ABSTRACT
The tumor suppressor BRCA1-associated protein 1 (BAP1) is a deubiquitinase that removes histone 2A ubiquitination. How BAP1 suppresses tumor development remains elusive. Our recent study identified the cystine transporter solute carrier family 7 member 11 (SLC7A11) as a critical BAP1 target, and showed that BAP1 promotes ferroptosis (a non-apoptotic cell death) through repressing SLC7A11 expression, resulting in tumor suppression.
KEYWORDS: BAP1, SLC7A11, metabolism, ferroptosis, H2A ubiquitination, tumor suppression
BRCA1-associated protein 1 (BAP1) encodes a deubiquitinase (DUB) which predominantly localizes in the nucleus. Although BAP1 was originally identified as a BRCA1-binding protein through a yeast two-hybrid screen, subsequent proteomic analyses from many studies have not identified BRCA1 as a major binding protein of BAP1. Instead, these studies showed that BAP1 mainly interacts with several transcription factors and chromatin-associated proteins, including ASXL1, ASXL2, FOXK1, FOXK2, HCFC1, KDM1B, MBD5, MBD6, and OGT, indicating a role of BAP1 in regulating gene transcription.1 A seminal study from Drosophila revealed that BAP1 and its associated proteins form the so called polycomb repressive deubiquitinase (PR-DUB) complex, which mainly functions to remove monoubiquitination of H2A (H2Aub) at lysine 118 in Drosophila and lysine 119 in vertebrates.2 Subsequent studies confirmed this finding in mammalian cell lines. These data thus suggest a model that the BAP1-containing PR-DUB complex regulates gene transcription through epigenetic mechanisms.
Research interest in BAP1 has substantially increased in recent years. A survey of PubMed revealed less than 15 BAP1-related publications per year before 2012, increasing to 40–60 per year between 2012 and 2014, and more than 100 per year since 2015. Arguably, this increase largely results from recent cancer genomic studies which identified prominent mutations of BAP1 in several human cancers, including 36–65% mesotheliomas, 32–47% uveal melanomas, 20–30% cholangiocarcinomas, and 10–15% clear cell renal cell carcinomas.1 More than 40% BAP1 mutations are inactivating mutations (such as truncating mutations), and BAP1 is located at the 3p21.1 locus which is frequently deleted in human cancers, indicating BAP1 is a tumor suppressor. Functional analyses showed that BAP1 restoration in BAP1-deficient cancer cells inhibited xenograft tumor development, while Bap1 deletion in mouse promoted tumor development in various contexts,1 further supporting BAP1’s tumor suppression function. It is conceivable that, similar to other tumor suppressors that regulate gene transcription such as TP53 (best known as p53), BAP1 exerts its tumor suppression function at least partly via its downstream transcriptional targets. However, the identity of such transcriptional targets and their associated biological processes in BAP1-mediated tumor suppression still remain largely unexplored.
To identify potential targets that underlie BAP1-mediated tumor suppression, we conducted both RNA sequencing (RNA-seq) and H2Aub chromatin immunoprecipitation coupled with high-throughput sequencing (ChIP-seq) analyses in BAP1-deficient cancer cells that stably express an empty vector or BAP1.3,4 Integration of both RNA-seq and H2Aub ChIP-seq data sets identified 541 potential target genes (including more than 300 BAP1-upregulated and around 200 BAP1-downregulated genes) with BAP1-dependent H2Aub reduction, suggesting that BAP1-mediated deubiquitination of H2Aub is associated with both transcriptional activation and repression of target genes. Gene ontology (GO) analysis revealed a striking enrichment of metabolism-related biological processes in BAP1 target genes. Cancer genomic analysis identified solute carrier family 7 member 11 (SLC7A11) as a top BAP1 target gene in human cancers, and SLC7A11 is also among “oxidative stress response”, a top ranking biological process from GO analysis. We subsequently confirmed that BAP1 decreases H2Aub occupancy on the SLC7A11 promoter and potently represses SLC7A11 expression in cancer cells. We further showed that other proteins in the PR-DUB complex, such as ASXL1, strongly bind on the SLC7A11 promoter, suggesting that these other proteins in the PR-DUB complex might function to recruit BAP1 to the SLC7A11 promoter to regulate H2Aub levels.
SLC7A11 is an amino acid transporter that imports extracellular cystine.5 Studies in recent years have linked this nutrient transporter to a new form of regulated cell death termed ferroptosis, which is caused by over-accumulation of intracellular lipid hydroperoxides in an iron-dependent manner.6 Glutathione peroxidase 4 (GPX4) uses glutathione as a cofactor to convert lipid hydroperoxides to lipid alcohols, therefore protecting cells from undergoing ferroptotic cell death. Since cystine is a critical precursor for glutathione biosynthesis, ferroptosis can be induced by cystine depletion or blockage of SLC7A11-mediated cystine transport by drugs such as erastin (Figure 1). Consistent with this, we showed that, through repressing SLC7A11 expression, BAP1 inhibits cystine uptake and glutathione synthesis, enhances lipid peroxidation, and sensitizes cancer cells to ferroptosis.
Figure 1.
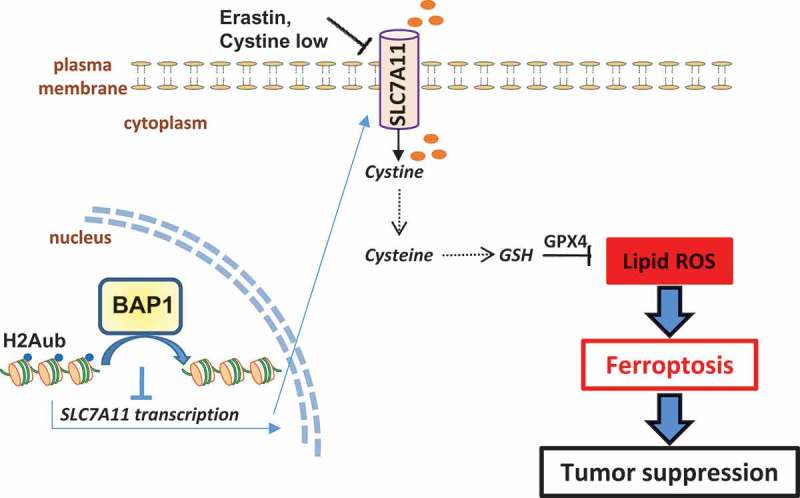
BAP1 suppresses tumor development through repressing SLC7A11 and inducing ferroptosis. Solute carrier family 7 member 11 (SLC7A11) imports extracellular cystine, which is subsequently converted to cysteine in cells. Cysteine is a rate-limiting precursor for glutathione (GSH) biosynthesis. GSH is used as a cofactor by glutathione peroxidase 4 (GPX4) to reduce lipid reactive oxygen species (ROS), particularly lipid hydroperoxides, to lipid alcohols. Overproduction of lipid ROS in cells results in ferroptosis. Similar to apoptosis, ferroptosis might serve as a tumor suppression mechanism. Cystine depletion or inhibition of SLC7A11-mediated cystine transport by erastin promotes lipid ROS and induces ferroptosis. BRCA1-associated protein 1 (BAP1) represses SLC7A11 transcription likely through deubiquitination of histone 2A monoubiquitination (H2Aub) at lysine 119 on the SLC7A11 gene, resulting in decreased cystine import, reduced GSH synthesis, enhanced sensitivity to ferroptosis, and tumor suppression.
Cell death resistance is one hallmark of cancer, and the role of apoptosis in tumor suppression is well established.7 However, whether ferroptosis plays any role in tumor suppression is less clear. Our studies provided several lines of evidence to support the model that ferroptosis indeed is critical for BAP1-mediated tumor suppression. First, electron microscopy analysis revealed that many tumor cells from BAP1-expressing xenograft tumors exhibit morphologic features of ferroptosis, and immunohistochemistry (IHC) analysis showed increased staining of 4-hydroxynonenal (a lipid peroxidation product) in BAP1-expressing tumors, confirming that BAP1 promotes lipid peroxidation and ferroptosis in vivo. In addition, restoration of SLC7A11 or treatment of a ferroptosis inhibitor partially restored tumor development in BAP1-expressing xenograft tumors, suggesting that BAP1 inhibits tumor development at least partly through repressing SLC7A11 and promoting ferroptosis. Finally, we showed that most cancer–associated BAP1 mutations we have analyzed are defective in inhibiting SLC7A11 and inducing ferroptosis.
In summary, our results show that BAP1 executes its tumor suppression function at least partly through its regulation of ferroptosis, and identify a hitherto unrecognized regulatory mechanism to link ferroptosis to tumor suppression (Figure 1). Our study also raises several important questions. Since H2Aub is generally associated with gene repression,8 it remains unclear how BAP1, a H2Aub DUB, represses the expression of its target genes including SLC7A11 through decreasing H2Aub levels. Our study, together with other recent studies,9 supports the emerging role of ferroptosis in tumor suppression; however, it remains to be determined how common this new tumor suppression mechanism occurs in human cancers. Finally, a convenient assay similar to cleaved caspase-3 IHC in apoptosis studies is needed to routinely characterize ferroptosis in tumor specimens. We envision that novel mechanistic insights will be revealed and important research tools be established in this emerging field in the upcoming years.
Funding Statement
This work was supported by the National Institutes of Health [CA181196];
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Carbone M, Yang H, Pass HI, Krausz T, Testa JR, Gaudino G.. BAP1 and cancer. Nat Rev Cancer. 2013;13:153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scheuermann JC, de Ayala Alonso AG, Oktaba K, Ly-Hartig N, McGinty RK, Fraterman S, Wilm M, Muir TW, Muller J. Histone H2A deubiquitinase activity of the Polycomb repressive complex PR-DUB. Nature. 2010;465:243–247. doi: 10.1038/nature08966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y, Shi J, Liu X, Feng L, Gong Z, Koppula P, Sirohi K, Li X, Wei Y, Lee H, et al. BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat Cell Biol. 2018;20:1181–1192. doi: 10.1038/s41556-018-0178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dai F, Lee H, Zhang Y, Zhuang L, Yao H, Xi Y, Xiao ZD, You MJ, Li W, Su X, et al. BAP1 inhibits the ER stress gene regulatory network and modulates metabolic stress response. Proc Natl Acad Sci U S A. 2017;114:3192–3197. doi: 10.1073/pnas.1619588114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koppula P, Zhang Y, Zhuang L, Gan B. Amino acid transporter SLC7A11/xCT at the crossroads of regulating redox homeostasis and nutrient dependency of cancer. Cancer Commun (Lond). 2018;38:12. doi: 10.1186/s40880-018-0288-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascon S, Hatzios SK, Kagan VE, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Weake VM, Workman JL. Histone ubiquitination: triggering gene activity. Mol Cell. 2008;29:653–663. doi: 10.1016/j.molcel.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Jiang L, Kon N, Li T, Wang SJ, Su T, Hibshoosh H, Baer R, Gu W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520:57–62. doi: 10.1038/nature14344. [DOI] [PMC free article] [PubMed] [Google Scholar]


