data of the experimental group and control group were comparable. The percentage of patients with hepatitis in TACE only group is 72.7% (16/22), similar to that of TACE + apatinib group 72% (18/25).
Figure 1.
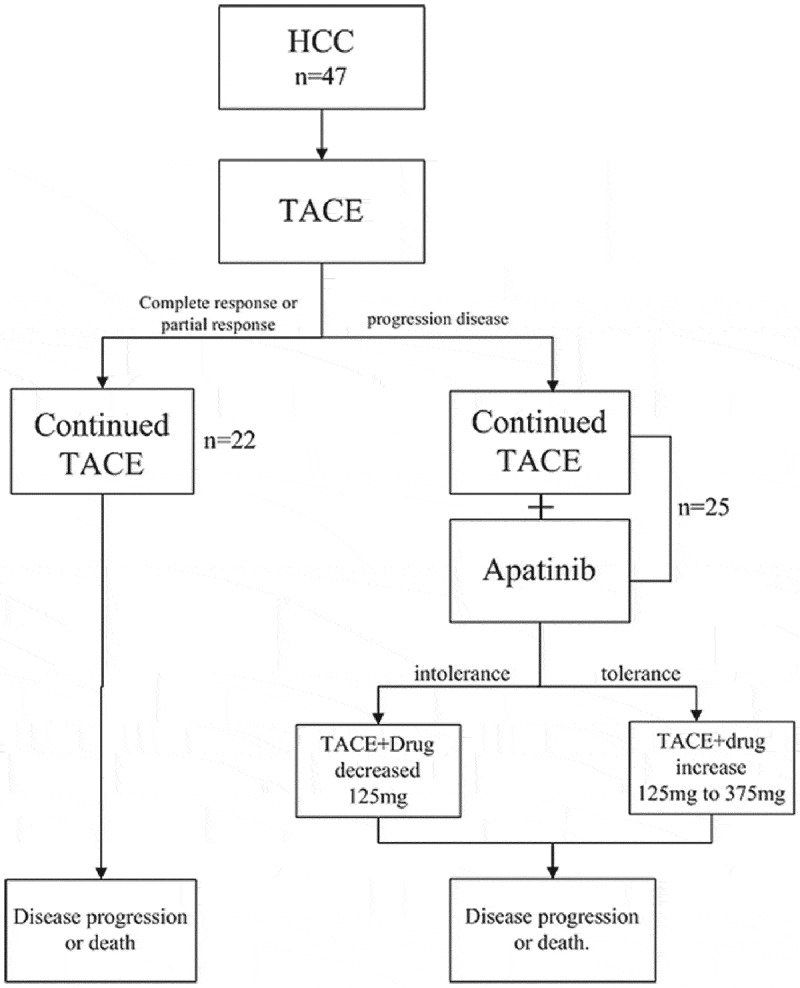
Flow diagram illustrating the treatment process.
Forty-seven patients with advanced hepatocellular carcinoma who has been treated with TACE between September 2016 and August 2017 were included. Among these patients, twenty-two patients with advanced hepatocellular carcinoma responded positively to TACE treatment alone. Twenty-five patients with advanced hepatocellular carcinoma who experienced progression after TACE were prescribed with apatinib. Apatinib was administered orally at an initial dose of 250mg once a day. The starting dose was determined on an individual basis according to patients’ performance status and comorbidities, as per clinician discretion. The dose of apatinib was reduced to 125mg/day if the patients are intolerant or increased to 375mg/day if the patients are tolerant.
Table 1.
The baseline characteristics of the 45 HCC patients.
| Demographics | Experimental group (N = 25) |
Control group (N = 22) |
|---|---|---|
| Gender | ||
| Male | 20 | 18 |
| Female | 5 | 4 |
| Age | ||
| >65 | 12 | 10 |
| ≤ 65 | 13 | 12 |
| Comorbidities | ||
| Hepatitis B | 18 | 14 |
| Hepatitis C | 0 | 2 |
| liver cirrhosis | 14 | 10 |
| BCLC algorithm | ||
| B(intermediate) | 10 | 10 |
| C(advanced) | 15 | 12 |
| Apatinib dose | ||
| Initial dose 250 mg qd | 24 | - |
| Initial dose 125 mg qd | 1 | - |
| Duration of Apatinib treatment, (days) | 133.7 | - |
| Number of interventional therapy | 6.3(1–24) | 6.1(1–17) |
| ECOG score | ||
| 0 | 8 | 12 |
| 1 | 11 | 10 |
| 2 | 6 | 9 |
Efficacy
At the end of follow-up, median OS was significantly improved in the apatinib plus TACE group compared with the TACE group (496 days; 95% CI, 421.1–890.1 vs 294 days; 95% CI, 220–742.4; P = 0.017). Similarly, apatinib in combination with TACE significantly prolonged median PFS compared with TACE monotherapy (135 days; 95% CI, 119.2–232.5 vs 54 days; 95% CI, 19.01–191.3; P = 0.004) (Figure 2). The evaluation of best response in each group was shown in Table 2 according to mRECIST criteria.
Figure 2.
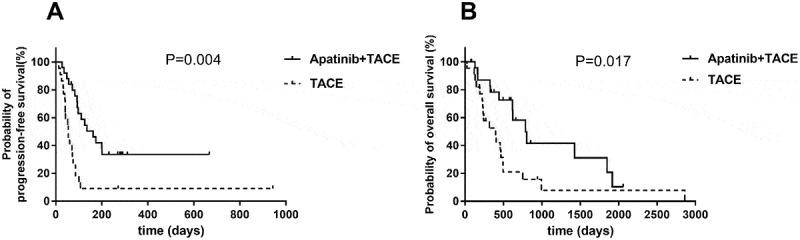
Efficacy of TACE and TACE plus apatinib treatment.
(A) Kaplan–Meier survival curve showing progression-free survival (PFS) with combination of TACE and apatinib compared to TACE alone. Apatinib in combination with TACE significantly prolonged median PFS compared with TACE monotherapy. (B) Kaplan–Meier survival curve showing overall survival (OS) with combination of TACE and apatinib compared to TACE alone. Median OS was significantly improved in the apatinib plus TACE group compared with the TACE group.
Table 2.
Best overall response according to mRECIST.
| Best response | Experimental group (N = 25) |
Control group (N = 22) |
|---|---|---|
| Complete response | 3 | 2 |
| Partial response | 6 | 5 |
| Stable disease | 5 | 5 |
| Progressive disease | 11 | 10 |
| Objective response rate | 36% | 31.8% |
| Disease control rate | 56% | 54.5% |
mRECIST, modified Response Evaluation Criteria in Solid Tumors
Objective Response Rate (ORR) = Complete Response (CR)+ Partial Response (PR)
Disease Control Rate (DCR) = Complete Response (CR)+ Partial Response (PR)+ Stable Disease (SD)
Predictive value of advanced hepatocellular carcinoma
Univariate analysis indicated that there was no significant association of survival benefits with age, gender and ECOG performance status in the experimental group (Table 3). Patients with Barcelona clinic liver cancer (BCLC) stage B have prolonged PFS and OS over patients with BCLC stage C (Figure 3). In BCLC-B group, the OS increased from only 399 days using TACE to 588 days using TACE and apatinib (P = 0.014) (Figure 4A). The PFS increased from 42 days to 273.5 days (P = 0.003) (Figure 4B). Similarly, in BCLC-C group, TACE combined apatinib improved OS from 233 days to 442 days (P = 0.031) (Figure 5A). TACE combined with apatinib also improved PFS from 62 days to 97 days (P = 0.005) (Figure 5B). The combination therapy had a dramatic effect on OS and PFS for patients at both BCLC B and BCLC C level (Table 4). Furthermore, we analyzed whether hypertension and hand-foot syndrome were associated with PFS and OS and found that those variables were not linked.
Table 3.
The Log Rank analysis of factors for survival benefit in the experimental group.
| OS |
PFS |
||||
|---|---|---|---|---|---|
| Variable | NO. | Median (95% CI) | P | Median (95% CI) | P |
| Age | 0.442 | 0.176 | |||
| >65 | 12 | 520 (257.9–955.6) | 127 (78.6–185.8) | ||
| ≤ 65 | 13 | 496 (335.7–1065.7) | 200 (116.2–316.4) | ||
| Gender | 0.783 | 0.873 | |||
| Male | 20 | 410 (348.8–874.9) | 144 (112.9–250.1) | ||
| Female | 5 | 663 (57.3–1603.9) | 135 (27.8–279.4) | ||
| BCLC | 0.001* | 0.001* | |||
| B | 10 | 588 (300.6–1261.6) | 273.5 (141.7–382.1) | ||
| C | 15 | 442 (298.0.6–845.8) | 97 (78.1–159.0) | ||
| ECOG score | 0.213 | 0.417 | |||
| 0–1 | 13 | 379 (227.3–866.66) | 126 (87.9–198.7) | ||
| ≥ 2 | 12 | 621 (386.8–1159.7) | 181 (102.3–320.0) | ||
| Hypertension | 0.121 | 0.869 | |||
| Yes | 17 | 579 (382.7–1070.4) | 135 (98.9–260.4) | ||
| No | 8 | 393 (324.9–684.6) | 144 (92.4–243.3) | ||
| Hand-foot syndrome | 0.056 | 0.838 | |||
| Yes | 15 | 343 (248.2–517.1) | 111 (94.4–215.1) | ||
| No | 10 | 737.5 (580.5–1549.5) | 154 (83.8–331.4) | ||
NO. = the number of the patients
Figure 3.
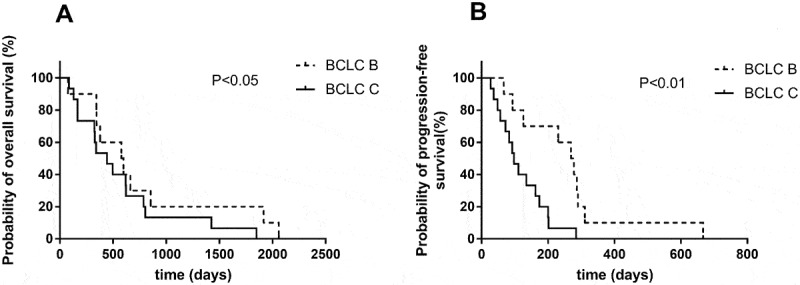
Efficacy of the combination of TACE and apatinib.
(A) Kaplan–Meier survival curve showing the probability of overall survival (OS) stratifed by BCLC B and BCLC C. (B) Probability of progression-free survival (PFS) stratifed by BCLC B and BCLC C. The result showed patients with BCLC B has longer PFS and OS than BCLC C.
Figure 4.
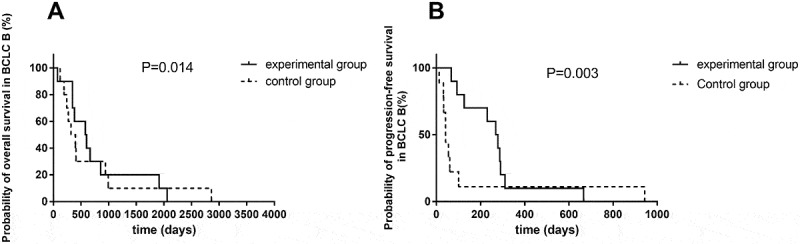
Survival curve of TACE and apatinib therapy in BCLC-B group.
(A) Kaplan–Meier survival curve showing improved overall survival with combination of TACE and apatinib compared to TACE alone in BCLC-B group. (B) Kaplan–Meier survival curve showing improved progression-free survival with combination of TACE and apatinib compared to TACE alone in BCLC-B group.
Figure 5.
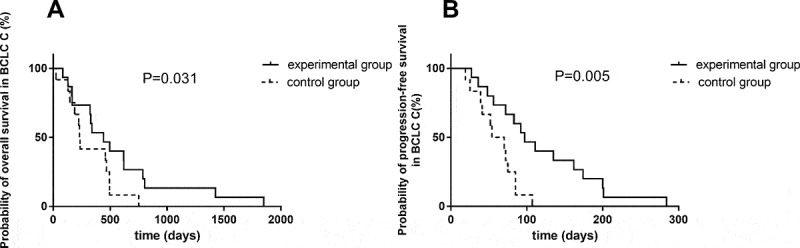
Survival curve of TACE and apatinib therapy in BCLC-C group.
(A) Kaplan–Meier survival curve showing improved overall survival with combination of TACE and apatinib compared to TACE alone in BCLC-C group. (B) Kaplan–Meier survival curve showing improved progression-free survival with combination of TACE and apatinib compared to TACE alone in BCLC-C group.
Table 4.
The analysis of BCLC B and BCLC C.
| OS |
PFS |
||||
|---|---|---|---|---|---|
| Variable | NO. | Median (95% CI) | P | Median (95% CI) | P |
| BCLC B | 0.014* | 0.003* | |||
| Experimental group | 10 | 588 (300.6–1261.6) | 273.5 (141.7–382.1) | ||
| Control group | 10 | 399 (276.5–521.5) | 42 (21.9–62.1) | ||
| BCLC C | 0.031* | 0.005* | |||
| Experimental group | 15 | 442 (298–845.8) | 97 (78.1–159.0) | ||
| Control group | 12 | 233 (187.3–453.2) | 62 (43.5–77.2) | ||
Safety
All 47 patients were included in the safety analysis set. Twenty-four patients took apatinib with an initial dose of 250mg and one patient took apatinib with an initial dose of 125mg. Toxicities were manageable and tolerable. Our results showed that out of 25 patients receiving apatinib, 6 patients developed Grade 1 toxicity, 15 developed Grade 2 toxicity, 4 developed Grade 3 toxicity and none of the patients developed Grade 4 toxicity. Once patients develop Grade 3 toxicity, the dose of apatinib was reduced to 125mg. In contrast, if the patients were tolerant to apatinib treatment, the dose of the drug was subsequently increased by 125mg from 250mg to 375mg. The main reasons for dose reduction in apatinib plus TACE group were hand-foot syndrome, hypertension, weight loss and fatigue. All toxicities occurring in both groups are shown in Table 5. The incidence of hypertension, anorexia, weight loss and fatigue was moderately higher in the experimental group.
Table 5.
Outcomes and adverse events.
| Adverse Events |
Experimental group (n = 25) |
Control group (n = 22) |
||
|---|---|---|---|---|
| Toxicity grade | 1/2 | 3/4 | 1/2 | 3/4 |
| Liver dysfunction (transaminitis) |
7 (28%) | 0 | 3 (13.6%) | 0 |
| Fatigue | 23 (92%) | 3 (12%) | 0 | 0 |
| Diarrhea | 9 (36%) | 0 | 0 | 0 |
| Anorexia | 20 (80%) | 4 (16%) | 8 (36.4%) | 3 (13.6%) |
| Hypertension | 17 (68%) | 2 (8%) | 0 | 0 |
| Hand-foot syndrome | 15 (60%) | 2 (8%) | 0 | 0 |
| Skin rash | 1 (4%) | 0 | 0 | 0 |
| Albuminuria | 5 (20%) | 1 (4%) | 0 | 0 |
| Hoarseness | 8 (32%) | 0 | 0 | 0 |
| Weight loss | 20 (80%) | 0 | 10 (45.5%) | 0 |
Discussion
Most cases of liver cancer are detected at advanced stages. Thus, only a small number of patients are suitable for curative therapy including liver resection, while the majority of patients are left with palliative treatments. Sorafenib is the first approved systemic therapy for liver cancer, but it only marginally increases median survival.13 Clearly, novel approaches are needed for more effective treatment of liver cancer. In this single-center retrospective study, we sought to investigate the safety and efficacy of TACE combined with apatinib on patients with TACE-refractory advanced hepatocellular carcinoma. Our study showed that the combined therapy significantly improved OS and PFS compared with TACE alone without introducing serious adverse effects.
Despite its effectiveness in HCC, TACE is generally not considered as a curative method. Factors likely jeopardizing the efficacy of TACE consist of a hypothetical neo-angiogenic response caused by ischemia, which is echoed by the elevated levels of vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (b-FGF) following TACE.14 When stimulated by VEGF, VEGFR-2 is auto-phosphorylated at the carboxy terminal tail and kinase-insert region, which results in a potent pro-angiogenic effect. The phosphorylation of specific sites generates binding sites for the SH2 domains of various signaling molecules and has subsequent effects of cell proliferation, migration, permeability, and survival on the vascular endothelium.15 In contrast, antiangiogenic agents that inhibit VEGFR-2 and block VEGF-stimulated endothelial cell migration and proliferation have been shown to decrease tumor microvascular density and promote apoptosis.16 Therefore, TACE in combination with targeted antiangiogenic drugs might be able to suppress the growth of both the tumor body as well as blood vessel simultaneously.
Indeed, Wu et al showed that TACE together with sorafenib significantly prolonged the 5-year OS in TACE-refractory advanced HCC compared with the TACE group.17 Joy Varghese et al also reported that the combination of TACE and sorafenib improved the outcomes of HCC patients with BCLC stage B compared to TACE.18 Although the combination of TACE with sorafenib has demonstrated certain clinical benefits, the adverse effects associated with sorafenib cannot be completely ignored. Notably, sorafenib is an inhibitor of multiple tyrosine kinases including PDGFR, Raf family kinases, and VEGFR1-3.19 Because of its relative lack of selectivity, sorafenib has been associated with severe adverse events which could jeopardize its clinical application.20 By contrast, apatinib selectively targets VEGF receptor 2 (VEGFR2), and its binding affinity to VEGFR2 is ten times higher than that of sorafenib.21 In addition, apatinib is much cheaper compared to sorafenib. The improved efficiency of VEGFR2 blocking together with its affordability renders apatinib a particularly attractive alternative to sorafenib for tumor management.
The antitumor activity of apatinib has been showcased in several phase II and Ⅲ clinical trials,22-24 and a previous single-arm retrospective study of 19 HCC patients with portal venous tumor thrombus found that the combination of apatinib and TACE led to prolonged overall survival.25 Here, we found that combination of apatinib and TACE significantly improved median OS and prolonged median PFS compared with TACE monotherapy. Meanwhile, the combined therapy achieved an objective response rate (ORR) of 36% and a disease control rate (DCR) of 56%. As a comparison, DCR of sorafenib trial was 35.3%-43.5%,26,27 suggesting that our combined therapy might be able to exert superior efficacy over sorafenib. Further subgroup analysis showed that the combination therapy dramatically prolonged mOS and mPFS in both BCLC-B and -C stages, indicating that the combination therapy could be effectively utilized irrespective of patients’ metastatic status.
Potential predictive indicators for treatment efficacy have been one of the exploration directions. A predictive indicator evaluation from the AVAGAST randomized phase III trial showed that baseline plasma VEGF-A level and tumor neuropilin-1 expression were potential predictors of bevacizumab efficacy.28 Besides, a retrospective study of 269 patients with advanced gastric cancer treated by apatinib indicated that hypertension, proteinuria and hand-foot syndrome occuring during the first cycle of apatinib therapy were identified as potential predictors of apatinib efficacy.29 In our study, the univariate analysis indicated that there was no significant association of mPFS and mOS with gender, age, ECOG performance status and extrahepatic disease. By contrast, we noticed that patients with BCLC stage B have prolonged mPFS and mOS over patients with BCLC stage C, thus validating BCLC stratification of intermediate vs. advanced tumor stage. Besides, we performed PFS and OS analysis based on adverse event, and found that there was no significant association of prolonged PFS and OS with hypertension and hand-foot syndrome.
Our subgroup analysis showed that TACE combined with apatinib provides a significant and clinically meaningful improvement in OS and PFS in both BCLC-B and BCLC-C groups. This finding was associated with an increase the OS from 399 days to 588 days in BCLC-B, from 233 days to 442 days in BCLC-C. Meanwhile, PFS increased from 42 days to 273.5 days in BCLC-B, from 62 days to 97 days in BCLC-C. In particular, the combination therapy might be able to provide patients with hepatocellular carcinoma with extrahepatic metastasis or macrovascular invasion with further therapeutic opportunities. In the present study, we used 250mg of apatinib as initial dose, and our results showed that only 16% patients developed grade 3/4 toxicities including anorexia, fatigue, hypertension, and hand-foot syndrome, which could be clinically managed by symptomatic treatment. Furthermore, the most common non-hematological toxicities related to apatinib treatment were mainly mild to moderate and could be well tolerated. Apatinib has been shown to be well tolerated at doses below 750 mg/day.23 In our current study, by combining apatinib with TACE, we managed to reduce the recommended dose of apatinib by more than half while still maintain its antitumor efficacy. The dose reduction of apatinib will not only diminish the chance of severe adverse events but also dramatically decrease the cost of long-term drug administration, which will potentially improve patients’ compliance during cancer treatment. However, because apatinib inhibits angiogenesis by selectively inhibiting VEGFR-2, we would still suggest that patients who had a history of coronary atherosclerosis or angina pectoris should be cautious about using apatinib to prevent collateral development and compensatory blood flow caused by cardiac ischemia.
In conclusion, apatinib combined with TACE revealed certain survival benefits for HCC patients who experienced tumor progression following TACE, and our findings provide an additional therapeutic regimen for HCC. However, our retrospective study is limited by its small sample size. Further large-scale prospective studies are required to confirm the effect of TACE together with apatinib in liver cancer.
Patients and methods
Patient information
Data were collected retrospectively for all patients diagnosed with HCC who received treatment with TACE combined with apatinib between September 2016 and August 2017 at Beijing Friendship Hospital, Capital Medical University. Of all the included patients, 25 patients received TACE combined with apatinib, and 22 patients received only TACE treatment. Disease classification and treatment response were assessed by investigators using the modified Response Evaluation Criteria in Solid Tumors (mRECIST).30 The evaluation period was one year.18
Therapeutic schedule
All patients were diagnosed with multiple lesions in liver or with distant metastasis, and unresponsive to previous non-TACE treatment or unsuitable for surgery. All patients received TACE once or several times. The patients received TACE combined with apatinib were defined as experimental group, and the patients received only TACE treatment were defined as control group.
Apatinib was administered orally in a 4-week (about 28 days) schedule, with starting dose and dose adjustments guided by protocol and clinician discretion based on empirical experience of apatinib in patients with HCC. Starting dose of apatinib is 250mg once a day, and reduced dose is 125 mg once a day. The most suitable starting dose was determined on an individual basis according to performance status and comorbidities, as per clinician discretion. Oral apatinib was stopped 3 days before TACE treatment and 3 days after TACE, Apatinib was again administrated (250mg once a day)(Figure 5). Apatinib was taken once a day, after meal at the same time each day. The treatment was repeated every 28 days.
Apatinib (Jiangsu Hengrui Medicine Co Ltd) has not been approved for HCC treatment in China. Since the treatment cost of apatinib for HCC was not covered by the health insurance, all patients were required to provide written informed consent for the off-label use of apatinib and to pay for the apatinib treatment by themselves.
Therapeutic evaluation
All patients underwent physical examination, laboratory tests, including liver functions, and hepatitis serologic tests. Contrast-enhanced computed tomography (CT) was performed before treatment and follow-up contrast-enhanced CT was performed every 4–6 weeks post-treatment to assess tumor response and guide timely decision making regarding subsequent therapies.
The tumor response was determined according to mRECIST 1.1 criteria.31 The overall survival was measured from the date of enrollment to the date of death or the date of last follow-up.
This study was approved by Beijing Friendship Hospital, Capital Medical University and was performed in compliance with the 1975 Declaration of Helsinki. All patients provided informed written consent for TACE and apatinib administration. The study was approved by institutional review board ethics committee and complied with Good Clinical Practice guidelines, the Declaration of Helsinki, and received license from the State Food and Drug administration (NO. CXHB1100041SU).
Response and toxicity assessment
The grade of adverse events was determined according to the National Cancer Institute Common Toxicity Criteria for Adverse Events version 4.0 (NCI CTC v4.0) and recorded. Consistent with previous studies, grade 2 was selected as the cut-off. We defined patients with skin toxicity ≥grade 2 as dermatologic responders and patients with skin toxicity <grade 2 as dermatologic non-responders.
Statistical analysis
SPSS version.19.0 (SPSS Inc., Chicago, IL) was used for analysis. The relationship features of tumor progression were analyzed using the Log Rank analysis. Kaplan–Meier survival curves were constructed in survival analysis. A P value of 0.05 was considered to be statistically significant for all analyses.
Funding Statement
This work was funded in part by funds from Beijing Municipal Administration of Hospital’s Mission Plan (No.SML20150101). The authors thank all the medical staff and patients at Beijing Friendship Hospital.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Ethical approval
The study was approved by centre’s ethics committee and institutional review board and complied with Good Clinical Practice guidelines, the Declaration of Helsinki, and received the license from the State Food and Drug administration (NO. CXHB1100041SU).
Informed consent
Informed consent for receiving TACE and apatinib treatment was obtained from all individual participants included in the study
References
- 1.Zhang J, Li H, Gao D, Zhang B, Zheng M, Lun M, Wei M, Duan R, Guo M, Hua J, et al. A prognosis and impact factor analysis of DC-CIK cell therapy for patients with hepatocellular carcinoma undergoing postoperative TACE. Cancer Biol Ther. 2018;19:475–483. doi: 10.1080/15384047.2018.1433501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meyer T, Palmer DH, Cheng AL, Hocke J, Loembe AB, Yen CJ.. mRECIST to predict survival in advanced hepatocellular carcinoma: analysis of two randomised phase II trials comparing nintedanib vs sorafenib. Liver Int. 2017;37:1047–1055. doi: 10.1111/liv.13359. [DOI] [PubMed] [Google Scholar]
- 3.Covey AM, Hussain SM.. Liver-directed therapy for hepatocellular carcinoma: an overview of techniques, outcomes, and posttreatment imaging findings. AJR Am J Roentgenol. 2017;209:67–76. doi: 10.2214/AJR.17.17799. [DOI] [PubMed] [Google Scholar]
- 4.Zhang H. Apatinib for molecular targeted therapy in tumor. Drug Des Devel Ther. 2015;9:6075–6081. doi: 10.2147/DDDT.S97235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Baere T, Tselikas L, Yevich S, Boige V, Deschamps F, Ducreux M, Goere D, Nguyen F, Malka D. The role of image-guided therapy in the management of colorectal cancer metastatic disease. Eur J Cancer. 2017;75:231–242. doi: 10.1016/j.ejca.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Liu K, Min XL, Peng J, Yang K, Yang L, Zhang XM. The changes of HIF-1alpha and VEGF expression after TACE in patients with hepatocellular carcinoma. J Clin Med Res. 2016;8:297–302. doi: 10.14740/jocmr2496w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang B, Xu H, Gao ZQ, Ning HF, Sun YQ, Cao GW. Increased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolization. Acta Radiol. 2008;49:523–529. doi: 10.1080/02841850801958890. [DOI] [PubMed] [Google Scholar]
- 8.Du H, Zhao J, Hai L, Wu J, Yi H, Shi Y. The roles of vasohibin and its family members: beyond angiogenesis modulators. Cancer Biol Ther. 2017;18:827–832. doi: 10.1080/15384047.2017.1373217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinter M, Sieghart W, Graziadei I, Vogel W, Maieron A, Königsberg R, Weissmann A, Kornek G, Plank C, Peck-Radosavljevic M. Sorafenib in unresectable hepatocellular carcinoma from mild to advanced stage liver cirrhosis. Oncologist. 2009;14:70–76. doi: 10.1634/theoncologist.2008-0191. [DOI] [PubMed] [Google Scholar]
- 10.Bruix J, Tak W-Y, Gasbarrini A, Santoro A, Colombo M, Lim H-Y, Mazzaferro V, Wiest R, Reig M, Wagner A, et al. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: multicentre, open-label, phase II safety study. Eur J Cancer. 2013;49:3412–3419. doi: 10.1016/j.ejca.2013.05.028. [DOI] [PubMed] [Google Scholar]
- 11.Han T, Luan Y, Xu Y, Yang X, Li J, Liu R, Li Q, Zheng Z. Successful treatment of advanced pancreatic liposarcoma with apatinib: A case report and literature review. Cancer Biol Ther. 2017;18:635–639. doi: 10.1080/15384047.2017.1345394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu W, Jin X-L, Yang C, Du P, Jiang F-Q, Ma J-P, Yang J, Xie P, Zhang Z. Comparison of efficacy between TACE combined with apatinib and TACE alone in the treatment of intermediate and advanced hepatocellular carcinoma: A single-center randomized controlled trial. Cancer Biol Ther. 2017;18:433–438. doi: 10.1080/15384047.2017.1323589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc J-F, de Oliveira AC, Santoro A, Raoul J-L, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 14.Sergio A, Cristofori C, Cardin R, Pivetta G, Ragazzi R, Baldan A, Girardi L, Cillo U, Burra P, Giacomin A, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103:914–921. doi: 10.1111/j.1572-0241.2007.01712.x. [DOI] [PubMed] [Google Scholar]
- 15.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 16.Lin D, Wang Z, Li J, Wang L, Wang S, Hu G-X, Liu X. The effect of apatinib on the metabolism of carvedilol both in vitro and in vivo. Pharmacology. 2016;97:31–37. doi: 10.1159/000441228. [DOI] [PubMed] [Google Scholar]
- 17.Wu J, Li A, Yang J, Lu Y, Li J. Efficacy and safety of TACE in combination with sorafenib for the treatment of TACE-refractory advanced hepatocellular carcinoma in Chinese patients: a retrospective study. Onco Targets Ther. 2017;10:2761–2768. doi: 10.2147/OTT.S131022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varghese J, Kedarisetty C, Venkataraman J, Srinivasan V, Deepashree T, Uthappa M, Ilankumaran K, Govil S, Reddy M, Rela M. Combination of TACE and sorafenib improves outcomes in BCLC stages B/C of hepatocellular carcinoma: a single centre experience. Ann Hepatol. 2017;16:247–254. doi: 10.5604/16652681.1231583. [DOI] [PubMed] [Google Scholar]
- 19.Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7:3129–3140. doi: 10.1158/1535-7163.MCT-08-0013. [DOI] [PubMed] [Google Scholar]
- 20.Blanchet B, Billemont B, Barete S, Garrigue H, Cabanes L, Coriat R, Francès C, Knebelmann B, Goldwasser F. Toxicity of sorafenib: clinical and molecular aspects. Expert Opin Drug Saf. 2010;9:275–287. doi: 10.1517/14740330903510608. [DOI] [PubMed] [Google Scholar]
- 21.Langer CJ, Mok T, Postmus PE. Targeted agents in the third-/fourth-line treatment of patients with advanced (stage III/IV) non-small cell lung cancer (NSCLC). Cancer Treat Rev. 2013;39:252–260. doi: 10.1016/j.ctrv.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Li J, Qin S, Xu J, Guo W, Xiong J, Bai Y, Sun G, Yang Y, Wang L, Xu N, et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol. 2013;31:3219–3225. doi: 10.1200/JCO.2013.48.8585. [DOI] [PubMed] [Google Scholar]
- 23.Hu X, Zhang J, Xu B, Jiang Z, Ragaz J, Tong Z, Zhang Q, Wang X, Feng J, Pang D, et al. Multicenter phase II study of apatinib, a novel VEGFR inhibitor in heavily pretreated patients with metastatic triple-negative breast cancer. Int J Cancer. 2014;135:1961–1969. doi: 10.1002/ijc.28829. [DOI] [PubMed] [Google Scholar]
- 24.Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y, Liu W, Tong J, Liu Y, Xu R, et al. Randomized, double-blind, placebo-controlled phase iii trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol. 2016;34:1448–1454. doi: 10.1200/JCO.2015.63.5995. [DOI] [PubMed] [Google Scholar]
- 25.Liu C, Xing W, Si T, Yu H, Guo Z. Efficacy and safety of apatinib combined with transarterial chemoembolization for hepatocellular carcinoma with portal venous tumor thrombus: a retrospective study. Oncotarget. 2017;8:100734–100745. doi: 10.18632/oncotarget.20140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng A-L, Kang Y-K, Chen Z, Tsao C-J, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang T-S, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 27.Bruix J, Raoul J-L, Sherman M, Mazzaferro V, Bolondi L, Craxi A, Galle PR, Santoro A, Beaugrand M, Sangiovanni A, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol. 2012;57:821–829. doi: 10.1016/j.jhep.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 28.Jiang T, Cheng R, Zhang G, Su C, Zhao C, Li X, Zhang J, Wu F, Chen X, Gao G, et al. Characterization of liver metastasis and its effect on targeted therapy in EGFR-mutant NSCLC: a multicenter study. Clin Lung Cancer. 2017;18:631–9 e2. doi: 10.1016/j.cllc.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 29.Liu X, Qin S, Wang Z, Xu J, Xiong J, Bai Y, Wang Z, Yang Y, Sun G, Wang L, et al. Early presence of anti-angiogenesis-related adverse events as a potential biomarker of antitumor efficacy in metastatic gastric cancer patients treated with apatinib: a cohort study. J Hematol Oncol. 2017;10:153. doi: 10.1186/s13045-017-0521-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 31.Seyal AR, Gonzalez-Guindalini FD, Arslanoglu A, Harmath CB, Lewandowski RJ, Salem R, Yaghmai V. Reproducibility of mRECIST in assessing response to transarterial radioembolization therapy in hepatocellular carcinoma. Hepatology. 2015;62:1111–1121. doi: 10.1002/hep.27915. [DOI] [PubMed] [Google Scholar]


