A 41‐year‐old woman was seen at the National Institutes of Health (NIH) Neuroimmunology Clinic in 2017 for recurrent episodes of fever, neck stiffness, and back and leg pain.
In 2002, at age 26 years, she had several episodes of back and neck pain, malaise, and fever, each lasting 1 to 3 days (Fig 1). A chest x‐ray and complete blood count were normal. Cerebrospinal fluid (CSF) during one of these episodes showed pleocytosis (60 white blood cells [WBC]/μl; 60% monocytes, 25% lymphocytes, 15% neutrophils), with elevated protein (96 mg/dl) and low glucose (26 mg/dl Table Supplementary Table 1). Magnetic resonance imaging (MRI) of the brain showed subtle fluid‐attenuated inversion recovery (FLAIR) hyperintensity in the sulcal CSF. MRI of the spine was normal. Extensive investigations including CSF Mycobacterium tuberculosis (TB) complex polymerase chain reaction (PCR) and culture, Coccidioides antibodies, histoplasma antigen, cryptococcal antigen, and herpes simplex virus and varicella zoster virus PCRs were negative (see Supplementary Table 1). Nonetheless, she was treated empirically with valacyclovir for 2 weeks. She had had a recent exposure to TB and had converted from a negative purified protein derivative (PPD) skin test in 2001 to a positive result at the time of her presentation in 2002. Thus, she was also treated empirically for TB meningitis (TBM) with rifampin, pyrazinamide, and ethambutol for 1 year. Isoniazid (INH) was started but was discontinued after several weeks due to transaminitis and nausea. She did not receive adjunctive steroids.
Figure 1.
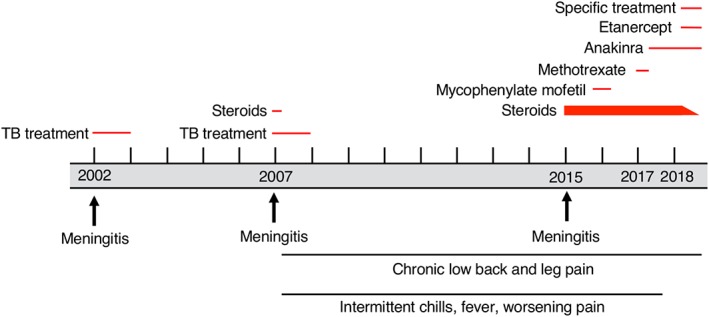
Clinical timeline. TB = Mycobacterium tuberculosis.
Her symptoms resolved until 2006 when, immediately following spinal epidural anesthesia during childbirth, she developed a fever with headache, neck stiffness, back pain, and night sweats. She was treated for endometritis but continued to have similar but less severe symptoms for several months. In early 2007, she acutely developed bilateral gluteal pain and left leg dysesthesias. CSF again showed pleocytosis (130 WBC/μl; 83% lymphocytes, 13% monocytes, 2% neutrophils, 2% other) with elevated protein (132 mg/dl) and low glucose (10 mg/dl). CSF TB PCR and culture, cryptococcal antigen, bacterial and fungal cultures, and viral PCRs, as well as CSF cytology and flow cytometry for malignant cells, were negative (see Supplementary Table 1). MRI of the lumbar spine now showed abnormal enhancement and nerve‐root thickening in the caudal thecal sac, indicating arachnoiditis (see Fig 2A, B); brain, cervical, and thoracic spine MRI was normal. Repeat lumbar spine MRI 2 months later showed more extensive enhancement and clumping of the cauda equina. Computed tomography (CT) scans of the chest, abdomen, and pelvis, and a gallium scan, were unremarkable.
Figure 2.
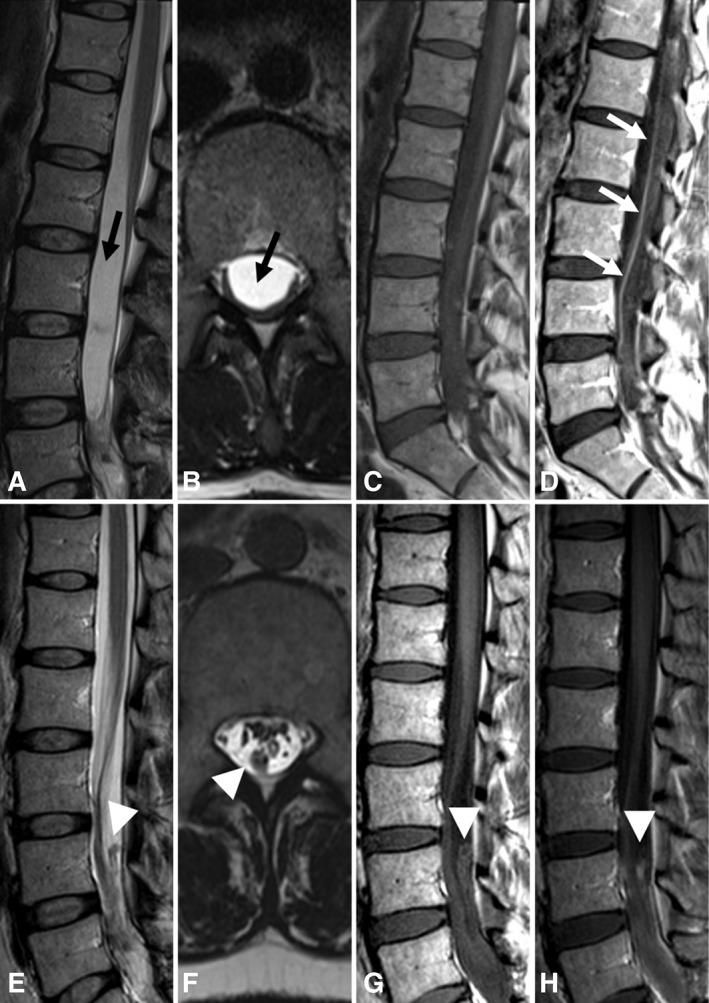
Lumbar spine magnetic resonance imaging (MRI) findings. (A, B) MRI of the lumbar spine in 2015 showed a cystlike structure in the lumbosacral sac (black arrows) seen on sagittal (A) and axial (B) T2‐weighted images. (C, D) Repeat MRI in early 2017 showed clumping of the nerve roots of the cauda equina and enhancement of the nerve roots on postcontrast T1‐weighted images (D, white arrows) compared to precontrast images (C). (E–H) In late 2017, soon after a symptom flare, lumbar spine MRI showed an extramedullary, intradural nodule (white arrowheads) on T2‐weighted (E, F) and T1‐weighted (G) images, which demonstrated contrast enhancement (H).
In April 2007, she had a laminectomy and biopsy at L5/S1. The dura was thick, and there were adhesions within the thecal sac and scar tissue surrounding the nerve roots. Histology showed lymphohistiocytic inflammation and a poorly formed non‐necrotizing granuloma. Mycobacterial cultures were negative. There was concern that premature discontinuation of INH in 2002 may have led to incomplete treatment of TBM, so she was again treated empirically for TB with rifampin, INH, pyrazinamide, ethambutol, and moxifloxacin for 1 year, as well as 3 weeks of prednisone 60 mg daily followed by a 3‐week prednisone taper. This time, pyrazinamide was stopped early due to transaminitis and nausea, and ethambutol was stopped after several months when her CSF profile improved. Repeat lumbar punctures (LPs) showed normalization of protein and glucose with mild residual CSF pleocytosis (5–10 WBC/μl). Her symptoms improved significantly, and she resumed her daily activities. However, she continued to have intermittent mild low back pain, sometimes accompanied by chills.
In 2015, at age 39 years, several days after a partial thyroidectomy for an incidentally discovered thyroid nodule, the patient again developed low back and leg pain, chills, headache, fever, and neck stiffness. She was treated with valacyclovir for possible herpes meningitis, as well as prednisone 60 mg daily for 5 weeks for arachnoiditis, with immediate improvement in symptoms. However, her pain recurred when prednisone was tapered gradually over the next several months, and she also developed pain with eye movement, urinary frequency and hesitancy, and subjective sensory changes in the left leg distally from the knee. MRI showed evidence of worsening lumbosacral arachnoiditis with thickening and enhancement of the cauda equina. There was also displacement of the cauda equina posteriorly and laterally by a loculated cystlike structure (see Fig 2A, B). She had another LP; however, only a small amount of fluid was obtained, possibly due to the loculation. The CSF was bloody (1,075 red blood cells [RBC]/μl) with 9 WBC/μl (78% lymphocytes, 22% neutrophils), elevated glucose (179 mg/dl), and low protein (13 mg/dl). Cryptococcal antigen and fungal cultures were negative. She had a whole body fluorodeoxyglucose positron emission tomography (FDG‐PET) CT that was normal. The etiology of the arachnoiditis was thought to be postinfectious or autoimmune. She was treated with a 3‐day course of intravenous methylprednisolone, followed by oral prednisone, with dramatic improvement; however, pain, neck stiffness, and fever again recurred when steroids were tapered several weeks later.
She was subsequently maintained on prednisone, and any attempt to decrease to <35 mg/day resulted in worsening of her back pain, fatigue, and intermittent low‐grade fevers and night sweats. Due to concern for neurosarcoidosis or another autoimmune disorder, she was placed on concomitant mycophenolate mofetil (up to 3,000 mg/day) for several months in 2016, without any improvement. While continuing the prednisone, she was transitioned from mycophenolate mofetil to methotrexate up to 15 mg/wk in early 2017, also with no improvement or ability to taper steroids. She developed bilateral cataracts attributed to chronic corticosteroid use.
At the time of her presentation to the NIH Neuroimmunology Clinic in 2017, she had constant dull, aching pain in her back and buttocks that worsened with prolonged activity or stress. Every 1 to 3 months, she had several days of malaise and fever up to 38.3°C accompanied by more severe back and buttock pain. She complained of a sensation of urinary retention, although postvoid residuals were normal. She denied constipation or bowel incontinence, weakness or numbness, or neurological symptoms in her arms. Treatment of pain with pregabalin was minimally effective and caused drowsiness. Her pain responded to nonsteroidal anti‐inflammatory drugs.
She was born in Mumbai, India, immigrated to Arizona at age 22 years, and later moved to New York and then Maryland. She returned to India once, in 2009, and had no other foreign travel. Her medical history was significant for thrombocytopenia during early childhood, hepatitis B virus infection at age 12 years that subsequently resolved, left facial palsy in her teens associated with a herpetic rash in her left ear canal, and fever, headache, and malaise in 1998 at age 22 years, for which she was treated for malaria despite a negative blood smear. She had a sister with breast cancer and several distant family members with cancer, including leukemia, neuroblastoma, lung cancer, and a hepatoma. She had no family history of autoimmune or neurological disease, and no family members with frequent or severe infections.
Her general physical and neurological evaluations were normal with the exception of mild atrophy in both legs without fasciculations and with preserved strength. Lumbar spine MRI again showed arachnoiditis with some extension superiorly compared to 2015 (see Fig 2C, D). Brain MRI showed a few small foci of leptomeningeal enhancement on postgadolinium FLAIR images. Blood was negative for rheumatologic testing, human immunodeficiency virus type 1 antibody, human T‐cell lymphotropic virus type 1 and 2 antibodies, and Lyme serology. Erythrocyte sedimentation rate (ESR) and C‐reactive protein (CRP) were normal (see Supplementary Table 1).
Given the patient's previously negative infectious workups as well as her response to steroids, her symptoms and MRI findings were thought to be secondary to an autoimmune process, perhaps triggered initially by an infection. As she had no response to therapies directed at lymphocytes (methotrexate, mycophenolate), she was given a 1‐month trial of the IL‐1 receptor antagonist anakinra (up to 200 mg/day), with no improvement in her chronic symptoms. However, about 1 week after discontinuation of anakinra, she developed her typical flare symptoms of malaise and worsening back pain. Her neurologic examination was stable. She had an LP at that time, which showed 156 RBC/μl, 30 WBC/μl (92% lymphocytes, 4% monocytes, 4% neutrophils), total protein 41 mg/dl, and glucose 47 mg/dl. Infectious studies, including mycobacterial culture, histoplasma antigen, and viral PCRs, were negative. She had an elevated IgG index of 2.41 (normal range = 0.26–0.62) and partially identical oligoclonal bands in CSF and serum (pattern 3). She had an elevated blood WBC count of 15,680 cells/μl (94% neutrophils) and normal ESR and CRP. Anakinra was restarted, and her flare symptoms improved over the next several weeks, although her chronic symptoms continued. Several months later, a surveillance lumbar spine MRI showed enlargement and new contrast enhancement of a subarachnoid nodule (see Fig 2E–H).
An additional test was performed, and a diagnosis was made.
Differential Diagnosis Discussion
This is a 41‐year‐old woman with a 15‐year history of relapsing meningitis that progressed to chronic lumbar arachnoiditis, with several notable relapses following episodes of physical stress. Despite multiple investigations, no definitive etiology was identified, and the disease recurred despite multiple empirical treatment regimens for TB, herpesvirus infections, and inflammatory conditions. Several possible diagnoses should be considered at this stage. We focus our discussion around the findings of chronic arachnoiditis and granulomatous disease.
Infectious
The patient's initial presentation at age 26 years was of a subacute prodrome followed by acute meningism. An infectious cause was appropriately at the top of the differential. Given the subacute history and the monocyte‐predominant CSF with WBC < 100 cells/μl, bacterial meningitis secondary to typical pathogens, although possible, was less likely.1, 2 Subacute causes of meningitis commonly occur in the setting of viral, parasitic, fungal, or atypical bacterial infections, such as TB.2 Multiple investigations for these types of pathogens were negative, and there was no evidence of systemic infection by CT or FDG‐PET CT. However, TB PCR and culture are insensitive, and up to half of people with TBM have no evidence of systemic disease.3, 4 Given her recent exposure to TB and subsequent PPD conversion, CSF pleocytosis, hypoglycorrhachia, and the poor sensitivity of diagnostic tests for TBM, empiric treatment was appropriate given the disease's high morbidity and mortality.5, 6, 7 Her prolonged remission after empiric TB therapy was also reassuring.
Her second attack 4 years later occurred acutely after an epidural anesthetic in the setting of pregnancy, when she was potentially more prone to infection. MRI showed new lumbar arachnoiditis. Efforts to culture or detect a pathogenic organism from the CSF were again unsuccessful, despite CSF pleocytosis and low glucose. Surgical biopsy revealed a poorly formed non‐necrotizing granuloma.
Necrotizing granulomas are a hallmark of TB; however, non‐necrotizing granulomas can be found in TB‐positive patients, and this finding should not dissuade the clinician from the diagnosis if clinical suspicion is high.8, 9 INH is a cornerstone medication in the therapy of TBM, and patients with INH resistance have significantly worse outcomes.10, 11, 12, 13 The patient's initial TBM treatment did not include an adequate course of INH, and there was concern for recurrence. TB affects the spine predominantly in the form of extramedullary disease.14 Spinal arachnoiditis can occur as a complication of TBM and can infrequently be an asymptomatic finding.14, 15, 16 Cases of delayed lumbar arachnoiditis, occurring up to 15 years after effective treatment of the initial TBM, have been reported.17, 18
There are a multitude of infectious, autoimmune, and neoplastic causes of granulomatous disease in the central nervous system (CNS) (Table 1).19 Most notably in this patient, other infectious etiologies to consider would include fungal infections, neurosyphilis, and a variety of parasitic infections. Many of these conditions can cause chronic meningitis with a relapsing component, and broad diagnostic tests, such as cultures, may detect some (but not all) of these pathogens.20
Table 1.
Causes of Central Nervous System Granulomatous Disease
| Infectious19, 70 | Immune19, 71, 72, 73, 74, 75, 76 |
|---|---|
| Bacteria | Common variable immunodeficiency |
| Bartonella henselae | Chronic granulomatous disease |
| Brucella sp. | Idiopathic pachymeningitis |
| Listeria monocytogenes | Kikuchi–Fujimoto disease |
| Mycobacterium leprae | Neurosarcoidosis |
| Mycobacterium tuberculosis | Rheumatoid arthritis |
| Nocardia sp.a | Vasculitis44, 45 |
| Treponema pallidum | Eosinophilic granulomatosis with polyangiitis |
| Tropheryma whipplei | Giant cell arteritis |
| Fungi | Granulomatosis with polyangiitis |
| Aspergillus sp.b | Primary angiitis of the central nervous system |
| Candida albicans b | Takayasu arteritis |
| Coccidioides sp.c | ANCA‐associated vasculitis |
| Cryptococcus neoformans | Malignancy 77, 78, 79 |
| Histoplasma capsulatum | Lymphomatoid granulomatosis |
| Mucormycosisa | Langerhans cell histiocytosis |
| Paracoccidioides brasiliensis d | Erdheim–Chester disease |
| Parasites | |
| Acanthamoeba sp.b | |
| Balamuthia mandrillaris | |
| Echinococcus sp.d | |
| Naegleria fowleri | |
| Paragonimus westermani c | |
| Plasmodium sp.c | |
| Schistosoma sp.c | |
| Taenia sp.c | |
| Toxoplasma gondii | |
| Trypanosoma cruzi |
In immunocompromised or diabetic people or intravenous drug users.
In immunocompromised hosts.
In people with history of travel to endemic areas.
In people with prolonged residence in endemic areas.
ANCA = antineutrophil cytoplasmic antibody.
Arachnoiditis is a rare condition characterized by chronic inflammation of the arachnoid and pia mater with increased production of collagen deposition between the two layers, leading to adhesions. Anatomically related cranial and radicular nerve roots become edematous and hyperemic before being entrapped and clumped together in the adhesive leptomeninges. Over time, the nerves atrophy due to diminished blood supply.21 Intracranial arachnoiditis can lead to cranial nerve abnormalities, with blindness possible in cases of optochiasmatic arachnoiditis.22 Lumbar arachnoiditis can cause back and lower limb pain, variable neurological deficits, and partial cauda equina syndrome. However, the clinical manifestations depend on the severity and location of disease.23 Any irritant or pathogen that causes chronic inflammation of the arachnoid mater can lead to adhesive arachnoiditis, and therefore, despite the rarity of the syndrome, the potential etiologies are broad. Older contrast agents used in CT myelograms (particularly ethyliodophentylate, which is no longer used), blood breakdown following subarachnoid hemorrhage, older anesthetic preservatives, and lumbar surgery have all been implicated.23 Other causes include infections, autoimmune conditions, and malignancy, as described below. Despite an extensive workup, a causative organism may not be found, due to poor sensitivities of diagnostic assays or unintentional omission of appropriate pathogen‐specific investigations.
Fungal infections such as Cryptococcus and Candida species can cause chronic meningitis and arachnoiditis.15, 22, 24, 25 However, this patient was not known to be immunocompromised or to have engaged in intravenous drug use, making fungal meningitis less likely. In addition, her clinical course and CSF profile were not consistent with coccidioidomycosis, which was suspected because she had lived in Arizona. Neurocysticercosis (NCC) can cause spinal arachnoid disease, usually in patients with basal arachnoid disease, and can be asymptomatic, although this was not considered in this patient (although she grew up in a country where NCC is endemic) because no parenchymal or subarachnoid cysts were detected on at least 8 brain MRIs over 15 years.26 She did have a cyst adjacent to the lumbar cord seen on MRI in 2015; however, in light of her overall presentation and lack of brain cysts, NCC was not investigated as a possible cause. Syphilis can rarely cause subacute meningitis with optochiasmal arachnoiditis and has also been implicated in lumbar arachnoiditis.27, 28 Schistosomiasis can manifest with spinal cord disease, either with transverse myelitis commonly involving the conus medullaris or with lumbar arachnoiditis.29 Neuroschistosomiasis occurs in endemic regions, such as Egypt, but neither India nor the USA is considered a high‐risk area.30 Meningitis can also be seen in angiostrongyliasis, gnathostomiasis, and sparganosis parasitic infections, but usually with a much more acute course.31, 32 Case reports of vertebral disease and lumbar arachnoiditis secondary to Echinococcus granulosus, a zoonotic parasitic infection transmitted from dogs, have also been described.33, 34 Amoebic infections such as Balamuthia mandrillaris can cause granulomatous meningitis, but the course is usually much more rapid.35 An occult or indolent infection in her lumbar canal may have incited a larger‐than‐expected immune response after the introduction of an epidural needle, which would otherwise cause only a mild local inflammatory response.
On a single LP in 2002, our patient had CSF eosinophilia, with eosinophils making up 1% of 147 WBC/μl. Eosinophilic meningitis, which is defined as the presence of at least 10% eosinophils of CSF WBC or at least 10 eosinophils/μl, is most often caused by a helminthic infection, most commonly Angiostrongylus catonensis. However, a wide variety of other infections as well as malignancy and autoimmune disease, including sarcoidosis, can also cause CSF eosinophilia, with variable blood eosinophilia.31
Inflammatory Conditions
Postinfectious autoimmune neurological conditions are common. Paradoxical worsening of TBM is an immune‐mediated response that can present up to a year after effective treatment, with 4% of patients developing spinal arachnoiditis.36 Postinfectious inflammatory lumbar arachnoiditis can also be seen following cryptococcal meningitis secondary to a postinfectious inflammatory response syndrome. Most cases have negative cryptococcal cultures in CSF.37
Neurosarcoidosis is an autoimmune non‐necrotizing granulomatous condition that can present with both systemic and neurological disease.38 Neurosarcoidosis commonly causes a relapsing chronic meningitis, and chronic lumbar arachnoiditis has been described.39, 40 Neurological symptoms can be the initial presentation of neurosarcoidosis in 50% of patients, with 85% ultimately developing systemic disease but with a significant percentage continuing to have isolated CNS involvement.41 Features that prompt the consideration of neurosarcoidosis in our patient were her presentation with chronic meningitis and arachnoiditis, CSF pleocytosis, low CSF glucose (which can occur in 14% of patients), non‐necrotizing granulomas on biopsy, and the relapsing nature of the disease in the same region of the CNS.41, 42, 43 Despite extensive investigation including CT of the chest, abdomen, and pelvis as well as FDG‐PET/CT, there was no evidence of systemic sarcoidosis. Her later relapses responded extremely well to steroid therapy, and she became dependent on steroids, further implicating an autoimmune etiology. Primary CNS vasculitis can also present with granulomatous inflammation, but it rarely involves the spinal cord,44, 45 and blood vessel wall inflammation was not identified on biopsy. Antineutrophil cytoplasmic antibody (ANCA)‐associated vasculitis can also have CNS involvement, but repeat serum ANCA testing was negative.
Malignancy
Invasive malignancy of the subarachnoid space is another important diagnostic consideration. Despite the patient's strong family history of cancer, there was no evidence of malignancy in repeated CSF cytology and flow cytometry examinations, on lumbar meningeal biopsy, or by whole body imaging. In addition, the sheer length of the 15‐year disease course with no manifestations of systemic malignancy make the diagnosis less likely. However, this diagnostic possibility should always still be considered, especially given that systemic glucocorticoids can temporarily improve hematologic malignancies, and CSF cytology is notoriously insensitive.
Research CSF Metagenomic Sequencing
Despite the presumption of an autoimmune etiology, lingering concerns about an occult infection prompted enrollment of the patient in a research study at University of California, San Francisco to investigate her CSF with metagenomic next generation sequencing (mNGS), an unbiased approach to the identification of neuroinfectious diseases. The use of mNGS has gained momentum over the past several years, with several notable case reports and case series showcasing mNGS's ability to capture a broad range of infections with a single assay.24, 46, 47, 48, 49, 50, 51, 52 Total RNA is extracted from a patient's CSF, and complementary DNA (cDNA) is generated by reverse transcription with random hexamer primers. The cDNA is then converted into a library of random fragments, which is then sequenced on a massively parallel scale.53 The resulting genomic data are then processed through a bioinformatics pipeline, which removes human, low‐complexity, redundant, and poor‐quality sequences. The remaining sequences are searched against all known organisms in the National Center for Biotechnology Information's GenBank database to identify the source of the high‐quality, nonredundant, high‐complexity, nonhuman sequences.54, 55, 56 As a result, it is possible to identify the vast majority of known organisms, including viral, fungal, bacterial, and parasitic infections, whether or not they are being considered as part of the treating physician's differential diagnosis.
In this case, mNGS of total RNA extracted from 500 μl of the patient's CSF generated 5,750,572 pairs of 135 nucleotide sequences. After computational filtering, there were 67,334 pairs of high‐quality, nonhuman, and nonredundant sequences. Of these, 2,725 sequence pairs unambiguously aligned to the genus Taenia with 99 to 100% similarity to Taenia solium. Based on a z score–based statistical model comparing the abundance of organisms in the sample to "no template" water controls and uninfected CSF samples, T. solium was the highest ranking organism, with all other identified microbes consistent with frequent environmental contaminants.24 The diagnosis of NCC was confirmed with a clinical CSF cestode antigen assay and serology, which previously had not been performed.57 Retrospective review of the patient's earlier meningeal biopsy did not reveal evidence of cysticerci.
Discussion of Pathology and Diagnosis
NCC is caused by infection with T. solium, the pork tapeworm. The condition leads to single or multiple intraparenchymal, ventricular, and subarachnoid cysts. NCC is endemic to Central America, South America, Sub‐Saharan Africa, and Asia.58 The hallmark of parenchymal NCC is the formation of a vesicular cyst that degenerates from a viable to a calcified form. During the viable stage, the parasite is thought to evade host defenses, and there is minimal to no immune response.59 Cyst degeneration occurs when the immune system detects the parasite. The pathogen can no longer evade the immune system, and a robust granulomatous inflammatory response occurs, which can lead to significant neurological morbidity.60, 61 The final, calcified stage contains a dead parasite and creates minimal inflammatory response.58, 62 In subarachnoid NCC, the parasite can lack typical cystic structures, making it more challenging to identify on imaging. Subarachnoid NCC can also cause pronounced inflammation, which can be difficult to control and treat.63, 64 Spinal NCC is a rarer manifestation of NCC, with extramedullary arachnoid disease constituting the majority of cases.26 Intracranial disease is also evident in most cases, but our patient only had subtle leptomeningeal enhancement on brain MRI and no intraparenchymal or intraventricular lesions.26 In retrospect, the cyst seen on the patient's lumbar spine MRI in 2015, and the enhancing intradural extramedullary lumbar nodule seen in 2017, likely represented degenerating cysts, although even after the diagnosis was made there was debate among neuroradiologists about whether the findings on MRI in 2015 represented a Taenia cyst versus arachnoid scarring. At the time, these were not identified as parasitic, given the lack of more typical brain cysts, as well as the larger clinical context of recurrent fever and constitutional symptoms, which are unusual features of NCC.65, 66 Nevertheless, NCC should be considered in any patient with chronic meningitis who has spent time in an endemic area, even without typical MRI findings.
Follow‐up
After the NCC diagnosis, the patient was started on dual antihelminthic therapy with praziquantel and albendazole. She was also started on the tumor necrosis factor α inhibitor etanercept to protect against an inflammatory reaction to degenerating cysts.67 On this therapy, she tolerated a steroid taper for the first time in 2 years, and after a year was able to discontinue steroids completely. Following 3 months of treatment, her MRI remained stable, CSF demonstrated reduced leukocytosis (10 WBC/μl; 91% lymphocytes, 7% monocytes, 2% neutrophils) with normal protein and glucose, and the CSF cestode antigen was no longer detectable. CSF cestode antigen was again undetectable after a year of treatment, and so antihelminthic treatment and etanercept were stopped, with the intention of stopping anakinra in the coming months. She continues to have fatigue and low back and buttock pain but has been able to increase her daily activities and has not had a severe symptom flare since starting specific therapy.
This case thus highlights the utility of mNGS for the diagnosis of atypical presentations of common infections.24, 68, 69 The case also vividly illustrates that either improvement or lack of clinical deterioration in the setting of immunosuppression does not rule out an underlying infectious etiology, even after years of treatment.
Author Contributions
E.S.B., A.V., A.N., J.L.D., and M.R.W. contributed to the conception and design of the study. E.S.B., E.M.O., T.N., D.S.R., A.V., A.N., L.M.K., H.A.S., K.C.Z., J.L.D., and M.R.W. contributed to the acquisition and analysis of data. E.S.B., P.S.R., and M.R.W. contributed to drafting the text and preparing the figures.
Potential Conflicts of Interest
Nothing to report.
Supporting information
Supporting Information Table
Acknowledgment
We thank the NIH neuroimmunology staff for their assistance in care of the patient and collection of samples, and the patient for her participation in the research study.
References
- 1. McGill F, Heyderman RS, Panagiotou S, et al. Acute bacterial meningitis in adults. Lancet 2016;388:3036–3047. [DOI] [PubMed] [Google Scholar]
- 2. Sulaiman T, Salazar L, Hasbun R. Acute versus subacute community‐acquired meningitis: analysis of 611 patients. Medicine (Baltimore) 2017;96:e7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bahr NC, Boulware DR. Methods of rapid diagnosis for the etiology of meningitis in adults. Biomark Med 2014;8:1085–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sutlas PN, Unal A, Forta H, et al. Tuberculous meningitis in adults: review of 61 cases. Infection 2003;31:387–391. [DOI] [PubMed] [Google Scholar]
- 5. Woldeamanuel YW, Girma B. A 43‐year systematic review and meta‐analysis: case‐fatality and risk of death among adults with tuberculous meningitis in Africa. J Neurol 2014;261:851–865. [DOI] [PubMed] [Google Scholar]
- 6. Thwaites GE, Nguyen DB, Nguyen HD, et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med 2004;351:1741–1751. [DOI] [PubMed] [Google Scholar]
- 7. Chiang SS, Khan FA, Milstein MB, et al. Treatment outcomes of childhood tuberculous meningitis: a systematic review and meta‐analysis. Lancet Infect Dis 2014;14:947–957. [DOI] [PubMed] [Google Scholar]
- 8. Chih‐Hui Tien M, Ramachandran P, White OB, Lee AG. Not all headaches are migraines. Surv Ophthalmol 2017;62:378–382. [DOI] [PubMed] [Google Scholar]
- 9. Litinsky I, Elkayam O, Flusser G, et al. Sarcoidosis: TB or not TB? Ann Rheum Dis 2002;61:385–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thwaites GE, Lan NT, Dung NH, et al. Effect of antituberculosis drug resistance on response to treatment and outcome in adults with tuberculous meningitis. J Infect Dis 2005;192:79–88. [DOI] [PubMed] [Google Scholar]
- 11. Vinnard C, King L, Munsiff S, et al. Long‐term mortality of patients with tuberculous meningitis in New York City: a cohort study. Clin Infect Dis 2017;64:401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vinnard C, Winston CA, Wileyto EP, et al. Isoniazid resistance and death in patients with tuberculous meningitis: retrospective cohort study. BMJ 2010;341:c4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tho DQ, Torok ME, Yen NT, et al. Influence of antituberculosis drug resistance and Mycobacterium tuberculosis lineage on outcome in HIV‐associated tuberculous meningitis. Antimicrob Agents Chemother 2012;56:3074–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Garg RK, Malhotra HS, Gupta R. Spinal cord involvement in tuberculous meningitis. Spinal Cord 2015;53:649–657. [DOI] [PubMed] [Google Scholar]
- 15. Hernandez‐Albujar S, Arribas JR, Royo A, et al. Tuberculous radiculomyelitis complicating tuberculous meningitis: case report and review. Clin Infect Dis 2000;30:915–921. [DOI] [PubMed] [Google Scholar]
- 16. Srivastava T, Kochar DK. Asymptomatic spinal arachnoiditis in patients with tuberculous meningitis. Neuroradiology 2003;45:727–729. [DOI] [PubMed] [Google Scholar]
- 17. Chang KH, Han MH, Choi YW, et al. Tuberculous arachnoiditis of the spine: findings on myelography, CT, and MR imaging. AJNR Am J Neuroradiol 1989;10:1255–1262. [PMC free article] [PubMed] [Google Scholar]
- 18. Kozlowski K. Late spinal blocks after tuberculous meningitis. Am J Roentgenol 1963;90:1220–1226. [PubMed] [Google Scholar]
- 19. Zumla A, James G. Granulomatous infections: etiology and classification. Clin Infect Dis 1996;23:146–158. [DOI] [PubMed] [Google Scholar]
- 20. Barenfanger J, Lawhorn J, Drake C. Nonvalue of culturing cerebrospinal fluid for fungi. J Clin Microbiol 2004;42:236–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Burton CV. Lumbosacral arachnoiditis. Spine (Phila Pa 1976) 1978;3:24–30. [DOI] [PubMed] [Google Scholar]
- 22. Thwaites GE, van Toorn R, Schoeman J. Tuberculous meningitis: more questions, still too few answers. Lancet Neurol 2013;12:999–1010. [DOI] [PubMed] [Google Scholar]
- 23. Rice I, Wee MY, Thomson K. Obstetric epidurals and chronic adhesive arachnoiditis. Br J Anaesth 2004;92:109–120. [DOI] [PubMed] [Google Scholar]
- 24. Wilson MR, O'Donovan BD, Gelfand JM, et al. Chronic meningitis investigated via metagenomic next‐generation sequencing. JAMA Neurol 2018;75:947–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wan CL, Chang CS, Wei CP, et al. Cryptococcal infection presenting with lumbosacral polyradiculopathy: report of a case. J Formos Med Assoc 1991;90:1218–1221. [PubMed] [Google Scholar]
- 26. Callacondo D, Garcia HH, Gonzales I, et al. High frequency of spinal involvement in patients with basal subarachnoid neurocysticercosis. Neurology 2012;78:1394–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bruetsch WL. Etiology of optochiasmatic arachnoiditis. Arch Neurol Psychiatry 1948;59:215–228. [DOI] [PubMed] [Google Scholar]
- 28. Wadia NH, Dastur DK. Spinal meningitides with radiculo‐myelopathy. 1. Clinical and radiological features. J Neurol Sci 1969;8:239–260. [DOI] [PubMed] [Google Scholar]
- 29. Haribhai HC, Bhigjee AI, Bill PL, et al. Spinal cord schistosomiasis. A clinical, laboratory and radiological study, with a note on therapeutic aspects. Brain 1991;114(pt 2):709–726. [DOI] [PubMed] [Google Scholar]
- 30. Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet 2014;383:2253–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sawanyawisuth K, Chotmongkol V. Eosinophilic meningitis. Handb Clin Neurol 2013;114:207–215. [DOI] [PubMed] [Google Scholar]
- 32. Holodniy M, Almenoff J, Loutit J, Steinberg GK. Cerebral sparganosis: case report and review. Rev Infect Dis 1991;13:155–159. [DOI] [PubMed] [Google Scholar]
- 33. Jacquier M, Piroth L. Vertebral hydatidosis. N Engl J Med 2018;379:e5. [DOI] [PubMed] [Google Scholar]
- 34. Singh S, Sardhara J, Singh AK, et al. Spinal intradural hydatid cyst causing arachnoiditis: a rare etiology of cauda equina syndrome. J Craniovertebr Junction Spine 2016;7:282–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bando Y, Takahashi T, Uehara H, et al. Autopsy case of amebic granulomatous meningoencephalitis caused by Balamuthia mandrillaris in Japan. Pathol Int 2012;62:418–423. [DOI] [PubMed] [Google Scholar]
- 36. Singh AK, Malhotra HS, Garg RK, et al. Paradoxical reaction in tuberculous meningitis: presentation, predictors and impact on prognosis. BMC Infect Dis 2016;16:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Panackal AA, Komori M, Kosa P, et al. Spinal arachnoiditis as a complication of cryptococcal meningoencephalitis in non‐HIV previously healthy adults. Clin Infect Dis 2017;64:275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gelfand JM. Demystifying neurosarcoidosis and informing prognosis. JAMA Neurol 2017;74:1296–1298. [DOI] [PubMed] [Google Scholar]
- 39. Jiang QL, Tomyanovich M, Flowers C. Spinal arachnoiditis as a manifestation of sarcoidosis (P06.229). Neurology 2013;80:P06.229. [Google Scholar]
- 40. Fritz D, Voortman M, van de Beek D, et al. Many faces of neurosarcoidosis: from chronic meningitis to myelopathy. Curr Opin Pulm Med 2017;23:439–446. [DOI] [PubMed] [Google Scholar]
- 41. Fritz D, van de Beek D, Brouwer MC. Clinical features, treatment and outcome in neurosarcoidosis: systematic review and meta‐analysis. BMC Neurol 2016;16:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gelfand JM, Bradshaw MJ, Stern BJ, et al. Infliximab for the treatment of CNS sarcoidosis: a multi‐institutional series. Neurology 2017;89:2092–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pawate S, Moses H, Sriram S. Presentations and outcomes of neurosarcoidosis: a study of 54 cases. QJM 2009;102:449–460. [DOI] [PubMed] [Google Scholar]
- 44. Marquez J, Flores D, Candia L, Espinoza LR. Granulomatous vasculitis. Curr Rheumatol Rep 2003;5:128–135. [DOI] [PubMed] [Google Scholar]
- 45. Salvarani C, Giannini C, Salvarani C, et al. Primary central nervous system vasculitis: pathology and mechanisms. Acta Neuropathol 2012;123:759–772. [DOI] [PubMed] [Google Scholar]
- 46. Chiu CY, Coffey LL, Murkey J, et al. Diagnosis of fatal human case of St. Louis encephalitis virus infection by metagenomic sequencing, California, 2016. Emerg Infect Dis 2017;23:1964–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Murkey JA, Chew KW, Carlson M, et al. Hepatitis E virus‐associated meningoencephalitis in a lung transplant recipient diagnosed by clinical metagenomic sequencing. Open Forum Infect Dis 2017;4:ofx121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wilson MR, Naccache SN, Samayoa E, et al. Actionable diagnosis of neuroleptospirosis by next‐generation sequencing. N Engl J Med 2014;370:2408–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wilson MR, Shanbhag NM, Reid MJ, et al. Diagnosing Balamuthia mandrillaris encephalitis with metagenomic deep sequencing. Ann Neurol 2015;78:722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wilson MR, Zimmermann LL, Crawford ED, et al. Acute West Nile virus meningoencephalitis diagnosed via metagenomic deep sequencing of cerebrospinal fluid in a renal transplant patient. Am J Transplant 2017;17:803–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fremond ML, Perot P, Muth E, et al. Next‐generation sequencing for diagnosis and tailored therapy: a case report of astrovirus‐associated progressive encephalitis. J Pediatric Infect Dis Soc 2015;4:e53–e57. [DOI] [PubMed] [Google Scholar]
- 52. Mongkolrattanothai K, Naccache SN, Bender JM, et al. Neurobrucellosis: unexpected answer from metagenomic next‐generation sequencing. J Pediatric Infect Dis Soc 2017;6:393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wilson MR, Suan D, Duggins A, et al. A novel cause of chronic viral meningoencephalitis: Cache Valley virus. Ann Neurol 2017;82:105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schubert RD, Wilson MR. A tale of two approaches: how metagenomics and proteomics are shaping the future of encephalitis diagnostics. Curr Opin Neurol 2015;28:283–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Naccache SN, Federman S, Veeraraghavan N, et al. A cloud‐compatible bioinformatics pipeline for ultrarapid pathogen identification from next‐generation sequencing of clinical samples. Genome Res 2014;24:1180–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Brown JR, Bharucha T, Breuer J. Encephalitis diagnosis using metagenomics: application of next generation sequencing for undiagnosed cases. J Infect 2018;76:225–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rodriguez S, Dorny P, Tsang VC, et al. Detection of Taenia solium antigens and anti‐T. solium antibodies in paired serum and cerebrospinal fluid samples from patients with intraparenchymal or extraparenchymal neurocysticercosis. J Infect Dis 2009;199:1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Garcia HH, Nash TE, Del Brutto OH. Clinical symptoms, diagnosis, and treatment of neurocysticercosis. Lancet Neurol 2014;13:1202–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hernandez JL, Leung G, McKay DM. Cestode regulation of inflammation and inflammatory diseases. Int J Parasitol 2013;43:233–243. [DOI] [PubMed] [Google Scholar]
- 60. Restrepo BI, Alvarez JI, Castano JA, et al. Brain granulomas in neurocysticercosis patients are associated with a Th1 and Th2 profile. Infect Immun 2001;69:4554–4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rajshekhar V, Joshi DD, Doanh NQ, et al. Taenia solium taeniosis/cysticercosis in Asia: epidemiology, impact and issues. Acta Trop 2003;87:53–60. [DOI] [PubMed] [Google Scholar]
- 62. Nash TE, Garcia HH. Diagnosis and treatment of neurocysticercosis. Nat Rev Neurol 2011;7:584–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. White AC Jr, Coyle CM, Rajshekhar V, et al. Diagnosis and treatment of neurocysticercosis: 2017 clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis 2018;66:1159–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Carpio A, Fleury A, Hauser WA. Neurocysticercosis: five new things. Neurol Clin Pract 2013;3:118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Del Brutto OH, Nash TE, White AC Jr, et al. Revised diagnostic criteria for neurocysticercosis. J Neurol Sci 2017;372:202–210. [DOI] [PubMed] [Google Scholar]
- 66. Del Brutto OH, Roman GC, Sotelo J. Neurocysticercosis. Abingdon, UK: Taylor & Francis, 1998. [Google Scholar]
- 67. Mahanty S, Orrego MA, Cangalaya C, et al. TNF‐alpha blockade suppresses pericystic inflammation following anthelmintic treatment in porcine neurocysticercosis. PLoS Negl Trop Dis 2017;11:e0006059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Liu P, Weng X, Zhou J, et al. Next generation sequencing based pathogen analysis in a patient with neurocysticercosis: a case report. BMC Infect Dis 2018;18:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fan S, Qiao X, Liu L, et al. Next‐generation sequencing of cerebrospinal fluid for the diagnosis of neurocysticercosis. Front Neurol 2018;9:471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tihan T. Pathologic approach to spinal cord infections. Neuroimaging Clin N Am 2015;25:163–172. [DOI] [PubMed] [Google Scholar]
- 71. Najem CE, Springer J, Prayson R, et al. Intra cranial granulomatous disease in common variable immunodeficiency: case series and review of the literature. Semin Arthritis Rheum 2018;47:890–896. [DOI] [PubMed] [Google Scholar]
- 72. Prayson RA, Cohen ML. Granulomatous inflammation In: Prayson RA, Cohen ML, eds. Practical differential diagnosis in surgical neuropathology. Totowa, NJ: Humana Press, 2000:161–164. [Google Scholar]
- 73. Walker DH, Okiye G. Chronic granulomatous disease involving the central nervous system. Pediatr Pathol 1983;1:159–167. [DOI] [PubMed] [Google Scholar]
- 74. Jasti DB, Naveen Prasad SV, Naveen T, Vengamma B. Kikuchi‐Fujimoto disease presenting as brainstem encephalitis with secondary blepharospasm. J Neurosci Rural Pract 2016;7:157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kupersmith MJ, Martin V, Heller G, et al. Idiopathic hypertrophic pachymeningitis. Neurology 2004;62:686–694. [DOI] [PubMed] [Google Scholar]
- 76. Tosa M, Hara M, Morita A, et al. Idiopathic hypertrophic spinal pachymeningitis. Intern Med 2015;54:1923–1926. [DOI] [PubMed] [Google Scholar]
- 77. Grois N, Prayer D, Prosch H, et al. Neuropathology of CNS disease in Langerhans cell histiocytosis. Brain 2005;128(pt 4):829–838. [DOI] [PubMed] [Google Scholar]
- 78. Mazor RD, Manevich‐Mazor M, Shoenfeld Y. Erdheim‐Chester disease: a comprehensive review of the literature. Orphanet J Rare Dis 2013;8:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Liu H, Chen J, Yu D, Hu J. Lymphomatoid granulomatosis involving the central nervous system: a case report and review of the literature. Oncol Lett 2014;7:1843–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Table


