Abstract
Purpose:
Obesity may alter mononuclear phagocyte system (MPS) function and the pharmacology and efficacy of nanoparticles therapies, such as PEGylated liposomal doxorubicin (PLD). We aimed to evaluate relationships between hormone and chemokine mediators of MPS function and the pharmacokinetic (PK) exposure of PLD in obese and normal weight patients with ovarian and endometrial cancer.
Methods:
Hormone and chemokine mediators in obese and normal weight ovarian and endometrial cancer patients were measured. A separate pharmacology study was performed that evaluated the relationship between serum hormone concentrations, MPS function, and PK disposition of PLD in refractory ovarian cancer patients.
Results:
Univariate analysis revealed a significant relationship between serum estradiol and body mass index (OR: 8.64, 95% CI: 2.67–28.0, p<0.001). Estrone and testosterone concentrations were positively correlated with MPS function (rho=0.57 and 0.53, p=0.14 and 0.18, respectively) and inversely correlated with PLD PK exposure (rho= −0.75 and −0.76, respectively, p=0.02 for both).
Conclusions:
Higher MPS function resulting in reduced PLD exposure is a potential mechanism for reduced efficacy of PLD and other nanoparticles observed in obese patients with cancer. PK simulations suggest higher doses of PLD are required in obese patients to achieve similar exposures as standard dosing in normal weight patients.
Keywords: Nanoparticle, Pharmacology, Obesity, Ovarian cancer, Endometrial cancer, Estradiol
Introduction
Nanotechnology offers a number of advantages over traditional drug delivery systems for the treatment of solid tumors [1,2]. Advantages of nanoparticles (NP) in solid tumor treatment include increased blood circulation time, enhanced delivery of entrapped drug to tumors, improved therapeutic time and a reduction in off target effects [1,2]. The abnormal blood and lymphatic vasculature allow for selective delivery and accumulation of NPs at tumor sites via the enhanced permeability and retention effect (EPR) [3]. Unfortunately, only a limited number of NPs have become clinically successful due to high inter-patient and intra-patient variability in the pharmacokinetics (PK) and pharmacodynamics (PD) of NPs [4,5].
Liposomes, lipid vesicles formed by a lipid bilayer surrounding an aqueous core, are the most common NP drug carriers approved by the FDA [6]. One such carrier approved for use in humans is PEGylated liposomal doxorubicin (PLD, Doxil®). In a meta-analysis of inter-patient variability in the PK of liposomal anticancer agents compared to small molecule formulations of the agent, the PK variability of liposomal drugs, measured as coefficient of variance (CV%) of area under the concentration versus time curve (AUC), was significantly greater [5]. This PK variability is significant as it has been associated with high variability in the efficacy and toxicity of NPs [5,7,8].
Bone marrow-derived progenitors, blood monocytes, and tissue macrophages comprise the mononuclear phagocyte system (MPS) [9]. The MPS recognizes, internalizes and eventually clears NPs. The immunological properties of NPs trigger the MPS [10]. Identifying factors to predict the PK and PD of NPs to allow for individualized dosing may further enhance tolerability while preserving or improving efficacy [11]. Associations between the PK variability of NPs and patient age, gender, type of cancer and the function of monocytes in patients with cancer have been previously reported [12–14]. In addition, the effects of body habitus, which is defined as the physical and constitutional characteristics of underweight, normal weight and overweight patients, on NP PK and PD have been evaluated [8,13–16]. In these studies, patients with a higher ratio of total to ideal body weight had increased clearance of PEGylated liposomal agents [15,16]. In addition, the PK variability of NPs and other carrier-mediated agents (CMAs) is even greater in obese patients. The factors affecting the PK of NPs and CMAs in obese patients are unclear but may be attributed to the effects of serum hormones on MPS function and ultimately CMA clearance [17]. This is supported by a series of studies demonstrating that serum hormones, such as estrogens and testosterone, modulate immune system activity and macrophage phagocytic and chemotactic function. Of particular interest are the reports of various estrogens stimulating the phagocytic activity of the MPS in vitro [18–20].
Obesity has reached epidemic proportions in the United States. Based on body mass index (BMI), over 30% of adults are obese and 65% are overweight [21–24]. Obesity has been linked to an increased risk of many cancers, including breast, colon, endometrial, ovarian and others [11]. Currently, there are approximately 1.5 million new cancer cases and half a million cancer-related deaths per year, with nearly one in five associated with obesity [11,25–28]. Obesity has been associated with increased risk and worse outcomes for both endometrial and ovarian cancer, which are both treated with PLD [11,29]. We hypothesize that obesity and obesity-related factors alter the PK and PD of NPs, such as PLD. Specifically, obese patients will have a higher distribution of NPs to adipose tissue and higher circulating levels of serum hormones and chemokines which lead to higher MPS function, higher NP clearance, and ultimately reduced NP tumor exposure. These hormone and chemokine mediators of MPS function and NP PK and PD have not been extensively evaluated in patients with cancer and especially not as related to body habitus. To test this hypothesis, we measured hormone and chemokine mediators in existing blood samples from obese and normal weight patients with ovarian and endometrial cancer enrolled on the UNC Health Registry/Cancer Survivorship Cohort (UNC CSC). The UNC CSC is a registry of cancer survivors with available interview data and clinically annotated biospecimens who consent to the use of their data and specimens for future research. Specific aims of this study included profiling hormone and chemokine mediators of MPS function and NP PK in obese (BMI > 30 kg/m2) and normal weight (BMI = 18.5–24.9 kg/m2) patients with ovarian and endometrial cancer. In addition, a separate pharmacology study was performed that evaluated the relationship between serum hormone concentrations, MPS function, and the PK exposure of PLD in patients with refractory ovarian cancer.
Materials & Methods
Exposure of Hormones and Chemokines.
Patients with ovarian and endometrial cancer were selected based on the high incidence of obesity in these patients and the potential lower response to NP therapy in obese compared with normal weight patients [11,29,38–42]. Post-menopausal Caucasian women were selected to maximize the ability to detect differences in hormone and chemokine exposures associated with obesity. Patients were classified as being either obese or normal weight based on BMI. According to the World Health Organization (WHO), BMI, a simple weight to height relationship, is used to classify body habitus in the adult population [43,44]. The resulting value (kg/m2) is used to define underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), and obese (BMI >30 kg/m2) people. Following these guidelines, our study defined obesity as a BMI >30 kg/m2 and normal weight as a BMI between 18.5–24.9 kg/m2. Patients that would be classified as overweight were not included.
Patient samples were obtained from the UNC CSC repository. The Biospecimen Processing Core Facility (BSP) functioned as the processing, biobanking, and repository management core for the UNC CSC. The concentration of estradiol, estrone, DHT and total testosterone was measured in serum (500uL) and the concentration of CCL2 and CCL5 chemokines was measured in plasma (500uL) from each patient at the UNC Cytokine and Biomarker Core Facility [27,28]. Hormones were measured using an existing ELISA assays [12,45]. A multiplex cytokine/chemokine assay (Millipore, Billerica, MA) was performed for quantification of CCL2 and CCL5 according to manufacturer’s protocol.
Pharmacology Studies of PLD in Patients with Ovarian Cancer.
This study was approved by the University of North Carolina IRB prior to enrolling any participants. Ten women ≥ 18 years of age receiving PLD, alone or in combination with carboplatin as the standard treatment for refractory ovarian cancer were enrolled on the study. All patients had previously undergone bilateral salpingo-oophorectomy prior to enrollment. Six patients received PLD monotherapy at a dose of 40 mg/m2 and four patients received PLD at a dose of 30 mg/m2 with concurrent carboplatin (AUC = 5 mg/mL/min).
The concentration of estradiol, estrone, DHT and total testosterone was measured in serum (500uL) and concentration of CCL2 and CCL5 chemokines was measured in plasma (500uL) prior to administration of PLD on cycle 1 [27,28]. The number and function of MPS cells (monocytes and dendritic cells) in blood were measured via flow cytometry in the University of North Carolina Flow Cytometry Core Facility using a Dako CyAn flow cytometer, and data were analyzed using FlowJo software (v7.6.5). For the oxidative burst assay, monocytes were gated based on light scatter properties (forward scatter versus side scatter) and subsequently plotted for histogram analysis. The proportion of positive cells was determined as those events which shifted to the right out of the “negative” region. Mean fluorescent intensity (MFI) of the positive cell population served as an index of oxidative burst. Oxidative burst of monocytes was assessed in response to opsonized non-fluorescent E. coli as a stimulus. Following a 10-minute exposure to the stimulus, non-fluorescent dihydrorhodamine 123 (Orpegen Pharma, San Diego, CA) was added to samples as a fluorogenic substrate, which, following intracellular oxidation was converted to fluorescent rhodamine 123. MFI of rhodamine 123 fluorescence served as a quantitative measure of intracellular oxidative activity. Hormone and chemokine concentrations were compared to MPS function and PLD PK parameters (i.e., clearance and plasma AUC).
Blood samples (5mL) for PK studies were obtained prior to PLD administration, at the end of infusion, and at hours 2, 6, 24, 48, 72, 96, 168, and on day 28 (prior to 2nd cycle of PLD) after administration of PLD. Samples were immediately placed on ice after collection, and centrifuged to plasma within 5 minutes at 4°C. Plasma was stored at 4°C until processed to encapsulated and released doxorubicin using solid phase separation and HPLC with fluorescence detection [12]. PK parameters were calculated using non-compartmental analysis (Phoenix WinNonlin, v6.03).
Statistical Analyses.
In the study in normal weight and obese patients with ovarian or endometrial cancer, correlations between serum hormone and chemokine concentration and BMI were analyzed using univariate and multivariate logistic regression models generated by SAS software (v9.4; SAS Institute Inc., Cary, NC). The concentration of hormones and chemokines were dichotomized at the median to convert them from a continuous to a discrete variable in order to use an odds ratio. Concentrations below the median were considered “low concentration”. Concentrations above the median were considered “high concentration”.
In the pharmacology study in patients with ovarian cancer, relationships between hormones, chemokines, MPS function and PLD PK were analyzed via exponential regression and spearman correlation using SAS.
Results
Exposure of Hormones and Chemokines.
A summary of patient demographics for this study are included in Table 1.The results of the univariate analysis for CCL2, CCL5, estrone, estradiol, total testosterone and dihydrotestosterone (DHT) are listed in Table 2. The odds ratio was significant for the relationship between estradiol and BMI (P=0.0003). The odds of a high concentration of estradiol among obese patients were 8.64-times that among normal weight patients (95% CI 2.67–28.0). A multivariate analysis was then performed in all patients to assess the effect of other factors, including BMI, age at diagnosis, stage and other hormones and chemokines on estradiol concentration. This multivariate analysis revealed a significant relationship between BMI and estradiol concentration (P=0.0023).
Table 1.
Patient demographics, hormone, and chemokine results for obese and non-obese patients with endometrial and ovarian cancer.
| Demographic or Result of Patients from UNC CSC | BMI Obese (n=33)Non-obese (n=28) | All Patients(n=61) | Patients with Endometrial Cancer(n=39) | Patients with Ovarian Cancer(n=22) |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| BMI (kg/m2) | Obese | 41.5 ± 7.2 | 46.2 ± 4.6 | 34.3 ± 3.6 |
| Non-obese | 22.7 ± 1.7 | 22.5 ± 2.0 | 23.1 ± 0.7 | |
| TBW (kg) | Obese | 110.2 ± 22.2 | 122.7 ± 17.8 | 91.0 ± 12.3 |
| Non-obese | 59.3 ± 6.7 | 58.1 ± 7.4 | 61.8 ± 3.9 | |
| BSA (m2) | Obese | 2.12 ± 0.2 | 2.22 ± 0.2 | 1.96 ± 0.2 |
| Non-obese | 1.62 ± 0.1 | 1.60 ± 0.1 | 1.67 ± 0.1 | |
| Estradiol (pg/mL) | Obese | 110.0 ± 73.2 | 126.6 ± 82.0 | 84.6 ± 50.0 |
| Non-obese | 61.7 ± 50.6 | 59.8 ± 47.7 | 65.9 ± 56.0 | |
| Estrone (pg/mL) | Obese | 73.1 ± 28.1 | 75.9 ± 30.4 | 68.6 ± 24.6 |
| Non-obese | 62.1 ± 20.7 | 59.5 ± 19.3 | 68.1 ± 22.5 | |
| Testosterone (pg/mL) | Obese | 4.8 ± 17.0 | 7.05 ± 21.7 | 1.2 ± 0.7 |
| Non-obese | 1.3 ± 1.3 | 1.3 ± 1.4 | 1.1 ± 0.9 | |
| 5-DHT (pg/mL) | Obese | 205.7 ± 141.2 | 237.6 ± 164.7 | 156.7 ± 76.9 |
| Non-obese | 147.0 ± 86.2 | 151.8 ± 92.3 | 136.3 ± 70.6 | |
| CCL2 (pg/mL) | Obese | 178.9 ± 84.4 | 153.9 ± 46.7 | 217.5 ± 113.5 |
| Non-obese | 174.1 ± 86.2 | 140.7 ± 69.7 | 248.4 ± 218.97 | |
| CCL5 (pg/mL) | Obese | 16,313.8 ± 14931.3 | 13,867.8 ± 14,137.6 | 19,770.4 ± 22,206.6 |
| Non-obese | 19,703.3 ± 25,396.7 | 19,673.2 ± 27,255.8 | 20,076.9 ± 15,897.7 |
Table 2.
Odds of high vs. low serum hormone or chemokine concentration based on BMI status (obese vs non-obese) for patients enrolled on the UNC Cancer Survivorship Cohort.
| Hormone, Chemokine* | Odds Ratio (95% CI) | P-value** |
|---|---|---|
| CCL2 | 2.48 (0.87–7.12) | 0.090 |
| CCL5 | 1.07 (0.39–2.98) | 0.895 |
| Estrone | 1.85 (0.66–5.21) | 0.242 |
| Estradiol | 8.64 (2.67–28.0) | < 0.001 |
| Testosterone | 2.14 (0.76–6.06) | 0.152 |
| DHT | 1.85 (0.66–5.21) | 0.242 |
Odds ratio reference point equals 1.0
Concentration of hormone, chemokines were dichotomized at the median.
Univariable logistic regression model
Obese group n=33, non-obese group n=28
The univariate analysis was repeated with obese and normal weight patients stratified by cancer type. In patients with ovarian cancer (13 obese, 9 normal weight), odds ratios were non-significant. In patients with endometrial cancer (20 obese, 19 normal weight), odds ratio for the relationship between serum estradiol and BMI increased, indicating a stronger relationship than that between estradiol and BMI in all patients (e.g. in patients with ovarian or endometrial) (OR 11.20, 95% CI 2.51–50.08, P=0.0016). These results can be found in Tables 2 and 3.
Table 3.
Multivariate Analysis on the effect of covariates on estradiol concentration among obese cancer cases: odds of high concentration of estradiol among obese patients.
| Odds Ratio**(95% CI) | P-Value | |
|---|---|---|
| BMI | 8.33(2.05–33.85) | 0.003 |
| Age at diagnosis | 0.58 (0.15–2.29) | 0.436 |
| Stage 3–4* | 2.53 (0.21–30.32) | 0.463 |
| Stage Unknown* | 0.69 (0.15–3.17) | 0.632 |
| CCL2 | 0.90 (0.18–4.50) | 0.893 |
| CCL5 | 0.65 (0.15–2.71) | 0.550 |
| Estrone | 0.44 (0.07–2.85) | 0.390 |
| Testosterone | 2.78 (0.24–35.51) | 0.416 |
| DHT | 1.62 (0.16–16.81) | 0.688 |
Reference group: Stage 1–2.
Adjusted for site, stage, age at diagnosis, and other hormone/chemokine concentrations.
Concentration of estradiol was dichotomized at the median.
Pharmacology Studies of PLD in Patients with Ovarian Cancer.
Ten patients with refractory ovarian cancer who received PLD as treatment participated in this pharmacology study. Estrogen and DHT concentrations were below the lower limit of quantification (10 pg/ml for estrogen and 50 pg/ml for DHT) for the majority of the patients and thus were not included in the analyses. A summary of the patient demographics, chemokine and hormone levels, and PLD PK for this study are included in Table 4.
Table 4.
Univariate Analysis: Odds of high hormone/chemokine concentration among obese vs. non-obese patients enrolled on Cancer Survivorship Cohort by cancer type.
| Mediator | Endometrial Cancer | Ovarian Cancer | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) | P-Value | Odds Ratio (95% CI) | P-Value | |
| CCL2 | 1.70(0.45–6.36) | 0.433 | 5.63 (0.75–42.36) | 0.094 |
| CCL5 | 0.89 (0.25–3.16) | 0.855 | 1.40 (0.23–8.46) | 0.714 |
| Estrone | 3.25 (0.87–12.13) | 0.080 | 0.714 (0.12–4.32) | 0.714 |
| Estradiol | 11.20 (2.51–50.08) | 0.002 | 5.33 (0.78–36.33) | 0.087 |
| Testosterone | 3.25 (0.87–12.13) | 0.080 | 1.14 (0.18–7.23) | 0.888 |
| DHT | 2.57 (0.71–9.36) | 0.152 | 1.04 (0.18–6.12) | 0.964 |
Odds ratio reference point equals 1.0
Concentration of hormone, chemokines were dichotomized at the median
Univariable logistic regression model
Endometrial cancer: obese group n=20, non-obese group n=19, Ovarian cancer: obese group n=13, non-obese group n=9
We also evaluated the relationship between serum hormone concentrations and MPS function. MPS function in monocytes and DCs in blood was measured as the generation of reactive oxygen species (ROS) [7]. Blood samples for MPS function studies were obtained prior to PLD administration. There was a positive relationship between estrone and testosterone concentrations and the generation of ROS in monocytes and DCs in blood (rho = 0.57 and 0.53, respectively) (Figure 1).
Figure 1. Serum hormone concentration versus baseline monocyte functional activity (E.coli-stimulated oxidative burst).
Individual patient values are represented by the solid squares and the regression is represented by the solid line. There was a the direct relationship between serum hormone concentrations of estrone (A) and total testosterone (B), and oxidative burst activity in monocytes stimulated with E.coli at baseline in patients with ovarian cancer treated with PLD alone or in combination with carboplatin.
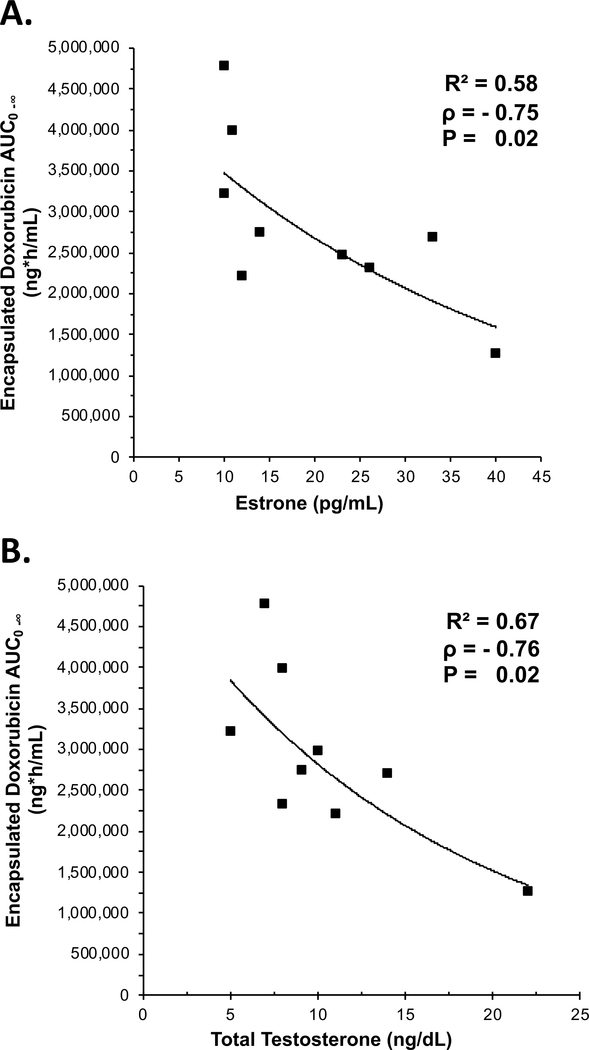
There also was an inverse relationship between estrone and testosterone and encapsulated doxorubicin AUC0−∞ in plasma (rho = −0.75 and −0.76, respectively) (Figure 2). There was a positive relationship between estrone and testosterone and encapsulated doxorubicin clearance in plasma (rho = 0.60 and 0.60, respectively; data not shown). When patients were subdivided based on PLD monotherapy (n=6), there was a stronger correlation between estrone and testosterone concentrations with encapsulated doxorubicin AUC (R2 = 0.57 and 0.59, respectively) and clearance (R2 = 0.86 and 0.88, respectively) (data not shown). No significant relationship was found between patient TBW, BMI, and hormone concentrations. In addition, there was no correlation between baseline exposures of chemokines and MPS function or PLD PK (data not shown).
Figure 2. Serum hormone concentration versus encapsulated doxorubicin AUC from plasma.
Individual patient values are represented by the solid squares and the regression is represented by the solid line. One patient did not have an estrone concentration available and another patient did not have a testosterone concentration available. There was an inverse relationship between estrone (A) and total testosterone (B) concentrations, and encapsulated doxorubicin area under the plasma concentration versus time curve (AUC) in patients with ovarian cancer treated with PLD alone or in combination with carboplatin.
The BSA (1.31-fold), BMI (1.86-fold) and estradiol concentrations (2.01-fold) were significantly higher in obese patients compared with normal weight patients with endometrial cancer (Table 1). In addition, the BSA (1.15-fold), BMI (1.38-fold) and estradiol concentrations (1.35-fold) were significantly higher in obese patients compared with normal weight patients with ovarian cancer (Table 1). As these factors are associated with reduced plasma exposure of PLD, PK simulations were performed to determine what dose of PLD would be required to obtain similar plasma AUC of encapsulated doxorubicin in obese patients compared with normal weight patients. The mean plasma AUC of encapsulated doxorubicin after administration of PLD at 40 mg/m2 (standard clinical dose of single agent PLD) in normal weight patients was 2,820 ug/mL•h. In obese patients with endometrial cancer, the dose of PLD required to achieve similar plasma exposures of PLD based on differences in BSA, BMI and estradiol were 52, 74 and 81 mg/m2, respectively. In obese patients with ovarian cancer, the dose of PLD required to achieve similar plasma exposures of PLD based on differences in BSA, BMI and estradiol were 46, 55 and 54 mg/m2, respectively. As there are significant differences in estrogen-like hormones between obese and normal weight patients and these hormones have been reported to significantly affect MPS function and the PK of PLD, the higher dose of PLD based on these hormonal factors in obese patients may be the most clinically relevant.
Discussion
Obese patients are at an increased risk of developing cancer, having a recurrence of their cancer, and dying from their disease [30]. Furthermore, inter-patient variability in the PK and PD of NPs is greater in obese patients. Moreover, the efficacy of NPs of anticancer agents appears to be reduced in obese patients [13,16,21,31]. Our group has demonstrated in mouse-models that NPs preferentially distribute to adipose tissue versus muscle in a study that evaluated the exposures of small molecule CKD-602, a camptothecin analogue, and a PEGylated liposomal formulation of CKD-602 (S-CKD-602). There was a 3.8-fold higher ratio of CKD-602 sum total exposure in fat to muscle after administration of S-CKD-602 as compared with CKD-602 [32]. The increased distribution of NPs to adipose results in an increased volume of distribution, reduced accumulation in tumors, and ultimately decreased efficacy. However, when comparing PK differences in obese and normal weight patients, nearly ten-fold differences were observed in plasma indicating a second, more dominant, mechanism associated with lower plasma exposure of NPs [13]. The second mechanism thought to be responsible for reduced plasma exposure of NPs in obese patients is related to higher blood clearance of NPs. Previous studies have demonstrated the impact MPS function and its mediators on NP PK and PD [14,32]. The current studies sought to test the hypothesis that obese patients with cancer have higher levels of hormones and chemokines and that these elevated factors alter MPS function and the PK and PD of NPs, such as PLD. These studies were performed in patients with ovarian and endometrial cancer who have a high incidence of obesity and in whom PLD is used as a treatment.
The results of the CSC study demonstrated a strong positive association between estradiol and BMI (OR 8.64, 95% CI 2.67–28.0, P=0.0003) in all patients. Moreover, this relationship was only significant between estradiol and BMI in patients with endometrial cancer (Table 3; OR 11.20, 95% CI 2.51–50.08, P=0.002). This supports our hypothesis that obese patients have higher circulating levels of hormones. While the odds ratios for the relationship between estrone and BMI (Table 2; OR 1.85, 95% CI: 0.66–5.21, P=0.242) and testosterone and BMI (Table 2; OR 2.14, 95% CI: 0.76–6.06, P=0.152) trended in the direction expected according to our hypothesis, we would have expected statically significant relationships between all hormones and BMI. Given that estrone and estradiol are readily interconverted by 17β-Hydroxysteroid dehydrogenase (17β-HSD) and that testosterone can be converted by Cytochrome P450 Family 19 (CYP19) into estradiol, a significant relationship between the concentration of estrone and BMI and the concentration of testosterone and BMI may be absent because enzyme kinetics in adipose tissue may favor conversion of these hormones to estradiol. A similar explanation is possible for the absence of a significant relationship between DHT and BMI. In adipocytes, testosterone can be converted by CYP19 into estradiol or by SR5A1 into DHT [33]. If testosterone is being shunted to the estradiol pathway, there may not be sufficient reserves for the generation of DHT, thus preventing the detection of a relationship between DHT and BMI.
The odds ratio for the relationship between estradiol and BMI was significant for patients with endometrial cancer, but not in patients with ovarian cancer, though this may be due to small sample size. This may also be due to a significant difference in the BMI of obese patients with endometrial cancer compared with ovarian cancer. The mean ± SD BMI for the obese patients with endometrial cancer and ovarian cancer were 46.26 ± 4.41 and 32.78 ± 3.48, respectively. All obese patients with endometrial cancer enrolled in the study were considered morbidly obese with BMIs > 40, ranging from 42.2 to 61.2. The BMIs for obese patients with ovarian cancer ranged from 30.2 to 40.7 with only one of the patients having a BMI > 40. The differences in the magnitude of obesity offer a possible explanation for the lack of continuity in the results between patients with endometrial and ovarian cancer. It may be that the relationship between hormone concentration and BMI does not become significant until patients are morbidly obese. Additional studies are need to evaluate these factors.
Based on the findings of the first observational study, we performed a pharmacology study in patients with refractory ovarian cancer to evaluate the relationship between serum hormone levels, MPS function in blood and PLD PK disposition. Our findings indicate that patients with higher serum estrone had higher MPS function (as measured by generation of ROS; Figure 1A) and lower plasma exposure of encapsulated doxorubicin (Figure 2A). Patient body weight and BMI were not found to be associated with estrone concentrations in patients with ovarian cancer, suggesting phenotypic and/or genotypic variations in estrone production in adipose tissue in these patients or that the influence of BMI only occurs in patients that are morbidly obese, which are consistent with the other results of this study.
The exact reasons for differences in these hormone levels between the observational and pharmacology studies are unknown. However, the difference in detectable hormone concentrations between the study populations may be indicative of differences in disease burden. Patients included in the pharmacology study had all previously undergone bilateral salpingo oopherectryomy, were recently relapsed, and were receiving active treatment at the time of study; whereas, patients included in the observational study had not received treatment in the 6 months prior to the samples being collected.
In all of the PK endpoints analyzed in the study, the relationship between serum hormone concentrations and PLD PK disposition appeared to be strongest in the 6 patients who received PLD monotherapy. This finding may be explained by a possible influence of concurrent carboplatin therapy on monocyte function and chemotaxis and subsequent alteration in the PK of PLD and other NPs, which would not have been measured by MPS function prior to drug administration [7,34].
Patients with ovarian cancer have a greater response to PLD than patients with endometrial cancer, as such PLD is approved for the treatment of ovarian cancer but not endometrial cancer [35,36]. We hypothesized that the decreased efficacy of PLD in patients with endometrial cancer, especially as compared to the efficacy of small molecule doxorubicin in endometrial cancer and efficacy of PLD in patients with ovarian cancer, may be due to an increased incidence of morbid obesity and higher serum estradiol, which leads to higher MPS function and PLD clearance and ultimately lower exposures of PLD in blood and tumors. The observational study reported significantly higher BMIs and a greater association between BMI and estradiol exposures in patients with endometrial cancer compared with ovarian cancer. In addition, the pharmacology study demonstrated a relationship between serum testosterone and estrone concentrations with MPS function and PLD PK among ovarian cancer cases. These studies suggest that serum hormone concentrations and MPS function are potentially useful for individualizing the dose of PLD and other NPs in obese patients with cancer. Moreover, these results suggest that obese patients with ovarian cancer, and especially endometrial cancer, require a higher dose of PLD to achieve similar plasma exposures to patients that are normal weight. These results are somewhat surprising given that it is standard for PLD to be dosed based on BSA (e.g. 40 mg/m2). Moreover, these results indicate that additional physiological factors associated with morbid obesity in the endometrial cancer patients may contribute to these findings. Our PK simulations based on differences in serum estradiol concentrations in obese and normal weight patients suggests a PLD dose of 54 to 81 mg/m2 is required in obese patients to achieve a similar plasma exposure as a PLD dose of 40 mg/m2 in normal weight patients. However, a limitation of the current study is that the small sample size did not allow enough power to stratify patients into categories of obese and morbidly obese. Thus, larger prospective studies are needed to confirm these results and to further understand the relationship between obesity, hormones, MPS function and the PK and PD of nanoparticles, such as PLD, in obese patients with different types of cancer. The American Society of Clinical Oncology (ASCO) reports that 40% of obese patients receive insufficient chemotherapy doses and exposures, which may lead to reduced efficacy, and recommends that PK studies are needed to guide appropriate dosing of anticancer agents in overweight and obese patients [37]. Our results suggest the magnitude of under dosing of liposomal, carrier-mediated and biological anticancer agents in obese patients may be significantly greater than for standard small molecule anticancer agents.
Table 5.
Demographics, Hormone, Chemokine and PLD PK results for the pharmacology study in patients with refractory ovarian cancer treated with PLD (N=10).
| Parameters (units) | Mean ± SD | |
|---|---|---|
| Patient Characteristics | Age (yrs) | 58.9 ± 10.9 |
| Height (cm) | 165.9 ± 7.0 | |
| Weight (kg) | 78.5 ± 19.9 | |
| BMI | 28.7 ± 8.1 | |
| BSA (m2) | 1.83 ± 0.17 | |
| PLD cycles received (#) | 3.2 ± 1.9 | |
| Baseline serum hormone concentrations | Estrone (pg/mL) | 22.7 ± 11.1 |
| Testosterone, Total (ng/dL) | 11.7 ± 5.0 | |
| Estrogen (pg/mL) | BQL* | |
| Dihydrotestosterone (pg/mL) | BQL* | |
| Baseline serum chemokine concentrations | CCL2 (pg/mL) | 242.3 ± 70.8 |
| CCL5 (pg/mL) | 2,175 ± 2764.1 | |
| Encapsulated Doxorubicin PK parameter | Clearance (ml/h) | 25.9 ± 12.0 |
| AUC 0-infinity (h*ng/mL) | 2,867,933 ± 973,734 | |
| AUC 0-infinity % extrapolated (%) | 14.6 ± 18.5 | |
| Volume of distribution (L) | 2.5 ± 0.6 | |
| Elimination rate constant (1/h) | 0.011 ± 0.008 | |
| T1/2 (h) | 76.3 ± 25.5 | |
One patient did not have an estrone concentration available and another patient did not have a testosterone concentration available due to a miscommunication in laboratory orders.
BQL: Below Quantitative Limit
Acknowledgments
The authors thank the UNC Health Registry/Cancer Survivorship Cohort (HR/CSC) participants for their important contributions. The HR/CSC is funded in part by the UNC Lineberger Comprehensive Cancer Center’s University Cancer Research Fund. This project was reviewed and approved by the Human Research Protections Program (IRB Number: 09–0605) at the University of North Carolina at Chapel Hill. The authors would also like to acknowledge the UNC Biospecimen Facility for our blood processing, and storage and sample disbursement (genome.unc.edu/bsp).
Financial Support:
The sera analyses as part of this study were funded by a Developmental Research Award from the University of North Carolina Lineberger Comprehensive Cancer Center. The pharmacology study as part of this study was funded by a University Cancer Research Fund grant.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Abbreviations
- 17β-HSD
17β-hydroxysteroid dehydrogenase
- ASCO
American Society of Clinical Oncology
- AUC
area under the concentration versus time curve
- BMI
body mass index
- BSP
Biospecimen Processing Core Facility
- CMA
carrier-mediated agents
- CV%
coefficient of variance
- CYP19
Cytochrome P450 Family 19
- DHT
dihydrotestosterone
- EPR
enhanced permeability and retention effect
- MFI
mean fluorescent intensity
- MPS
mononuclear phagocyte system
- NP
nanoparticles
- PD
pharmacodynamics
- PK
pharmacokinetics
- PLD
PEGylated liposomal doxorubicin
- ROS
reactive oxygen species
- S-CKD-602
PEGylated liposomal formulation of CKD-602
- UNC CSC
UNC Health Registry/Cancer Survivorship Cohort
- WHO
World Health Organization
Compliance with Ethical Standards
Conflicts of Interest:
Brittney Roberts Starling has no conflicts of interest to report.
Parag Kumar has no conflicts of interest to report.
Andrew T. Lucas has no conflicts of interest to report.
David Barrow has no conflicts of interest to report.
Laura Farnan has no conflicts of interest to report.
Laura Hendrix has no conflicts of interest to report.
Hugh Giovinazzo has no conflicts of interest to report.
Gina Song has no conflicts of interest to report.
Paola Gehrig has no conflicts of interest to report.
Jeannette T. Bensen has no conflicts of interest to report.
William C. Zamboni has no conflicts of interest to report.
Ethical Approval:
This article does not contain any studies with animals performed by any of the authors.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Studies were approved by the University of North Carolina Institutional Review Board (UNC IRB# 08–1204 & UNC IRB# 14–2078).
Informed consent:
Informed consent was obtained from all individual participants included in the study.
References
- 1.Farokhzad OC, Langer R. (2009) Impact of nanotechnology on drug delivery. ACS Nano. 3(1):16–20. [DOI] [PubMed] [Google Scholar]
- 2.Peer D, Karp JM, Hong S, et al. (2007) Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2(12):751–760. [DOI] [PubMed] [Google Scholar]
- 3.Maeda H, Wu J, Sawa T, Matsumura Y, et al. (2000) Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J Control Release. 65(1–2):271–284. [DOI] [PubMed] [Google Scholar]
- 4.Zamboni WC. (2008) Concept and clinical evaluation of carrier-mediated anticancer agents. Oncologist. 13(3):248–260. [DOI] [PubMed] [Google Scholar]
- 5.Schell RF, Sidone BJ, Caron WP, et al. (2014) Meta-analysis of inter-patient pharmacokinetic variability of liposomal and non-liposomal anticancer agents. Nanomedicine Nanotechnology, Biol Med. 10(1):109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song G, Wu H, Yoshino K, et al. (2012) Factors affecting the pharmacokinetics and pharmacodynamics of liposomal drugs. J Liposome Res. 22(3):177–192. [DOI] [PubMed] [Google Scholar]
- 7.Caron WP, Song G, Kumar P, et al. (2012) Interpatient pharmacokinetic and pharmacodynamic variability of carrier-mediated anticancer agents. Clin Pharmacol Ther. 91(5):802–812. [DOI] [PubMed] [Google Scholar]
- 8.Wu H, Ramanathan RK, Zamboni BA, et al. (2011) Population Pharmacokinetics of Pegylated Liposomal CKD-602 (S-CKD602) in Patients With Advanced Malignancies. J Clin Pharmacol. 52(2): 180–194. [DOI] [PubMed] [Google Scholar]
- 9.Hume DA. (2006) The mononuclear phagocyte system. Curr Opin Immunol. 18(1):49–53. [DOI] [PubMed] [Google Scholar]
- 10.Moghimi SM, Murray JC, Hunter AC. (2001) Long-circulating and target specific nanoparticles: Theory to practice. Pharmacol Rev. 53(2):283–318. [PubMed] [Google Scholar]
- 11.Calle EE, Rodriguez C, Walker-Thurmond K, et al. (2003) Overweight, Obesity, and Mortality from Cancer in a Prospectively Studied Cohort of U.S. Adults. N Engl J Med. 348(17):1625–1638. [DOI] [PubMed] [Google Scholar]
- 12.Song G, Tarrant TK, White TF, et al. (2015) Roles of chemokines CCL2 and CCL5 in the pharmacokinetics of PEGylated liposomal doxorubicin in vivo and in patients with recurrent epithelial ovarian cancer. Nanomedicine Nanotechnology, Biol Med. 11(7):1797–1807. [DOI] [PubMed] [Google Scholar]
- 13.Zamboni WC, Strychor S, Joseph E, et al. (2007) Plasma, tumor, and tissue disposition of STEALTH liposomal CKD-602 (S-CKD602) and nonliposomal CKD-602 in mice bearing A375 human melanoma xenografts. Clin Cancer Res. 13(23):7217–7223. [DOI] [PubMed] [Google Scholar]
- 14.Song G, Moore S, Tarrant T, et al. (2012) Relationship between complement factors and CC chemokines and the pharmacokinetics (PK) and pharmacodynamics (PD) of PEGylated liposomal doxorubicin (Doxil) in patients with refractory epithelial ovarian cancer (EOC). In: Proceedings of 24th EORTC-NCI-AACR Symposium on “Molecular Targets and Cancer Therapeutics Abstract 126. doi: 10.1016/S0959–8049(12)71924-X. [Google Scholar]
- 15.Zamboni WC, Strychor S, Maruca L, et al. (2009) Pharmacokinetic study of pegylated liposomal CKD-602 (S-CKD602) in patients with advanced malignancies. Clin Pharmacol Ther. 86(5):519–526. [DOI] [PubMed] [Google Scholar]
- 16.Wu H, Infante JR, Keedy VL, et al. (2015) Factors affecting the pharmacokinetics and pharmacodynamics of PEGylated liposomal irinotecan (IHL-305) in patients with advanced solid tumors. Int J Nanomedicine. 10:1201–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nielsen H (1984) Effect of cis-platinum on human blood monocyte function in vitro. Cancer Immunol Immunother. 18(3): 223–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicol T, Bilbey D, Charles L, et al. (1964) Oestrogen: the natural stimulant of body defence. J Endocrinol. 30:277–291. [DOI] [PubMed] [Google Scholar]
- 19.Chao TC, Phuangsab A, Van Alten PJ, et al. (1996) Steroid sex hormones and macrophage function: regulation of chemiluminescence and phagocytosis. Am J Reprod Immunol AJRI Off J Am Soc Immunol Reprod Int Coord Comm Immunol Reprod. 35(2):106–113. [DOI] [PubMed] [Google Scholar]
- 20.Vernon-Roberts B (1969) The effects of steroid hormones on macrophage activity. Int Rev Cytol. 25:131–159. [DOI] [PubMed] [Google Scholar]
- 21.Ligibel J (2011) Obesity and breast cancer. Oncology. 25:994–1000. [PubMed] [Google Scholar]
- 22.Cleary MP, Grossmann ME. (2009) Minireview: Obesity and breast cancer: The estrogen connection. Endocrinology. 150(6):2537–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Connolly BS, Barnett C, Vogt KN, et al. (2002) A Meta-Analysis of Published Literature on Waist-to-Hip Ratio and Risk of Breast Cancer. Nutr Cancer. 44(2):127–138. [DOI] [PubMed] [Google Scholar]
- 24.Harvie M, Hooper L, Howell AH. (2003) Central obesity and breast cancer risk: a systematic review. Obes Rev. 4(3):157–173. [DOI] [PubMed] [Google Scholar]
- 25.Johnson AR, Justin Milner J, Makowski L. (2012) The inflammation highway: Metabolism accelerates inflammatory traffic in obesity. Immunol Rev. 2012;249(1):218–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flegal KM, Carroll MD, Ogden CL, et al. (2010) Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 303(3):235–241. [DOI] [PubMed] [Google Scholar]
- 27.Harris MI, Klein R, Welborn TA, et al. (1992) Onset of NIDDM occurs at least 4–7 yr before clinical diagnosis. DiabetesCare. 15(7):815–819. [DOI] [PubMed] [Google Scholar]
- 28.Jemal A, Siegel R, Xu J, Ward E. (2010) Cancer statistics, 2010. CA Cancer J Clin. 60(5):277–300. [DOI] [PubMed] [Google Scholar]
- 29.Steiner E, Plata K, Interthal C, et al. (2007) Diabetes mellitus is a multivariate independent prognostic factor in endometrial carcinoma: A clinicopathologic study on 313 patients. Eur J Gynaecol Oncol. 28(2):95–97. [PubMed] [Google Scholar]
- 30.Demark-Wahnefried W, Platz EA, Ligibel JA, et al. (2012) The Role of Obesity in Cancer Survival and Recurrence. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 21(8):1244–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.La-Beck NM, Zamboni BA, Gabizon A, et al. (2012) Factors affecting the pharmacokinetics of pegylated liposomal doxorubicin in patients. Cancer Chemother Pharmacol. 69(1):43–50. [DOI] [PubMed] [Google Scholar]
- 32.Song G, Terrant TK, Barrow DA, et al. (2012) The effect of CC chemokine ligand-2 (CCL2/MCP-1) and CC chemokineligand-5 (CCL5/RANTES) on the pharmacokinetics (PK) and pharmacodynamics (PD) of PEGylated liposomal CKD-602 (S-CKD602) in patients with advanced solid tumors. In: Proceedings of American Association for Cancer Research Abstract 754. doi: 10.1158/1538–7445.AM2012–754. [Google Scholar]
- 33.Li J, Papadopoulos V, Vihma V. (2015) Steroid biosynthesis in adipose tissue. Steroids. 103:89–104. [DOI] [PubMed] [Google Scholar]
- 34.Giovinazzo H, Kumar P, Sheikh A, et al. (2016) Technetium Tc 99m sulfur colloid phenotypic probe for the pharmacokinetics and pharmacodynamics of PEGylated liposomal doxorubicin in women with ovarian cancer. Cancer Chemother Pharmacol. 77(3):565–573. [DOI] [PubMed] [Google Scholar]
- 35.Gordon AN, Tonda M, Sun S, et al. (2004) Long-term survival advantage for women treated with pegylated liposomal doxorubicin compared with topotecan in a phase 3 randomized study of recurrent and refractory epithelial ovarian cancer. Gynecol Oncol. 95(1):1–8. [DOI] [PubMed] [Google Scholar]
- 36.Homesley HD, Blessing JA, Sorosky J, et al. (2005) Phase II trial of liposomal doxorubicin at 40 mg/m(2) every 4 weeks in endometrial carcinoma: A Gynecologic Oncology Group Study. Gynecol Oncol. 98(2):294–298. [DOI] [PubMed] [Google Scholar]
- 37.Griggs JJ, Mangu PB, Temin S, et al. (2012) Appropriate chemotherapy dosing for obese adult patients with cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 30(13):1553–1561. [DOI] [PubMed] [Google Scholar]
- 38.Reed MJ, Cheng RW, Noel CT, et al. (1983) Plasma Levels of Estrone, Estrone Sulfate, and Estradiol and the Percentage of Unbound Estradiol in Postmenopausal Women with and without Breast Disease. Cancer Res. 43(8):3940–3943. [PubMed] [Google Scholar]
- 39.Hankinson SE, Manson JE, Willett WC, et al. (1995) Reproducibility of Plasma Hormone Levels in Postmenopausal Women over a 2–3-year Period. Cancer Epidemiol Biomarkers Prev. 4(6):649–654. [PubMed] [Google Scholar]
- 40.Andreopoulou E, Gaiotti D, Kim E, et al. (2007) Pegylated liposomal doxorubicin HCL (PLD; Caelyx/Doxil): Experience with long-term maintenance in responding patients with recurrent epithelial ovarian cancer. Ann Oncol. 18(4):716–721. [DOI] [PubMed] [Google Scholar]
- 41.Muggia FM, Blessing JA, Sorosky J, et al. (2002) Phase II trial of the pegylated liposomal doxorubicin in previously treated metastatic endometrial cancer: A gynecologic oncology group study. J Clin Oncol. 20(9):2360–2364. [DOI] [PubMed] [Google Scholar]
- 42.Gadducci A, Cosio S, Genazzani AR. (2006) Old and new perspectives in the pharmacological treatment of advanced or recurrent endometrial cancer: Hormonal therapy, chemotherapy and molecularly targeted therapies. Crit Rev Oncol Hematol. 58(3):242–256. [DOI] [PubMed] [Google Scholar]
- 43.Gray DS, Fujioka K. (1991) Use of relative weight and Body Mass Index for the determination of adiposity. J Clin Epidemiol. 44(6):545–550. [DOI] [PubMed] [Google Scholar]
- 44.WHO. Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation. Vol 894; 2000. doi: 10.1016/S0140-6736(57)91352-1. [DOI] [PubMed]
- 45.Caron WP, Lay JC, Fong AM, et al. (2013) Translational Studies of Phenotypic Probes for the Mononuclear Phagocyte System and Liposomal Pharmacology. J Pharmacol Exp Ther. 347:599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]