Abstract
Although the thymus has long been recognized as a key organ for T cell selection, the intricate details linking these selection events to human autoimmunity have been challenging. Over the last two decades, there has been rapid progress in understanding the role of thymic tolerance mechanisms in autoimmunity through genetics. Here, we review some of the recent progress in our understanding of key thymic tolerance processes that are critical for preventing autoimmune disease.
INTRODUCTION
The prevention of autoimmunity is a major challenge for the adaptive immune system. The stochastic generation of the T cell receptor in individual thymocytes during thymic development will invariably result in the generation of individual clones with the potential for autoreactivity. Although it has long been hypothesized that the removal of such clones from the T cell repertoire in the thymus is crucial for the prevention of autoimmunity, it has only been in the last two decades that we have achieved clear evidence for a key role in thymic central tolerance in the prevention of autoimmunity. In this review, we highlight some of the recent advances in our understanding of the cellular and molecular processes that control central T cell tolerance and the growing evidence for a relationship between thymic central tolerance and autoimmune diseases.
The Autoimmune Regulator (Aire): A direct link of central tolerance with autoimmunity
During their development in the thymus, it has long been appreciated that thymocytes with high self-reactivity are deleted and removed from the T cell repertoire through negative selection 1,2. In spite of this knowledge, directly linking a breakdown in this important mechanism to the development of clinical autoimmunity had been a persistent challenge until the identification of the Autoimmune Regulator (Aire) gene as a key promoter of the expression of self-antigens within the thymus 3–5. Aire was originally identified as the defective gene in the Mendelian autoimmune syndrome called Autoimmune Polyglandular Syndrome Type 1 (APS1) in a positional cloning effort 6,7. Patients with APS1 develop autoimmunity that targets many individual organs with a predilection for destruction of the adrenal glands and the parathyroid glands 8,9. Interestingly, the patients also frequently develop susceptibility to mucocutaneous candidiasis which is now associated with an autoimmune response to Th17 related cytokines 10,11. Anti-cytokine autoantibodies to Type 1 interferons are found in > 99% of APS1 patients, and anti-IFNα antibodies are virtually diagnostic for the syndrome 12. Whether these interferon autoantibodies are pathogenic or protective remains unclear, though recent correlation of neutralizing interferon antibodies with reduced incidence of Type 1 diabetes in APS1 patients suggests that such autoantibodies may mediate a potential protective role in part 13.
Major clues into Aire function came from mapping its gene expression pattern and the development of a knockout mouse model. Aire is highly expressed in medullary thymic epithelial cells (mTECs) and to a lesser extent in thymic B cells and in a peripheral dendritic cell population 14–17. While such Aire-expressing peripheral dendritic cells and thymic B cells can serve as APCs to mediate antigen-specific deletional tolerance mechanisms 16–18, it remains unclear what the relative contribution of these smaller cell subsets may to enforcing thymic tolerance measures. Within mTECs, Aire helps promote the expression of thousands of tissue specific self-antigens (TSAs) for display to the developing T cell repertoire and we now have evidence that this display drives both the deletion of autoreactive T cells 19–21 and the positive selection of a subset of self-reactive T regulatory cells (Tregs) 22–24. Similar to the mouse model, individuals with APS1 demonstrate autoantibodies to a wide array of TSAs 13,25 with development of targeted organ-specific autoimmunity 26–28 though the specific antigenic targets and spectrum of organ-specific autoimmunity differs somewhat from the mouse model 29. Thus, the Aire/APS1 model system has provided the strongest evidence to date for a relationship between a defect in central tolerance the generation of autoimmunity in humans.
Thymic medullary epithelial cells, the display of self-antigens, and the activity of Aire
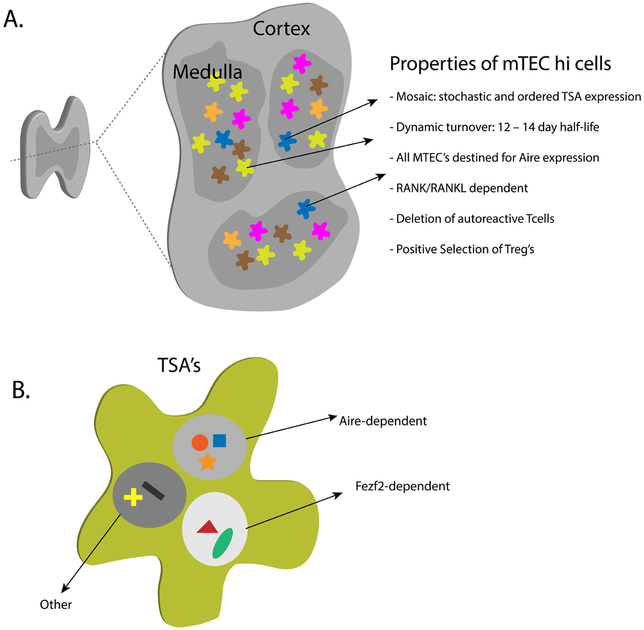
The mTEC population of cells has many unique properties and study of their development and gene expression pattern has been a renewed area of interest. The mTEC compartment can be broadly segregated into MHC Class II low versus MHC Class II high expressing sets of cells (mTEClo and mTEChi) 30–33. It now appears that a subset of mTEClo cells are destined to mature into mTEChi cells, which then also acquire Aire expression through RANK/RANK-Ligand mediated signals 34–36 In adult mice, this developmental pathway is remarkably dynamic with the half-life of Aire-expressing mTEChi cells being about 12–14 days 31,37. Thus, every two weeks, a new pool of mTEChi cells is generated within the medulla. This dynamic turnover may be in place to help ensure the consistent display of TSAs to developing thymocytes, especially because of heterogeneity in TSA-expression in the mTEC pool (see below). Interestingly, in vivo blockade of RANK-Ligand with monoclonal antibody (mAb) treatment in adult mice over two weeks can lead to selective depletion of mTECs from the thymus and an induced defect in negative selection 38. Consistent with these findings, recent studies with an Aire-driven reporter system also suggest that the majority of mTECs are on a developmental pathway to acquire Aire expression 39, and thus continued blockade of RANK-Ligand would be expected to significantly deplete the mTEC pool. Despite the defect in negative selection in RANK-Ligand treated mice, they do not succumb to spontaneous autoimmunity, and this may have to do with other peripheral tolerance mechanisms such as Tregs in the tissues that hold newly generated autoreactive cells in check in the periphery 24. Regardless, it will be interesting to determine if patients that are treated with anti-RANK-Ligand mAbs, which are widely used in the treatment of osteoporosis, show an increased susceptibility to autoimmunity. While an increased frequency of sinus and upper respiratory infections is noted with the anti-RANK-Ligand agent denosumab, which has been in use since 2010 for treatment of postmenopausal osteoporosis or skeletal-related complications in cancers with metastatic bone disease, reports of autoimmunity have been lacking thus far though absence of autoimmunity may be confounded by the advanced age of patients, relatively infrequent dosing (e.g. every 6 months) or differences in dose effect on different cell subtypes. A more intriguing possibility is whether it may be possible to also harness the effects of RANK-Ligand blockade to improve immune responses against tumor antigens 38,40.
The properties of the mTEChi pool of cells have been a source of intense study (see Figure 1) and recent studies using single cell RNA-Sequencing (RNA-Seq) have revealed that the nature of expression of Aire-dependent TSAs within these cells is both stochastic and ordered 41,42. It now appears that only a small fraction of mTECs express a particular TSA (1–3%), which highlights the stochastic feature of expression within the pool of mTEChi cells. At the same time, within individual cells, a general pattern of multiple co-expression of sets of TSAs can be identified. For the most part, these co-expression sets often have little in common and do not reflect how these TSAs are expressed in the peripheral tissues. Epigenetic studies have revealed that these TSAs are in open chromatin conformations and that looping of the chromatin between chromosomes correlates with co-expression properties 43. Recently, Aire was shown to be greatly enriched in “super” enhancers within mTECs along with other chromatin regulators, particularly the topoisomerases TOP1 and TOP2 44,45. Aire had previously been implicated to interact with Topoisomerase family members but through depletion studies it now appears that TOP1 is the primary Topoisomerase that Aire binds to44 and this interaction helps drive Aire’s localization to super enhancers where other factors that include elements involved in DNA-Double stranded break repair can interact to promote transcription of Aire target genes 46. Finally, Aire has also been demonstrated to promote transcriptional elongation of RNA-Polymerase II through its interaction with pTEFb 47,48. Thus, through localization of Aire to “super” enhancers that include chromosomal looping, a wide number of genes in this local environment can be transcribed. Taken together, a picture is emerging that a complex series of epigenetic events are required for Aire to promote the expression of TSAs (for further detail see 49,50).
Figure 1.
Properties of mTECs and drivers of TSA-expression. A) Shown is a schematic of a cross section of the thymus. Highlighted are some of the unique and unusual features of mTECs that may help poise them for promoting display of TSA’s and the induction of tolerance. B) Shown is a single representative mTEC and an array of TSA’s being expressed. It now appears that Aire and Fezf2 are unique promoters of TSA expression in mTECs and that there are likely to be other factors beyond this transcriptional pair that drive this process.
Fezf2: an Aire-like transcription factor
Studies on gene expression of mTECs have also revealed that a wide array of TSAs continue to be expressed in Aire−/− mTECs 3,51. This observation has led to the attractive hypothesis that other transcription factors are present and operate in mTECs to promote the expression of TSAs (Figure 1). Recently, forebrain expressed zinc finger 2 (Fezf2) has been implicated as such a transcription factor 52. Fezf2 was identified by screening for transcription factors that are differentially expressed between cortical thymic epithelial cells (cTECs) and mTECs. Fezf2 is highly expressed in the brain and plays an important role in the differentiation and development of corticospinal neurons 53. Germline knockout mice of Fezf2 die shortly after birth and the role of Fezf2 in the thymus or immune system had not been studied until recently 52. The thymus in Fezf2−/− neonatal mice is reported to be relatively normal with appropriate expression of Aire, distribution of mTEClo and mTEChi cells, and normal thymocyte population numbers and percentages. Within mTECs, Fezf2 appears to promote the expression of an array of TSAs that is distinct from the Aire-dependent TSAs. Furthermore, in either thymic grafting experiments with Fezf2−/− thymi or in TEC-specific knockouts of Fezf2, mice developed spontaneous autoimmunity with evidence of tissue organ infiltrates and autoantibodies in a spectrum distinct from those seen with Aire−/− mice. In addition, it was suggested that the induction of Fezf2 expression in mTECs was dependent on lymphotoxin beta signaling while that of Aire-expressing mTECs was mainly dependent on RANK induced signals. Recently, conflicting data to this was put forth by Anderson and colleagues, where it was found that TEC-specific deletion of the lymphotoxin beta receptor (LTβRTEC) did not ablate the development of either Fezf2 or Aire expressing mTECs 54. Here, it was found that RANK/RANK-Ligand signals were crucial for the development of both Aire and Fezf2 expressing cells. Nonetheless, the medulla of LTβRTEC mice showed a decreased frequency of mTECs and smaller islands of medullary areas. Interestingly in the same study, in contrast to the TEC specific loss of LTβR, mice with germline inactivation of LTβR (Ltβr−/−) showed evidence of generating autoimmunity in thymic grafting experiments and a defect in both medullary organization and the frequency of medullary dendritic cell populations. These results suggest a complex role of LTβR in both the mTEC and dendritic cell compartments in the medulla. Overall, further work will be needed to determine the exact mechanisms by which Fezf2 operates within mTECs, directly linking Fezf2 defects to human autoimmune disease, and if there are other similar factors that promote the expression of self within the medulla.
Thymic selection of Tregs, Aire, and autoimmunity
Although there is extensive data for a clear role for Aire-induced TSA display in promoting the negative selection of autoreactive T cells 19–21, its relationship with promoting Treg selection has come back into focus. In previous work, forced expression of model self-antigens in Aire-expressing mTECs could induce the positive selection of Foxp3-expressing Tregs in a TCR-transgenic model 55. In contrast, the frequency and general function of Tregs in the thymus and periphery of adult Aire-deficient mice appear relatively normal 24,56. Recently, more detailed studies on the TCR repertoire of Tregs from Aire-deficient versus wildtype hosts with a limited repertoire system have provided new evidence that a subset of the Treg repertoire is dependent on Aire 22,23. At the same time, many Treg specific TCRs do not appear at all to rely on Aire for their positive selection within the thymus and interestingly, may rely on the passive transfer of antigens from mTECs to thymic dendritic cells 23. Recently, the specificity of an Aire-dependent Treg TCR was identified and determined to be the prostate self-antigen Tcaf3 57. This particular Treg clone was originally identified by sequencing TCRs of individual Tregs in implanted prostate tumors in Aire+/+ mice 22. The expression of Tcaf3 within the thymus has been examined and determined to be Aire-dependent. Interestingly, tetramer analysis of CD4+ T cells from Aire+/+ versus Aire−/− mice for this specificity show great enrichment of Tregs in Aire+/+ mice. This data suggests that there is something potentially peculiar about the display of Tcaf3 within the thymus that promotes Treg induction. In contrast, a previously identified Aire-dependent CD4+ T cell epitope for an eye antigen called IRBP does not show this propensity for Treg induction in Aire+/+ hosts 21; rather, IRBP-specific cells appear to be absent suggesting a strong induction of negative selection. Clearly, more work will be needed to determine the nature of TSA-display within the thymus by mTECs that induces negative selection versus Treg selection and how such selection drives or prevents autoimmunity. Likewise, there has also been recent work that has shown that during the neonatal period in mice, there is a decreased frequency of Tregs within the thymus of Aire-deficient hosts 24. Elaborate Treg transfer studies in this time window suggest that this may be part of the cellular mechanism by which autoimmunity ensues in the Aire-deficient model. At the same time, previous work by two independent groups showed that simultaneous co-transfer of Aire+/+ and Aire−/− thymi into nude mouse hosts did not protect against autoimmunity which favors defects in deletional mechanisms rather than a dominant tolerance mechanism as the primary trigger for autoimmunity in the model 20,58. Again, more detailed study of the TCR specificities that emerge in the Treg and T conventional pool and their relationship with autoimmunity will have to be worked out to resolve these issues.
PSMB11, positive selection of CD8 T cells, and links to autoimmunity
Within the cortex of the thymus, the display of MHC-peptide ligands is crucial for the positive selection of developing thymocytes. Interestingly, the generation of peptide ligands for MHC class I are likely to be unique because of the expression of a thymus specific proteasome subunit called PSMB11 mainly in cTECs. PSMB11 is a catalytic subunit of the proteasome that has chymotrypsin-like activity and whose presence is critical for positive selection by cTECs 59 PSMB11 has lower chymotrypsin-like activity than other such subunits in the immunoproteosome and thus it likely helps produce a different array of peptide ligands for MHC Class I loading in cTECs than those present outside of the thymus. Recently, Nitta et al. took a translational approach to model human variants of PSMB11 in mice 60. Here they found that variants could indeed alter thymic positive selection and the array of peptides being displayed by cTECs. Interestingly, this process may have a link to autoimmunity as a SNP that results in the G49S variant is associated with the development of Sjogren’s disease and also impairs CD8+ T cell positive selection in the mouse model. More work will have to be done on the specific selection events that give rise to autoreactive T cells with this specificity, but this work underscores a relationship of cTEC-driven positive selection with autoimmune disease.
Impaired T cell signaling and defective thymic tolerance
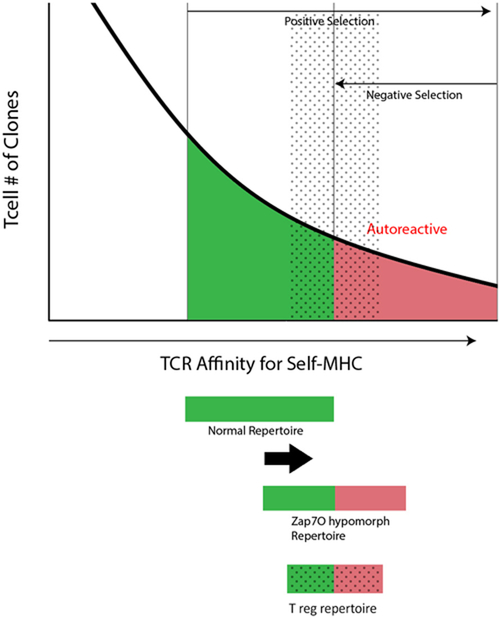
During their transit through the thymic maturation process, T cells display dynamic changes in the levels of cell surface TCR and signaling components to help guide the positive and negative selection process. Zap-70 is a tyrosine kinase and critical T cell signaling molecule whose expression is upregulated during thymocyte maturation. Loss of Zap-70 is associated with severe immune-deficiency with the absence of CD8 T cells and impaired CD4 T cells. Previous work by the Sakaguchi group on a spontaneous mutant mouse line termed Skg, identified a mutation in Zap-70 that was associated with autoimmunity and a defect in thymic selection 61,62. In a large body of work it now appears that this allele has hypomorphic activity that impairs but does not block Zap-70’s activity. Within the thymus, this results in an increased threshold for positive selection, such that only more autoreactive T cells can pass through this selection step. At the same time, negative selection is also impaired and in the end a more autoreactive repertoire is generated (see Figure 2). This elegant model has now been extended into a series of Zap70 alleles that cover a spectrum of hypomporphic activity in mouse models and highlight how altering TCR signaling thresholds in the thymus can promote the production of an autoreactive T cell repertoire, an altered Treg repertoire (Figure 2), and autoimmunity (see for a more thorough discussion 63,64). Recently, a novel human autoimmune syndrome was described in a single human kindred with two hypomorphic Zap-70 alleles that underscores this mechanism in promoting autoimmunity 65. The affected siblings in this kindred developed autoimmunity in the kidneys and the skin. Whole exome sequencing (WES) of the family revealed that the affected siblings harbored two mutant Zap-70 alleles, one with a R192W mutation and the other with a R360P mutation. Detailed analysis of these mutant alleles revealed that the R192W mutation in the SH2 domain of Zap-70 had reduced binding to the ζ-chain and the R360P mutation in the kinase domain affected autoinhibitory function. Thus, when both alleles are present Zap-70 signaling is likely to be somewhat hypomorphic and likely follows the autoimmune model developed in the mouse studies on hypomorphic Zap-70. Of course, in this human model it is difficult to determine the contribution of thymic versus peripheral defects in generating the autoimmune phenotype seen here and factors such as lymphopenia could play an important triggering role. Nonetheless, the preponderance of mouse data would suggest that at least in part, an altered thymic repertoire is part of the break in tolerance (Figure 2).
Figure 2.
Model of hypomorphic ZAP70 and altered T cell selection. Shown is a theoretical curve for positive selection and negative selection of T cell clones that results in a tolerant repertoire (green shading). In the case of hypomorphic ZAP70 activity the thresholds for both positive and negative selection result in a shift in the repertoire that now includes autoreactive T cell clones (red shading). The regulatory T cell repertoire is likely to fall in the interface between tolerant and autoreactive clones based on the model of induction of Treg fate in moderately self-reactive clones (gray dotted overlay).
Altered thymic architecture and autoimmunity
The role of central tolerance is notable also in examples of disease states associated with altered thymic architecture or function (reviewed in 66), such as seen with thymoma and in myasthenia gravis. Thymomas are highly associated with a wide variety of organ-specific and systemic autoimmune diseases (reviewed in 67). Notably, 95% of thymomas fail to express AIRE, suggesting that disordered thymic selection contributes to the paraneoplastic autoimmunity in these cases through the loss of AIRE-mediated effects on thymic epithelial development and TSA expression 68–70. Myasthenia gravis is frequently seen in association with thymoma along with the presence of anti-cytokine autoantibodies to type 1 interferons 71 Although the loss of AIRE-expression is thought to contribute to the autoimmune predilection in these subjects, it is worth noting that they rarely develop the pattern of autoimmunity associated with APS1. Interestingly, thymic hyperplasia is also associated with myasthenia gravis and these observations have led to the development of thymectomy as a clinical treatment for the disorder. Somewhat remarkably, thymectomy seems to be effective in significantly improving outcomes and reducing need for immunosuppression in many patients with myasthenia gravis, even in absence of overt thymoma 72. The mechanism(s) by which this technique provides clinical benefit still remains to be worked out but could include the blockade of the generation of new autoreactive T cells.
Links to more common autoimmune disease settings
Finally, the discovery of common risk variants through directed genetic studies and GWAS studies provides further clues as to contribution of thymic selection and central tolerance. Polymorphisms of the VNTR region of the insulin promoter lead to variable thymic expression of insulin are linked to risk for development of Type 1 diabetes 73, and Aire-mediated expression of CHRNA1 is linked to risk for development of myasthenia gravis 74. It is also worth noting that recent GWAS studies on myasthenia gravis have identified SNP variants in RANK, as a risk factor in myasthenia gravis 75. In all of these cases, the relationship of these more common risk variants with thymic selection have been greatly strengthened through the detailed study of the rare Mendelian forms of autoimmunity like APS1/APECED highlighted above. For example, it is now clear that Aire promotes the expression of insulin in the thymus and this provides a more detailed mechanism of how this likely connects the VNTR risk variant in insulin to diabetes risk. Likewise the myasthenia gravis risk variant in RANK can be more closely tied to mTEC’s, given what we have learned in its key role in mTEC maturation outlined above.
It is also worth noting the pattern of autoimmunity in the human conditions that are linked to the mechanisms highlighted here. For instance, APS1 patients tend to develop parathyroid and adrenal autoimmunity but thymoma patients develop myasthenia gravis. Likewise, the autoimmune clinical pattern in patients with ZAP70 hypomorphic activity is also distinct. Clearly, there is more work to do in unraveling the mechanisms and steps in play here that ultimately lead to these autoimmune phenotypes. Overall, the last two decades have seen major progress in unraveling key events in the thymic tolerance and linking them to autoimmune predisposition. Looking forward, the challenge remains of how to harness this information for the treatment and prevention of autoimmunity. Although it may be a difficult path forward, one can now imagine strategies that selectively target mTEC’s and TSA-expression through targeting RANK/RANK-Ligand or reconstruction and repair of the thymus through stem cell approaches 76. In the case of the latter, this could also be coupled with genetic engineering approaches such as CRISPR/Cas9 to enhance TSA expression or Aire function. Together, such approaches could have major implications for the induction of tolerance and the prevention of autoimmunity.
ACKNOWLEDGEMENTS
MSA is supported by the NIH, The Helmsley Charitable Trust, The California Institute of Regenerative Medicine, and the Larry L. Hillblom Foundation.
REFERENCES
- 1.Kappler JW, Roehm N & Marrack P T cell tolerance by clonal elimination in the thymus. Cell 49, 273–280 (1987). [DOI] [PubMed] [Google Scholar]
- 2.Kisielow P, Blüthmann H, Staerz UD, Steinmetz M & Boehmer, von, H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature 333, 742–746 (1988). [DOI] [PubMed] [Google Scholar]
- 3.Anderson MS et al. Projection of an immunological self shadow within the thymus by the aire protein. Science 298, 1395–1401 (2002). [DOI] [PubMed] [Google Scholar]
- 4.Derbinski J, Schulte A, Kyewski B & Klein L Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol 2, 1032–1039 (2001). [DOI] [PubMed] [Google Scholar]
- 5.Derbinski J Promiscuous gene expression in thymic epithelial cells is regulated at multiple levels. Journal of Experimental Medicine 202, 33–45 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aaltonen J et al. An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat Genet 17, 399–403 (1997). [DOI] [PubMed] [Google Scholar]
- 7.Nagamine K et al. Positional cloning of the APECED gene. Nat Genet 17, 393–398 (1997). [DOI] [PubMed] [Google Scholar]
- 8.Perheentupa J Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. Journal of Clinical Endocrinology & Metabolism 91, 2843–2850 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Husebye ES, Perheentupa J, Rautemaa R & Kämpe O Clinical manifestations and management of patients with autoimmune polyendocrine syndrome type I. J. Intern. Med. 265, 514–529 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Kisand K et al. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. Journal of Experimental Medicine 207, 299–308 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puel A et al. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. Journal of Experimental Medicine 207, 291–297 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meloni A et al. Autoantibodies against type I interferons as an additional diagnostic criterion for autoimmune polyendocrine syndrome type I. Journal of Clinical Endocrinology & Metabolism 93, 4389–4397 (2008). [DOI] [PubMed] [Google Scholar]
- 13.Meyer S et al. AIRE-Deficient Patients Harbor Unique High-Affinity Disease-Ameliorating Autoantibodies. Cell 166, 582–595 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heino M et al. RNA and protein expression of the murine autoimmune regulator gene (Aire) in normal, RelB-deficient and in NOD mouse. Eur J Immunol 30, 1884–1893 (2000). [DOI] [PubMed] [Google Scholar]
- 15.Liston A et al. Gene dosage--limiting role of Aire in thymic expression, clonal deletion, and organ-specific autoimmunity. J Exp Med 200, 1015–1026 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gardner JM et al. Deletional tolerance mediated by extrathymic Aire-expressing cells. Science 321, 843–847 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gardner JM et al. Extrathymic Aire-Expressing Cells Are a Distinct Bone Marrow-Derived Population that Induce Functional Inactivation of CD4(+) T Cells. Immunity 39, 560–572 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamano T et al. Thymic B Cells Are Licensed to Present Self Antigens for Central T Cell Tolerance Induction. Immunity 42, 1048–1061 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Liston A, Lesage S, Wilson J, Peltonen L & Goodnow CC Aire regulates negative selection of organ-specific T cells. Nat Immunol 4, 350–354 (2003). [DOI] [PubMed] [Google Scholar]
- 20.Anderson MS et al. The cellular mechanism of Aire control of T cell tolerance. Immunity 23, 227–239 (2005). [DOI] [PubMed] [Google Scholar]
- 21.Taniguchi RT et al. Detection of an autoreactive T-cell population within the polyclonal repertoire that undergoes distinct autoimmune regulator (Aire)-mediated selection. Proc Natl Acad Sci USA 109, 7847–7852 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malchow S et al. Aire-Dependent Thymic Development of Tumor-Associated Regulatory T Cells. Science 339, 1219–1224 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perry JSA et al. Distinct Contributions of Aire and Antigen-Presenting-Cell Subsets to the Generation of Self-Tolerance in the Thymus. Immunity 41, 414–426 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; Here Perry et al. examine Treg-specific TCR’s in a limited repertoire system and identify TCRs that depend on Aire for their selection.
- 24.Yang S, Fujikado N, Kolodin D, Benoist C & Mathis D Regulatory T cells generated early in life play a distinct role in maintaining self-tolerance. Science 348, 589–594 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study invokes another layer of a tolerance defect in the Aire-deficient model where Tregs are inappropriately selected early in life that then help protect against autoimmune responses in the tissues.
- 25.Landegren N et al. Proteome-wide survey of the autoimmune target repertoire in autoimmune polyendocrine syndrome type 1. Nature Publishing Group 1–11 (2016). doi: 10.1038/srep20104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alimohammadi M et al. Autoimmune polyendocrine syndrome type 1 and NALP5, a parathyroid autoantigen. N Engl J Med 358, 1018–1028 (2008). [DOI] [PubMed] [Google Scholar]
- 27.Alimohammadi M et al. Pulmonary autoimmunity as a feature of autoimmune polyendocrine syndrome type 1 and identification of KCNRG as a bronchial autoantigen. Proc Natl Acad Sci USA 106, 4396–4401 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shum AK et al. BPIFB1 Is a Lung-Specific Autoantigen Associated with Interstitial Lung Disease. Sci Transl Med 5, 206ra139 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pöntynen N et al. Aire deficient mice do not develop the same profile of tissue-specific autoantibodies as APECED patients. Journal of Autoimmunity 27, 96–104 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Gäbler J, Arnold J & Kyewski B Promiscuous gene expression and the developmental dynamics of medullary thymic epithelial cells. Eur J Immunol 37, 3363–3372 (2007). [DOI] [PubMed] [Google Scholar]
- 31.Gray D, Abramson J, Benoist C & Mathis D Proliferative arrest and rapid turnover of thymic epithelial cells expressing Aire. J Exp Med 204, 2521–2528 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamazaki Y et al. Medullary thymic epithelial cells expressing Aire represent a unique lineage derived from cells expressing claudin. Nat Immunol 8, 304–311 (2007). [DOI] [PubMed] [Google Scholar]
- 33.Gray DHD et al. Developmental kinetics, turnover, and stimulatory capacity of thymic epithelial cells. Blood 108, 3777–3785 (2006). [DOI] [PubMed] [Google Scholar]
- 34.Rossi SW et al. RANK signals from CD4(+)3(−) inducer cells regulate development of Aire-expressing epithelial cells in the thymic medulla. J Exp Med 204, 1267–1272 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akiyama T et al. The tumor necrosis factor family receptors RANK and CD40 cooperatively establish the thymic medullary microenvironment and self-tolerance. Immunity 29, 423–437 (2008). [DOI] [PubMed] [Google Scholar]
- 36.Hikosaka Y et al. The cytokine RANKL produced by positively selected thymocytes fosters medullary thymic epithelial cells that express autoimmune regulator. Immunity 29, 438–450 (2008). [DOI] [PubMed] [Google Scholar]
- 37.Metzger TC et al. Lineage Tracing and Cell Ablation Identify a Post-Aire-Expressing Thymic Epithelial Cell Population. Cell Rep (2013). doi: 10.1016/j.celrep.2013.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khan IS et al. Enhancement of an anti-tumor immune response by transient blockade of central T cell tolerance. Journal of Experimental Medicine 211, 761–768 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawano H et al. Aire Expression Is Inherent to Most Medullary Thymic Epithelial Cells during Their Differentiation Program. The Journal of Immunology 195, 5149–5158 (2015). [DOI] [PubMed] [Google Scholar]
- 40.Bakhru P et al. Combination central tolerance and peripheral checkpoint blockade unleashes antimelanoma immunity. JCI Insight 2, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; Bakhru et al. show that central tolerance imposes a barrier to robust tumor responses and combination therapy of RANK-ligand blockade and peripheral tolerance blockade can enhance tumor immune responses.
- 41.Meredith M, Zemmour D, Mathis D & Benoist C Aire controls gene expression in the thymic epithelium with ordered stochasticity. Nat Immunol 16, 942–949 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brennecke P et al. Single-cell transcriptome analysis reveals coordinated ectopic gene-expression patterns in medullary thymic epithelial cells. Nat Immunol 16, 933–941 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pinto S et al. Overlapping gene coexpression patterns in human medullary thymic epithelial cells generate self-antigen diversity. Proc Natl Acad Sci USA 110, E3497–505 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bansal K, Yoshida H, Benoist C & Mathis D The transcriptional regulator Aire binds to and activates super-enhancers. Nat Immunol 18, 263–273 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; Bansal et al. demonstrate that Aire appears to target genomic regions enriched for superenhancers to help drive its activity to promote TSA-expression. Furthermore, biochemical evidence that an interaction with Topoisomerase1 may be part of this targeting property.
- 45.Guha M et al. DNA breaks and chromatin structural changes enhance the transcription of autoimmune regulator target genes. J Biol Chem 292, 6542–6554 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abramson J, Giraud M, Benoist C & Mathis D Aire’s partners in the molecular control of immunological tolerance. Cell 140, 123–135 (2010). [DOI] [PubMed] [Google Scholar]
- 47.Oven I et al. AIRE Recruits P-TEFb for Transcriptional Elongation of Target Genes in Medullary Thymic Epithelial Cells. Mol. Cell. Biol. 27, 8815–8823 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giraud M et al. Aire unleashes stalled RNA polymerase to induce ectopic gene expression in thymic epithelial cells. Proc Natl Acad Sci USA 109, 535–540 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anderson MS & Su MA AIRE expands: new roles in immune tolerance and beyond. Nature Publishing Group 1–12 (2016). doi: 10.1038/nri.2016.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abramson J & Anderson G Thymic Epithelial Cells. Annu Rev Immunol 35, 85–118 (2017). [DOI] [PubMed] [Google Scholar]
- 51.Sansom SN et al. Population and single-cell genomics reveal the Airedependency, relief from Polycomb silencing, and distribution of self-antigen expression in thymic epithelia. Genome Research 24, 1918–1931 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takaba H et al. Fezf2 Orchestrates a Thymic Program of Self- Antigen Expression for Immune Tolerance. Cell 163, 975–987 (2015). [DOI] [PubMed] [Google Scholar]; Here Fezf2 is identified as an additional transcriptional factor that promotes thymic TSA expression for the induction of immune tolerance.
- 53.Guo C et al. Fezf2 Expression Identifies a Multipotent Progenitor for Neocortical Projection Neurons, Astrocytes, and Oligodendrocytes. Neuron 80, 1167–1174 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cosway EJ et al. Redefining thymus medulla specialization for central tolerance. Journal of Experimental Medicine 161, jem.20171000 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study helps clarify the potential role for lymphotoxin beta signaling in thymic medulla specification. Here, a more complex role of lymphotoxin receptor beta signaling was unraveled including a role for both mTEC’s and dendritic cell populations.
- 55.Aschenbrenner K et al. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol 8, 351–358 (2007). [DOI] [PubMed] [Google Scholar]
- 56.Lei Y et al. Aire-dependent production of XCL1 mediates medullary accumulation of thymic dendritic cells and contributes to regulatory T cell development. Journal of Experimental Medicine 208, 383–394 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leonard JD et al. Identification of Natural Regulatory T Cell Epitopes Reveals Convergence on a Dominant Autoantigen. Immunity 47, 107–117.e8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; Leonard et al. identify the antigen specificity for a Treg-specific clone which was originally identified in prostate tumors. Tetramer analysis for Tcaf-specific CD4 T cells shows a large skewing to the Treg lineage and dependency on Aire.
- 58.Kuroda N et al. Development of autoimmunity against transcriptionally unrepressed target antigen in the thymus of Aire-deficient mice. J. Immunol. 174, 1862–1870 (2005). [DOI] [PubMed] [Google Scholar]
- 59.Murata S et al. Regulation of CD8+ T cell development by thymus-specific proteasomes. Science 316, 1349–1353 (2007). [DOI] [PubMed] [Google Scholar]
- 60.Nitta T et al. Human thymoproteasome variations influence CD8 T cell selection. Sci Immunol 2, (2017). [DOI] [PubMed] [Google Scholar]; In this translational study, Nitta et al. find evidence for correlating a SNP risk variant for Sjogren’s with activity of the thymoproteosome for positive selection. These results, suggest that this could be part of the mechanism of how autoimmunity is triggered in this disease.
- 61.Sakaguchi N et al. Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature 426, 454–460 (2003). [DOI] [PubMed] [Google Scholar]
- 62.Tanaka S et al. Graded Attenuation of TCR Signaling Elicits Distinct Autoimmune Diseases by Altering Thymic T Cell Selection and Regulatory T Cell Function. The Journal of Immunology 185, 2295–2305 (2010). [DOI] [PubMed] [Google Scholar]
- 63.Hsu L-Y, Tan YX, Xiao Z, Malissen M & Weiss A A hypomorphic allele of ZAP-70 reveals a distinct thymic threshold for autoimmune disease versus autoimmune reactivity. Journal of Experimental Medicine 206, 2527–2541 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sakaguchi S, Benham H, Cope AP & Thomas R T-cell receptor signaling and the pathogenesis of autoimmune arthritis: insights from mouse and man. 90, 277–287 (2012). [DOI] [PubMed] [Google Scholar]
- 65.Chan AY et al. A novel human autoimmune syndrome caused by combined hypomorphic and activating mutations in ZAP-70. Journal of Experimental Medicine 213, 155–165 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; Chan et al. identified a single family with inheritance of an autoimmune syndrome that correlated with the presence of two mutant ZAP70 alleles. This family provides evidence of hypomorphic ZAP70 activity in the generation of autoimmunity.
- 66.Bruserud Ø, Oftedal BE, Wolff AB & Husebye ES ScienceDirectAIRE-mutations and autoimmune disease. Curr Opin Immunol 43, 8–15 (2016). [DOI] [PubMed] [Google Scholar]
- 67.Marx A et al. Thymoma and paraneoplastic myasthenia gravis. Autoimmunity 43, 413–427 (2010). [DOI] [PubMed] [Google Scholar]
- 68.Marx A et al. The autoimmune regulator AIRE in thymoma biology: autoimmunity and beyond. J Thorac Oncol 5, S266–72 (2010). [DOI] [PubMed] [Google Scholar]
- 69.Cheng MH et al. Acquired autoimmune polyglandular syndrome, thymoma, and an AIRE defect. N Engl J Med 362, 764–766 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu Y et al. Autoimmune regulator expression in thymomas with or without autoimmune disease. Immunol. Lett. 161, 50–56 (2014). [DOI] [PubMed] [Google Scholar]
- 71.Meager A et al. Anti-cytokine autoantibodies in autoimmunity: preponderance of neutralizing autoantibodies against interferon-alpha, interferon-omega and interleukin-12 in patients with thymoma and/or myasthenia gravis. Clin Exp Immunol 132, 128–136 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wolfe GI et al. Randomized Trial of Thymectomy in Myasthenia Gravis. N Engl J Med 375, 511–522 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vafiadis P et al. Insulin expression in human thymus is modulated by INS VNTR alleles at the IDDM2 locus. Nat Genet 15, 289–292 (1997). [DOI] [PubMed] [Google Scholar]
- 74.Giraud M et al. An IRF8-binding promoter variant and AIRE control CHRNA1 promiscuous expression in thymus. Nature 448, 934–937 (2007). [DOI] [PubMed] [Google Scholar]
- 75.Renton AE et al. A Genome-Wide Association Study of Myasthenia Gravis. JAMA Neurol 72, 396 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Parent AV et al. Generation of functional thymic epithelium from human embryonic stem cells that supports host T cell development. Cell Stem Cell 13:219–229, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]