Abstract
Background:
Early initiation of alcohol drinking has been associated with increased risk of alcohol dependence in adulthood. Although negative affect mediated in part by corticotropin-releasing factor (CRF) is a strong motivator for alcohol consumption in adults, comparisons of alcohol withdrawal in adolescents and adults generally have not included CRF-related measures like anxiety. The purpose of the present study was to compare withdrawal signs including anxiety-like behavior after a brief multi-day alcohol treatment in adolescent and adult male and female rats.
Methods:
Animals were treated with a 5-day regimen of alcohol injections (3 daily i.p. injections of 1.5 g/kg at 3-hour intervals, total of 15) starting on PN 28 or PN 70. Spontaneous withdrawal signs and anxiety-like behavior (light/dark box) were assessed 18 hours after the last injection as described. One cohort of rats was treated with alcohol, killed 18 hours after the last injection, and blood collected to assess corticosterone. Another cohort of rats was treated with alcohol or vehicle, given 1, 2 or 3 alcohol injections (1.5 g/kg) and killed 1 hour after final injection to determine blood alcohol concentration (BAC). Finally, adult and adolescent males and females received 5 days of alcohol or vehicle treatment followed by a final challenge with alcohol (3 g/kg), and blood was collected for corticosterone.
Results:
BAC was comparable in adolescents and adults. Spontaneous withdrawal signs were comparable in adolescents and adults, and no sex differences were observed. Anxiety-like behaviors (time and distance in light, latency to emerge and light entries) differed in alcohol- and vehicle-treated adults but not adolescents. Corticosterone was not elevated at withdrawal. Alcohol increased corticosterone significantly in vehicle-treated animals, but both adolescents and adults were tolerant to alcohol-induced elevation of corticosterone after 5 days of alcohol treatment.
Conclusions:
These findings suggest that adolescents experience milder negative affect during withdrawal from brief alcohol exposures relative to adults but comparable suppression of HPA axis function.
Keywords: adolescence, withdrawal, anxiety, sex differences
Introduction
Onset of alcohol use during the teenage years predicts higher levels of adult alcohol abuse, especially in individuals with genetic and environmental vulnerabilities (Hingson and Zha, 2009, Windle et al., 2008). Both men and women who begin drinking alcohol early in adolescence have higher levels of adult alcohol abuse and dependence than those who start in adulthood (Pitkanen et al., 2005, Livingston and Room, 2009, Clark et al., 1998). Similarly, compared to adults, adolescent rodents drink more alcohol, either under conditions of single bottle consumption or choice drinking (Doremus et al., 2005, Vetter et al., 2007, Wills et al., 2008, Schramm-Sapyta et al., 2008). Understanding the factors which motivate alcohol consumption during adolescence may facilitate the development of effective treatments of alcohol abuse during adolescence and prevent the development of persisting alcohol dependence in adulthood. Our lab and others have shown that the acute aversive effects of alcohol are less in adolescent rodents than adults (Schramm-Sapyta et al., 2010, Schramm-Sapyta et al., 2014, Anderson et al., 2010). However, the intensity of the aversive effects of repeated alcohol exposure such as the withdrawal syndrome have not been well characterized in adolescents. One particular gap in our understanding is the potential role played by corticotropin-releasing factor (CRF) and corticosterone.
Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis is thought to mediate the negative affect component of withdrawal, and contribute to relapse and continued consumption (Tunstall et al., 2017, Koob et al., 2014, Blaine and Sinha, 2017). Both acute alcohol exposure and acute withdrawal activate the HPA axis and production of glucocorticoids. There is a growing appreciation that preventing alcohol withdrawal with glucocorticoid- or CRF-targeted strategies to block the aversive effects of protracted abstinence may be useful in adults (Zorrilla et al., 2013, Vendruscolo et al., 2015). However, as the responsiveness of the HPA axis to stressors like alcohol withdrawal changes from adolescence to adulthood (Romeo et al., 2016), it is difficult to predict efficacy of these different targets in adolescent alcohol users.
The main purpose of the present study was to evaluate the negative affect experienced during alcohol withdrawal in adolescent and adult rats to determine whether negative affect represents an appropriate therapeutic target in adolescent alcohol abusers. Most published studies of withdrawal after adolescent exposure involved treatments that continue to adulthood and withdrawal that was tested in adulthood. While these studies achieve the goal of understanding the persevering effects of adolescent alcohol exposure, they do not provide insight into the process of withdrawal in adolescence, the most vulnerable time for initiation of alcohol intake. We used a model of brief but intense alcohol exposure in which adolescents and adults were exposed to the same alcohol regimen and achieved comparable blood alcohol concentrations (BAC). In this paradigm younger animals remained within the adolescent developmental window during withdrawal testing. We evaluated acute withdrawal signs and corticosterone secretion during withdrawal, as both have been proposed to contribute to negative affect during withdrawal. Finally, as glucocorticoid secretion has been proposed to contribute to alcohol withdrawal intensity (Sharrett-Field et al., 2013, Strong et al., 2009, Vendruscolo et al., 2012, Vendruscolo et al., 2015), we evaluated both basal corticosterone during early withdrawal, and alcohol-stimulated corticosterone secretion in vehicle- and alcohol-treated animals.
Materials and Methods
Experimental subjects
Adolescent (PN 28–30) or adult (PN 70–73) Sprague-Dawley rats were received from Charles River Laboratories (Raleigh NC) on PN 21 and PN 63 respectively, and group-housed in a 12/12-hour light/dark cycle with free access to water and standard lab chow. All treatments were approved by Duke University’s Institutional Animal Care and Use Committee.
Repeated alcohol treatment
Dosing occurred over 5 days for a total of 15 doses (Figure 1). Animals received intraperitoneal injections at approximately 1000, 1300, and 1600 hours. Experimental animals received 1.5 g/kg doses of alcohol (14.25% v/v alcohol in deionized water mixture). Control animals received a vehicle (0.9% saline) injection of a similar volume at each of these time points. Treatment duration and alcohol dose were selected based on previous studies which induced dependence in a relatively short time frame (Zhang et al., 2007).
Figure 1.
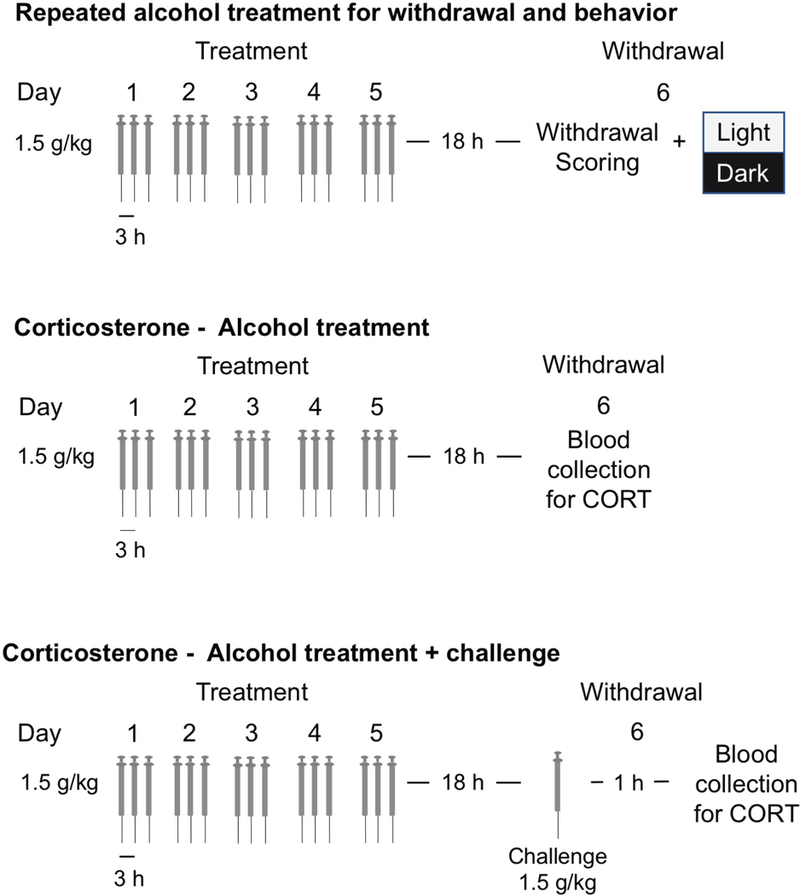
Schematic of Alcohol injection paradigms
Spontaneous withdrawal testing
Spontaneous behaviors during withdrawal were measured and scored by an experimenter blind to drug treatments 18 hours after the final dose of alcohol or vehicle. This time point was selected as the middle of the time period when animals demonstrate maximal spontaneous withdrawal across varying routes of administration (Macey et al., 1996). Behaviors scored included tail rigidity, vocalizations when handled, abnormal posture and/or gait, and tremor (including “wet dog shakes”). Scores are taken on a 0–2-point scale (0 - no sign, 1 - moderately severe, 2 - severe) for a total of 8 possible points.
Light/dark box anxiety testing
Anxiety-like behavior was measured 18 hours after the final dose via light/dark box testing. This test was performed with Kinder Inc. activity monitors (40 × 40 × 40 cm) fitted with “dark” IR transparent inserts (20 × 40 × 40 cm). The room was illuminated by incandescent lamps, resulting in 85–125 lx on the light side and 0–2 lx on the dark side. A small opening allowed free movement between the two sides. The floor of both halves of the box was covered with corncob bedding. Each rat was initially placed in the dark section and was monitored for 15 minutes in the box, with free choice to move between and within sections of the box.
Anxiety-related measures (including time spent in the light compartment, distance traveled in the light compartment, latency to enter the light compartment, and number of light side entries) and locomotion measures were determined using position and movement data collected during light-box dark testing. Immediately after light/dark box testing, animals were euthanized.
Corticosterone measurement
Sera from trunk blood samples were used for analysis of circulating levels of corticosterone via radioimmunoassay (Coat-A-Count, Siemens Medical Solutions Diagnostics, Los Angeles, CA) as previously reported (Schramm-Sapyta et al., 2008). To assess stress-induced HPA axis activation during withdrawal, trunk blood was collected from adolescent and adult males and females 18 hours after the end of alcohol treatment and plasma corticosterone determined. Slightly more alcohol-treated and females were run in anticipation of variance due to changes elicited by alcohol and potential estrous cycle effects that were not observed. To evaluate further the influence of alcohol treatment on HPA axis function, we treated cohorts of adolescent and adult male and female rats with vehicle or alcohol according to the described protocol. Animals were killed one hour after the last injection, and blood collected for determination of corticosterone
Blood alcohol concentration (BAC)
To determine BAC following acute administration by injection, adult and adolescent rats were injected intraperitoneally (14.25% v/v alcohol in deionized water mixture) at a dose of 1.5 g/kg. Three separate experimental groups were used: one group received just one injection; the second group received two injections with three hours between injections; and the third group received three injections, also with three hours between injections. This timeframe mirrored the injection administration paradigm described above. Trunk blood was collected from each experimental group one hour following the final injection they received. This timing captures BAC peaks following each of the three daily injections delivered in our experiment. For blood collection, animals were deeply anesthetized via isoflurane and immediately sacrificed. Trunk blood was collected, and serum was removed from these samples for BAC analysis. BAC analysis was performed with an Analox GL-6 analyzer.
Body weight
Animals were weighed daily, and body weight assessed by repeated measures ANOVA. Body weight varied by age and sex but there was no effect of alcohol treatment in adults or adolescents (data not shown).
Statistics
Data were analyzed by 3-way ANOVA (age, sex and treatment as factors) using NCSS software (NCSS, Kaysville, UT). Interactions were further analyzed by follow-up lower level ANOVAs and post-hoc Fisher’s LSD test for multiple comparisons.
Results
Blood alcohol concentration (BAC)
BAC 1 hour after 1, 2 or 3 injections in adolescent and adult male and female rats is shown in Table 1 Results reported as g/dl ± SEM. N = 6–10 for adolescents, 10–20 for adults. ANOVA indicated a significant effect of sex (F (1,124) = 9.02, p < 0.003), and injection (F (1,124) = 10.8, p < 0.001) with gradually incrementing levels across the 3 injections and a marginal effect of age (F (1,124)= 4.14, p < 0.044). Females had significantly higher BAC than males, and BAC after injection 3 was higher than after injections 1 and 2 by post-hoc test. No significant effect of age, or interactions among these terms were observed.
Table 1.
Blood Alcohol Concentration in Adolescent and Adult Males and Females after 1, 2 or 3 Alcohol Injections
 |
Spontaneous withdrawal at 18 hours
Spontaneous withdrawal signs were measured as described in Methods 18 hours after the last alcohol dose in adults and adolescents (Figure 2). Alcohol withdrawal was associated with modest but significant withdrawal signs in both adults and adolescents, and no sex differences were observed. Alcohol treatment had a significant effect on observed spontaneous withdrawal (F (1,119) = 106, p < 0.001) and there was a significant effect of age (F = 4.52, p < 0.035), showing lower values from both controls and alcohol-treated adolescents compared to adults. No main effect of sex or interaction with sex was observed.
Figure 2.
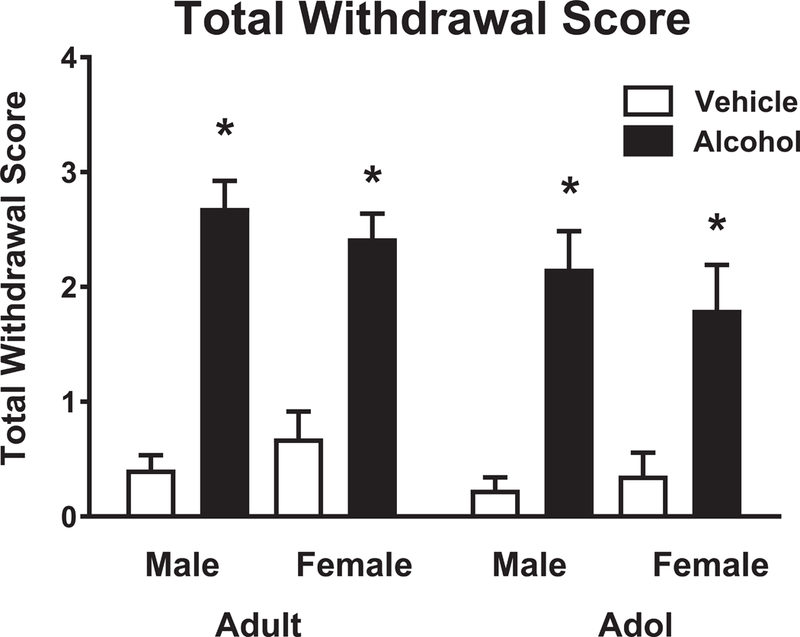
Total withdrawal scores in adults and adolescents 18 hours after withdrawal from alcohol. Results are expressed as mean ± SEM. N = 22–32 for adults, 15–19 for adolescents. White columns = vehicle, solid columns = alcohol. * indicates p < .05 or better relative to vehicle. * indicates p < .05 or better relative to vehicle by Fisher’s LSD post hoc test.
Anxiety measures
Time in light and latency to enter light were used as measures in the light/dark test to best reflect anxiety-like behavior in adolescent and adult rats (Arrant et al., 2013c). More anxious animals are expected to spend less time in light and have longer latencies to enter the light side of the box. For time in light (Figure 3a), ANOVA indicated an overall effect of treatment (F (1,164) = 12.41, p < 0.001) and age (F = 218.02, p < .00001). Lower level ANOVA indicated that in adult rats, 5 days of alcohol treatment resulted in less time in light relative to vehicle-treated controls (F (1, 70) = 7.94, p < 0.01), with no effect of sex and no interactions. In adolescents, no significant treatment effects were observed. For latency (Figure 3b), ANOVA indicated a significant effect of treatment (F (1,164) = 120.4, p < 0.0001), and age (F = 52.27, p < 0.00001), age x treatment interaction (F = 6.18, p < 0.013). Adolescents exhibited shorter latencies to emerge than adults in both vehicle- and alcohol-treated groups. Lower level ANOVA demonstrated a significant effect of treatment (F (1,70) =13.798, p < 0.004) but not sex in adults. There was no interaction between sex and treatment. In adolescents, ANOVA indicated an effect of treatment (F (1, 94) = 4.08, p < 0.05), with treated adolescents exhibiting longer latencies to emerge than controls, but no effect of sex.
Figure 3.

Light-dark data during alcohol withdrawal. Results are expressed as mean ± SEM. N = 23–27 for adults, 16–24 for adolescents. White columns = vehicle, black columns = alcohol. * indicates p < .05 or better relative to vehicle, age-matched control by Fisher’s LSD test in lower level ANOVA. Bracket = groups significantly different by Fisher’s LSD test. a = Time in light, b = latency to emerge into light (seconds), c = distance in light, d = light entries. N = 15 for adult male vehicle, 24 for adult male alcohol, 16 for adult female vehicle, 23 for adult female alcohol, 24 for adolescent male vehicle, 27 for adolescent male alcohol, 16 for adolescent female vehicle, 19 for adolescent female alcohol.
Two additional measures of anxiety-like behavior, distance in light and entries into light showed a similar pattern of treatment and age effects. For distance in light (Figure 3c), ANOVA showed a significant effect of treatment (F (1,164) = 6.74, p < 0.01) and a significant effect of age (F =15.22, p < 0.00001) but no interactions. Lower order ANOVA indicated that for adults, there was a significant effect of treatment with treated adults locomoting less in the light than controls (F (1,70) = 9.040, p <0.003) and a significant effect of sex (F = 6.72, p < 0.011), with females showing more locomotion, but no interaction of sex and treatment. For adolescents, there was no significant effect of treatment or sex. ANOVA for light entries (Figure 3d) indicated a significant effect of treatment (F (1,164) =19.03, p < 0.00021) and age (F = 18.71, p < 0.0003) and age x treatment interaction (F(1,164) = 5.14, p < 0.02), but no effect of sex. Lower level ANOVA indicated that for adults, there was a significant effect of treatment (F(1, 70) = 14–03, p < 0.0003) but no effect of sex, and no interaction between sex and treatment. For adolescents, ANOVA indicated no significant effects of treatment or sex.
Basal corticosterone: 18 hours withdrawal
Basal corticosterone was measured 18 hours after withdrawal (Figure 4). Adult females had higher corticosterone than males, but corticosterone was not elevated in alcohol-treated animals relative to vehicle-treated animals. ANOVA revealed a significant effect of sex (F (1,94 = 8.41, p < 0.005), but no effects of age or treatment, and no interactions among terms.
Figure 4.
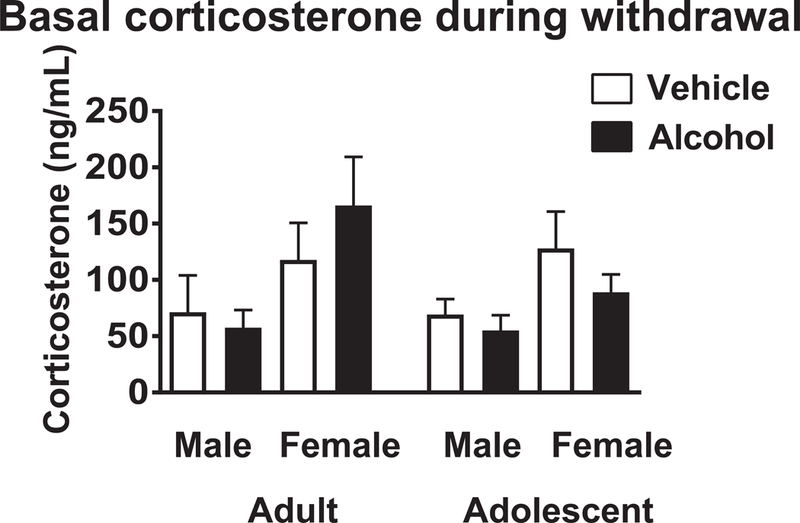
Corticosterone 18 hours after withdrawal from 5 days of vehicle or alcohol. White columns = vehicle, black columns = alcohol. * indicates p < .05 relative to vehicle, age-matched control by Fisher’s LSD test. N = 9 for adult male vehicle, 15 for adult male alcohol, 10 for adult female vehicle, 17 for adult female alcohol, 9 for adolescent male vehicle, 14 for adolescent male alcohol, 8 for adolescent female vehicle, 13 for adolescent female alcohol.
Corticosterone challenge: 18 hours withdrawal
Eighteen hours after the final injection of the 5-day treatment paradigm, animals received a challenge injection of alcohol (3 g/kg) and blood was collected 1 h later (Figure 5). N’s were slightly larger in vehicle-treated groups than alcohol-treated groups, as pilot studies indicated that the alcohol response in dependent animals was highly suppressed, and we could use fewer experimental animals to test our hypothesis. This alcohol challenge resulted in high corticosterone levels in vehicle-treated animals, but levels indistinguishable from the basal levels reported in Figure 6 in animals that had received 5 days of alcohol treatment. ANOVA revealed a significant effect of treatment (F (1,75) = 58.9, p < 0.001) but no effects of age or sex, and no interactions among terms.
Figure 5.

Final day alcohol challenge after 5 days of vehicle or alcohol. White columns = vehicle, black columns = alcohol. * indicates p < .05 relative to vehicle, age-matched control by Fisher’s LSD test. N = 14 for adult male vehicle, 6 for adult male alcohol, 6 for adult female vehicle, 17 for adult female alcohol, 12 for adolescent male vehicle, 8 for adolescent male alcohol, 12 for adolescent female vehicle, 5 for adolescent female alcohol. Results reported as g/dl ± SEM. N = 6–10 for adolescents, 10–20 for adults
Discussion
The main finding of this study is that brief alcohol exposure results in comparable acute spontaneous withdrawal signs in both adolescents and adults, while light/dark box-measured anxiety-like behavior was significantly less in adolescents than adults 18 hours after alcohol treatment. Both adolescents and adults exhibited profound tolerance to alcohol-induced corticosterone secretion after this brief exposure. The only sex difference observed was a significant effect on distance in light in the light/dark test (but no interaction with treatment). These results suggest that the CRF-mediated withdrawal syndrome thought to motivate craving, relapse and escalated alcohol consumption in alcohol-dependent adults (Koob, 2014) may be less intense during early chronic use in adolescents.
The risk of developing alcohol dependence is significantly related to early onset of alcohol consumption (Spear and Swartzwelder, 2014). Additionally, a rapidly expanding body of research shows that binge alcohol consumption throughout adolescence has persevering effects on brain and behavior (Crews et al., 2016, Spear, 2015). Despite these risks, the behavioral and neurobiological mechanisms which mediate the enhanced binge alcohol consumption typical of adolescence are not well understood. In adults, the rewarding effects of alcohol are thought to be important during initiation of alcohol use. For those who progress to elevated levels of consumption, growing alcohol tolerance motivates increased drinking. In the subset of adults who progress to high levels of consumption, aversive consequences of withdrawal play an increasing role in consumption (Koob, 2013). The goal of the present study was to investigate the latter mechanisms, which may be mediated in part by enhanced release of CRF, dynorphin, glucocorticoids and perhaps other neuromodulators during alcohol withdrawal (Koob, 2013).
Blood alcohol concentration (BAC) 1 hour after each of the three injections did not differ by age. These findings are consistent with other studies in which alcohol was delivered by intraperitoneal injection (Varlinskaya and Spear, 2002) but contrast with previous results showing that adolescents achieve slightly lower BAC after intragastric delivery (Morris et al., 2010). The injection route was selected for the present study to determine effects resulting from comparable alcohol exposure in adults and adolescents. Previous studies utilizing either intragastric administration or administration in a liquid diet produced significantly different alcohol exposures in adolescents and adults (Morris et al., 2010, Harper et al., 2017).
Withdrawal signs in alcohol-treated animals were mild, consistent with previous work using higher-intensity alcohol exposure, but statistically significant compared to withdrawal signs in controls. Withdrawal signs were comparable in adolescents and adults, a result consistent with other studies that have demonstrated that adolescent rats exhibit acute withdrawal comparable to adults so long as they are treated with enough alcohol to achieve comparable blood alcohol concentrations (Majchrowicz, 1975, Morris et al., 2010).
The absence of robust sex differences in any of the withdrawal measures were consistent with the literature if BACs are considered. Alcohol-withdrawal seizures are milder in female than in male rats (Reilly et al., 2009, Alele and Devaud, 2007), and comparable anxiety responses have been reported after repeated withdrawal from alcohol delivered in a liquid diet (Overstreet et al., 2004). As females in the present study experienced slightly higher BAC values, the comparable spontaneous withdrawal and anxiety-like behavior observed are consistent with the former studies. Alcohol-induced corticosterone elevation was comparable in males and females. This result was somewhat surprising, as female rodents are reported to exhibit higher HPA responses to acute alcohol treatment (Rivier, 1993) as they do to many stressful stimuli relative to males (Handa et al., 1994). One possible explanation is that the high-dose alcohol paradigm we used precluded sufficient sensitivity to detect sex differences in alcohol-induced corticosterone elevation.
The significant age differences in alcohol withdrawal effects on the measures of anxiety-like behavior in the light/dark box test contrasted with above-cited findings for spontaneous withdrawal. Alcohol-treated adults spent less time in the open field, exhibited significantly longer latency to emerge into the open field, traveled less distance in the open field and made fewer light entries than vehicle-treated adults. Compared to controls, alcohol-treated adolescents only demonstrated a significant difference in latency to enter the light side of the box relative to controls, and this difference was less robust than that observed for adults. Similar findings have been reported recently by Lee et al., who showed that adolescent mice that consumed alcohol (drinking in the dark for 2 hours a day for 2 weeks) exhibited less marble-burying at the end of treatment, while adults showed increased marble burying after the same duration of alcohol consumption (Lee et al., 2016). However, conflicting outcomes have been reported by others. Spear’s group found that social interaction was decreased comparably at both ages after chronic intermittent alcohol exposure (4 g/kg every 48 hours for 10 days) during adolescence and adulthood (Broadwater et al., 2011). Adolescents and adults that consumed a liquid diet containing alcohol showed similar decrements in social interaction (Wills et al., 2008) and more withdrawal anxiety in adolescents than adults (Harper et al., 2017).
There are several potential explanations for the discrepancies between the present studies and these previous studies. In the present study, we employed a brief treatment window to assure that adolescent animals remained within the window of adolescence at the time of testing. Studies showing comparable withdrawal in adolescents and adults involved treatment that was intermittent and longer, allowing more time for adaptation and necessitating that animals were tested at a later developmental stage. In the liquid diet studies, adolescents consumed more alcohol and achieved higher blood alcohol concentrations than adults, and withdrawal effects were only significant after multiple cycles, as adolescents approached adulthood (Harper et al., 2017). In addition, the social interaction task likely taps into different behavioral domains than the light/dark task (File, 1990).
The demonstration of more robust anxiety-like behavior in the light/dark task in alcohol-withdrawn adults than adolescents may reflect immaturity in the neural circuits related to risk assessment and avoidance behavior. Other studies from our laboratory have shown that adolescent rats exhibit substantially less anxiety-like behavior in this task after a range of pharmacological stimuli including a serotonergic agonist, the alpha 2- adrenergic agonist yohimbine and the inverse benzodiazepine agonist beta carboline (Arrant et al., 2013a, Arrant et al., 2013b, Arrant et al., 2013c). It is possible that immaturity in amygdala→cortical connections during early adolescence mediates this age-difference in anxiety-like responding. A recent finding that glutamate receptor adaptations in the nucleus accumbens are present in adult but not adolescent mice after 2 weeks of alcohol consumption further implicate the amygdala and cortical projections to the nucleus accumbens that are thought to mediate withdrawal-related negative affect and escalation of alcohol consumption (Lee et al., 2016, Gilpin et al., 2015). Substantial research shows that adolescent rodents are considerably less sensitive to the acute effects of alcohol (Spear and Varlinskaya, 2005). The same phenomenon may influence behavioral responses during alcohol withdrawal in young adolescents.
Glucocorticoids such as corticosterone may enhance CRF release in the extended amygdala, and thus contribute to alcohol withdrawal-related behaviors (Koob, 2013, Zorrilla et al., 2014, Gilpin et al., 2015, Vendruscolo et al., 2012). To probe the potential role of the HPA axis in the lower sensitivity of adolescents to anxiety-like behavior during alcohol withdrawal, we evaluated alcohol-induced corticosterone release in alcohol-naïve and dependent rats. Alcohol evoked robust and comparable increases in blood corticosterone levels in adolescents and adults that were absent in both adolescents and adults after 5 days of treatment. Withdrawal-induced increases in corticosterone were not significant in either adolescent and adults. Therefore, the different withdrawal-induced anxiety responses in adolescents and adults do not likely reflect differences in HPA axis function. These findings are concordant with a literature showing stimulation after acute alcohol but suppression of the HPA axis in adult rats after chronic treatment (Lee and Rivier, 1997, Richardson et al., 2008). One study in adolescent rats demonstrated that corticosterone responses to alcohol even remained suppressed in adults that were exposed to alcohol as adolescents (Allen et al., 2016). However, the predictive value of blood glucocorticoid levels in evaluating alcohol dependence is unclear, as both excessive cortisol release and suppressed cortisol release have been reported in withdrawing alcoholics or heavy drinking populations (Groote Veldman and Meinders, 1996, Stalder et al., 2010). Glucocorticoid potentiation may be important during extreme stress when glucocorticoid levels are high, during the protracted stage of withdrawal during which CRF mechanisms are important (Koob and Volkow, 2010, Zorrilla et al., 2014), or after a robust alcohol exposure that stimulates the HPA axis adequately to change expression of glucocorticoid-mediated genes (Repunte-Canonigo et al., 2015, Edwards et al., 2015).
There are several aspects of the experimental design which must be considered in interpreting the present data. The degree of alcohol exposure was high and comparable in adults and adolescents, but brief. While the route of administration provided good control of BAC, it did not simulate human ingestion of alcohol. Additionally, this approach did not provide information about protracted abstinence or adult consequences following adolescent exposure. Future studies with increasing duration of exposure and a dose-response approach to exposure will be needed to provide ideal characterization of protracted abstinence in adolescents.
In summary, the present findings show that, compared to adults, adolescent rats experience less withdrawal-related anxiety behavior. One question for future studies raised by these results is whether the enhanced voluntary consumption in adolescence is mediated by enhanced reinforcing effects of alcohol and/or diminished sensitivity to the aversive effects of both alcohol intoxication and its withdrawal. These answers would have significant implications for the development of appropriate strategies to treat adolescent alcohol dependence. The brief alcohol treatment and withdrawal time frame used in the present study to guarantee evaluation during adolescence raises the caveat that these conclusions may not hold true for more extensive alcohol exposures during adolescence.
Acknowledgments
Support: This paper was supported by grant # AA017621 from the National Institute of Alcohol Abuse and Alcoholism
References
- ALELE PE & DEVAUD LL 2007. Sex differences in steroid modulation of ethanol withdrawal in male and female rats. J Pharmacol Exp Ther, 320, 427–36. [DOI] [PubMed] [Google Scholar]
- ALLEN CD, GRIGOLEIT JS, HONG J, BAE S, VAUGHAN J & LEE S 2016. Exposure to alcohol during adolescence exerts long-term effects on stress response and the adult brain stress circuits. Neuroscience, 339, 64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDERSON RI, VARLINSKAYA EI & SPEAR LP 2010. Ethanol-induced conditioned taste aversion in male sprague-dawley rats: impact of age and stress. Alcohol Clin Exp Res, 34, 2106–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARRANT AE, COBURN E, JACOBSEN J & KUHN CM 2013a. Lower anxiogenic effects of serotonin agonists are associated with lower activation of amygdala and lateral orbital cortex in adolescent male rats. Neuropharmacology, 73, 359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARRANT AE, JEMAL H & KUHN CM 2013b. Adolescent male rats are less sensitive than adults to the anxiogenic and serotonin-releasing effects of fenfluramine. Neuropharmacology, 65, 213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARRANT AE, SCHRAMM-SAPYTA NL & KUHN CM 2013c. Use of the light/dark test for anxiety in adult and adolescent male rats. Behav Brain Res, 256, 119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLAINE SK & SINHA R 2017. Alcohol, stress, and glucocorticoids: From risk to dependence and relapse in alcohol use disorders. Neuropharmacology, 122, 136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROADWATER M, VARLINSKAYA EI & SPEAR LP 2011. Chronic intermittent ethanol exposure in early adolescent and adult male rats: effects on tolerance, social behavior, and ethanol intake. Alcohol Clin Exp Res, 35, 1392–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARK DB, KIRISCI L & TARTER RE 1998. Adolescent versus adult onset and the development of substance use disorders in males. Drug Alcohol Depend, 49, 115–21. [DOI] [PubMed] [Google Scholar]
- CREWS FT, VETRENO RP, BROADWATER MA & ROBINSON DL 2016. Adolescent Alcohol Exposure Persistently Impacts Adult Neurobiology and Behavior. Pharmacol Rev, 68, 1074–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOREMUS TL, BRUNELL SC, RAJENDRAN P & SPEAR LP 2005. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res, 29, 1796–808. [DOI] [PubMed] [Google Scholar]
- EDWARDS S, LITTLE HJ, RICHARDSON HN & VENDRUSCOLO LF 2015. Divergent regulation of distinct glucocorticoid systems in alcohol dependence. Alcohol, 49, 811–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FILE SE 1990. New strategies in the search for anxiolytics. Drug Des Deliv, 5, 195–201. [PubMed] [Google Scholar]
- GILPIN NW, HERMAN MA & ROBERTO M 2015. The central amygdala as an integrative hub for anxiety and alcohol use disorders. Biol Psychiatry, 77, 859–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROOTE VELDMAN R & MEINDERS AE 1996. On the mechanism of alcohol-induced pseudo-Cushing’s syndrome. Endocr Rev, 17, 262–8. [DOI] [PubMed] [Google Scholar]
- HANDA RJ, BURGESS LH, KERR JE & O’KEEFE JA 1994. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav, 28, 464–76. [DOI] [PubMed] [Google Scholar]
- HARPER KM, KNAPP DJ, PARK MA & BREESE GR 2017. Age-related differences in anxiety-like behavior and amygdalar CCL2 responsiveness to stress following alcohol withdrawal in male Wistar rats. Psychopharmacology (Berl), 234, 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HINGSON RW & ZHA W 2009. Age of drinking onset, alcohol use disorders, frequent heavy drinking, and unintentionally injuring oneself and others after drinking. Pediatrics, 123, 1477–84. [DOI] [PubMed] [Google Scholar]
- KOOB GF 2013. Theoretical frameworks and mechanistic aspects of alcohol addiction: alcohol addiction as a reward deficit disorder. Curr Top Behav Neurosci, 13, 3–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOOB GF 2014. Neurocircuitry of alcohol addiction: synthesis from animal models. Handb Clin Neurol, 125, 33–54. [DOI] [PubMed] [Google Scholar]
- KOOB GF, BUCK CL, COHEN A, EDWARDS S, PARK PE, SCHLOSBURG JE, SCHMEICHEL B, VENDRUSCOLO LF, WADE CL, WHITFIELD TW JR. & GEORGE O 2014. Addiction as a stress surfeit disorder. Neuropharmacology, 76 Pt B, 370–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOOB GF & VOLKOW ND 2010. Neurocircuitry of addiction. Neuropsychopharmacology, 35, 217–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE KM, COELHO MA, MCGREGOR HA, SOLTON NR, COHEN M & SZUMLINSKI KK 2016. Adolescent Mice Are Resilient to Alcohol Withdrawal-Induced Anxiety and Changes in Indices of Glutamate Function within the Nucleus Accumbens. Frontiers in Cellular Neuroscience, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE S & RIVIER C 1997. An initial, three-day-long treatment with alcohol induces a long-lasting phenomenon of selective tolerance in the activity of the rat hypothalamic-pituitary-adrenal axis. J Neurosci, 17, 8856–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIVINGSTON M & ROOM R 2009. Variations by age and sex in alcohol-related problematic behaviour per drinking volume and heavier drinking occasion. Drug Alcohol Depend, 101, 169–75. [DOI] [PubMed] [Google Scholar]
- MACEY DJ, SCHULTEIS G, HEINRICHS SC & KOOB GF 1996. Time-dependent quantifiable withdrawal from ethanol in the rat: effect of method of dependence induction. Alcohol, 13, 163–70. [DOI] [PubMed] [Google Scholar]
- MAJCHROWICZ E 1975. Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacologia, 43, 245–54. [DOI] [PubMed] [Google Scholar]
- MORRIS SA, KELSO ML, LIPUT DJ, MARSHALL SA & NIXON K 2010. Similar withdrawal severity in adolescents and adults in a rat model of alcohol dependence. Alcohol, 44, 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OVERSTREET DH, KNAPP DJ & BREESE GR 2004. Similar anxiety-like responses in male and female rats exposed to repeated withdrawals from ethanol. Pharmacol Biochem Behav, 78, 459–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PITKANEN T, LYYRA AL & PULKKINEN L 2005. Age of onset of drinking and the use of alcohol in adulthood: a follow-up study from age 8–42 for females and males. Addiction, 100, 652–61. [DOI] [PubMed] [Google Scholar]
- REILLY W, KOIRALA B & DEVAUD LL 2009. Sex differences in acoustic startle responses and seizure thresholds between ethanol-withdrawn male and female rats. Alcohol Alcohol, 44, 561–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REPUNTE-CANONIGO V, SHIN W, VENDRUSCOLO LF, LEFEBVRE C, VAN DER STAP L, KAWAMURA T, SCHLOSBURG JE, ALVAREZ M, KOOB GF, CALIFANO A & SANNA PP 2015. Identifying candidate drivers of alcohol dependence-induced excessive drinking by assembly and interrogation of brain-specific regulatory networks. Genome Biol, 16, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDSON HN, LEE SY, O’DELL LE, KOOB GF & RIVIER CL 2008. Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. Eur J Neurosci, 28, 1641–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIVIER C 1993. Female rats release more corticosterone than males in response to alcohol: influence of circulating sex steroids and possible consequences for blood alcohol levels. Alcohol Clin Exp Res, 17, 854–9. [DOI] [PubMed] [Google Scholar]
- ROMEO RD, PATEL R, PHAM L & SO VM 2016. Adolescence and the ontogeny of the hormonal stress response in male and female rats and mice. Neurosci Biobehav Rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHRAMM-SAPYTA NL, DIFELICEANTONIO AG, FOSCUE E, GLOWACZ S, HASEEB N, WANG N, ZHOU C & KUHN CM 2010. Aversive effects of ethanol in adolescent versus adult rats: potential causes and implication for future drinking. Alcohol Clin Exp Res, 34, 2061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHRAMM-SAPYTA NL, FRANCIS R, MACDONALD A, KEISTLER C, O’NEILL L & KUHN CM 2014. Effect of sex on ethanol consumption and conditioned taste aversion in adolescent and adult rats. Psychopharmacology (Berl), 231, 1831–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHRAMM-SAPYTA NL, KINGSLEY MA, REZVANI AH, PROPST K, SWARTZWELDER HS & KUHN CM 2008. Early ethanol consumption predicts relapse-like behavior in adolescent male rats. Alcohol Clin Exp Res, 32, 754–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHARRETT-FIELD L, BUTLER TR, BERRY JN, REYNOLDS AR & PRENDERGAST MA 2013. Mifepristone pretreatment reduces ethanol withdrawal severity in vivo. Alcohol Clin Exp Res, 37, 1417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPEAR LP 2015. Adolescent alcohol exposure: Are there separable vulnerable periods within adolescence? Physiol Behav. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPEAR LP & SWARTZWELDER HS 2014. Adolescent alcohol exposure and persistence of adolescent-typical phenotypes into adulthood: a mini-review. Neurosci Biobehav Rev, 45, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPEAR LP & VARLINSKAYA EI 2005. Adolescence. Alcohol sensitivity, tolerance, and intake. Recent Dev Alcohol, 17, 143–59. [PubMed] [Google Scholar]
- STALDER T, KIRSCHBAUM C, HEINZE K, STEUDTE S, FOLEY P, TIETZE A & DETTENBORN L 2010. Use of hair cortisol analysis to detect hypercortisolism during active drinking phases in alcohol-dependent individuals. Biological Psychology, 85, 357–360. [DOI] [PubMed] [Google Scholar]
- STRONG MN, KAUFMAN KR, CRABBE JC & FINN DA 2009. Sex differences in acute ethanol withdrawal severity after adrenalectomy and gonadectomy in Withdrawal Seizure-Prone and Withdrawal Seizure-Resistant mice. Alcohol, 43, 367–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TUNSTALL BJ, CARMACK SA, KOOB GF & VENDRUSCOLO LF 2017. Dysregulation of Brain Stress Systems Mediates Compulsive Alcohol Drinking. Curr Opin Behav Sci, 13, 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VARLINSKAYA EI & SPEAR LP 2002. Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcohol Clin Exp Res, 26, 1502–11. [DOI] [PubMed] [Google Scholar]
- VENDRUSCOLO LF, BARBIER E, SCHLOSBURG JE, MISRA KK, WHITFIELD TW JR., LOGRIP ML, RIVIER C, REPUNTE-CANONIGO V, ZORRILLA EP, SANNA PP, HEILIG M & KOOB GF 2012. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci, 32, 7563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VENDRUSCOLO LF, ESTEY D, GOODELL V, MACSHANE LG, LOGRIP ML, SCHLOSBURG JE, MCGINN MA, ZAMORA-MARTINEZ ER, BELANOFF JK, HUNT HJ, SANNA PP, GEORGE O, KOOB GF, EDWARDS S & MASON BJ 2015. Glucocorticoid receptor antagonism decreases alcohol seeking in alcohol-dependent individuals. J Clin Invest, 125, 3193–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VETTER CS, DOREMUS-FITZWATER TL & SPEAR LP 2007. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcohol Clin Exp Res, 31, 1159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLS TA, KNAPP DJ, OVERSTREET DH & BREESE GR 2008. Differential dietary ethanol intake and blood ethanol levels in adolescent and adult rats: effects on anxiety-like behavior and seizure thresholds. Alcohol Clin Exp Res, 32, 1350–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINDLE M, SPEAR LP, FULIGNI AJ, ANGOLD A, BROWN JD, PINE D, SMITH GT, GIEDD J & DAHL RE 2008. Transitions into underage and problem drinking: developmental processes and mechanisms between 10 and 15 years of age. Pediatrics, 121 Suppl 4, S273–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG Z, MORSE AC, KOOB GF & SCHULTEIS G 2007. Dose- and time-dependent expression of anxiety-like behavior in the elevated plus-maze during withdrawal from acute and repeated intermittent ethanol intoxication in rats. Alcohol Clin Exp Res, 31, 1811–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZORRILLA EP, HEILIG M, DE WIT H & SHAHAM Y 2013. Behavioral, biological, and chemical perspectives on targeting CRF(1) receptor antagonists to treat alcoholism. Drug Alcohol Depend, 128, 175–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZORRILLA EP, LOGRIP ML & KOOB GF 2014. Corticotropin releasing factor: A key role in the neurobiology of addiction. Front Neuroendocrinol, 35, 234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]


