Abstract
Background:
among the intervention and control groups (78% vs. 77%; OR=1.09, 95% CI: 0.43–2.76). Among returners, 26% had an oncogenic HPV type detected in their sample. Women who returned their self-test reported high levels of satisfaction and positive experiences with the self-Human papillomavirus (HPV) self-testing is an emerging cervical cancer screening strategy, yet few mail-based HPV self-testing programs have been implemented in the United States. We report the results of a pilot study of a mail-based program, the Health Outcomes through Motivation and Education (HOME) Project.
Methods:
In 2015–2016, we recruited 103 women from Appalachian Ohio who were ages 30–65 and had not received a Pap test in at least three years. Women were mailed an HPV self-test and randomized to receive either: a) self-test instructions developed by the device manufacturer and a standard information brochure about cervical cancer (control group); or b) self-test instructions developed by the HOME Project and a photo story information brochure about cervical cancer (intervention group). Logistic regression compared study arms on HPV self-test return and receipt of a Pap test.
Results:
Overall, 80 (78%) women returned their HPV self-test. Return was similar testing process. Few women overall received a Pap test (11%), and Pap testing was similar among the intervention and control groups (14% vs. 8%; OR=1.91, 95% CI: 0.52–6.97).
Conclusions:
Mail-based HPV self-testing programs are a potentially promising strategy for reaching underscreened women in Appalachia. Efforts are needed to better understand how to optimize the success of such programs.
Keywords: HPV, cervical cancer, screening, Appalachia
INTRODUCTION
Almost all cervical cancers are caused by persistent infection with high-risk (i.e., oncogenic) human papillomavirus (HPV) types (mainly types 16 and 18).1 Cervical cancer is, however, largely preventable through regular screening and follow-up care for precancerous lesions. For women ages 30–65, current cervical cancer screening guidelines in the United States (US) recommend a combination of cytology (i.e., Pap testing) and HPV testing every five years as the preferred screening strategy.2 Cytology alone every three years is recommended for women ages 21–29 and is also an acceptable strategy for women ages 30–65.2 The HPV testing included in the current guidelines involves samples collected by healthcare providers in a clinic setting. Interest in HPV testing as a primary screening strategy is increasing. Indeed, the Food and Drug Administration (FDA) approved an HPV test as a first-line screening test,3 and the US Preventive Services Task Force recently released draft guidelines recommending HPV testing alone every five years as a screening option for women ages 30–65.4
HPV self-testing is an emerging cervical cancer screening strategy that involves women collecting their own cervicovaginal sample. The sensitivity and specificity of HPV self-tests for detecting cervical disease is comparable to provider-collected samples.5,6 Mail-based HPV self-testing programs that send women a self-test in the mail and ask them to use the self-test at home and return it via mail have shown great promise. In large international studies, up to about 40% of underscreened women used and returned an HPV self-test that was mailed to them.7,8 Given this success, multiple countries recently integrated mail-based HPV self-testing for underscreened women into national screening programs.9,10
HPV self-testing is currently not an approved or recommended screening strategy in the US, but research studies have produced encouraging results. Focus group and survey studies have shown that most US women would be willing to use a self-test at home, though willingness differs by self-test device type.11–16 For example, willingness was higher for devices that use brushes to collect samples compared to those that function as lavages.15,16 A few recent studies have begun implementing mail-based HPV self-testing programs in the US.17,18 In these studies, at least 64% of women used and returned an HPV self-test that was mailed to them.
It is important to continue to examine the implementation of mail-based HPV self-testing programs in the US and begin to identify factors that may increase self-test return. One factor is the materials sent with the HPV self-test, including the instructions for using and returning the self-test. Such materials may help address several common concerns that women have about HPV self-testing (e.g., fear of using the self-test incorrectly, privacy concerns of returning the self-test via mail).14,19,20 We report the results of a pilot study of the Health Outcomes through Motivation and Education (HOME) Project, which aimed to implement a mail-based HPV self-testing program in Appalachian Ohio and examine how materials sent with the HPV self-test affect women’s screening behaviors.
MATERIALS AND METHODS
Recruitment
Appalachian Ohio is a 32 county region in southern and eastern Ohio that has higher cervical cancer incidence rates compared to the rest of Ohio and the US.21 We recruited participants for the HOME Project from the Valley View Health Centers, a group of Federally Qualified Health Centers in Appalachian Ohio. At the time of our study, there were six health center locations in four Appalachian Ohio counties (Adams, Jackson, Pike, and Scioto counties). We identified and randomly selected potentially eligible women from all six health center locations via medical records. We mailed selected women recruitment materials that included a form to confirm eligibility and a consent form.
Upon receipt of these completed forms, we confirmed study eligibility. Women were eligible if they: a) were ages 30–65; b) had no Pap test in the last three years; c) resided in an Appalachian Ohio county; d) were not currently pregnant and were not pregnant in the previous three months; e) had no history of invasive cervical cancer; and f) had no history of hysterectomy. We required women to read English, which was inferred upon completion and return of these forms. We limited participation to one woman per household.
We mailed eligible and consenting women a baseline survey (hereafter the T1 survey) and a medical records release (MRR) form. Women who returned the T1 survey were randomized using a 1:1 allocation ratio to either the intervention or control arm (described below). Randomization was stratified by clinic site. We did not require return of the MRR form for randomization and continued study participation.
We sent recruitment materials to a total 1,967 women, of whom 157 had a non-deliverable address (Figure 1). Of the remaining 1,810 women, 276 returned their eligibility and consent forms. A total of 106 women were eligible and consented (response rate=15%).22 We report data on 103 randomized women (52 in the control group and 51 in the intervention group). Three eligible and consented women were not randomized due to not returning the T1 survey. All 103 randomized women returned a MRR form.
Figure 1.
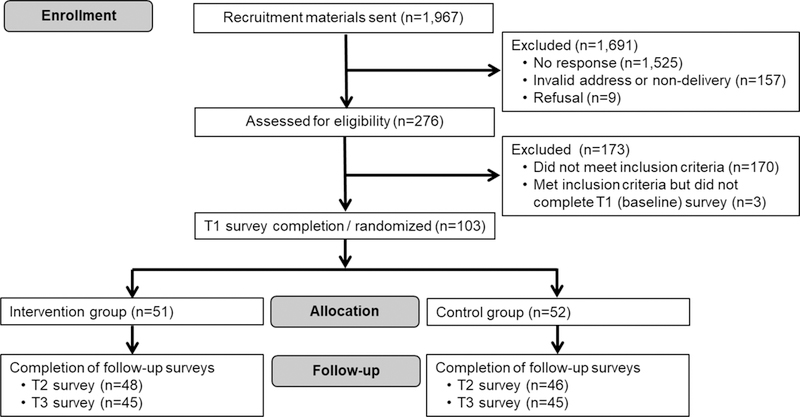
CONSORT Flow Diagram for the Health Outcomes through Motivation and Education (HOME) Project
Women were sent up to $85 in gift cards for this study, including a $5 gift card for returning their eligibility and consent forms (regardless of eligibility and consent status), a $5 gift card for returning their MRR form, and a $25 gift card for returning each of three surveys (described below). Women did not receive a gift card for using and returning their HPV self-test. The Institutional Review Board of The Ohio State University approved this study, which occurred from November 2015 through October 2016.
HPV Self-Test Study Kits
We sent all randomized women a study kit that included: a) an introduction letter; b) HPV self-test device; c) plastic sample bag; and d) a postage-paid, pre-addressed return box. The HPV self-test device was the Evalyn® Brush (Rovers Medical Devices, The Netherlands), which has the ability to collect and transport a dry sample. To return the HPV self-test after use, women placed the self-test inside the plastic sample bag and then placed the sample bag in the return box. If needed, we sent a reminder letter about returning the HPV self-test two weeks after study kits were mailed.
As part of their study kit, participants in the control group also received: a) HPV self-test instructions developed by the device manufacturer to show the step-by-step process for using and returning the self-test; and b) an information brochure about HPV, cervical cancer, and cervical cancer screening created by the Centers for Disease Control and Prevention (CDC).23 We considered these materials to be “usual care.”
As part of their study kit, participants in the intervention group also received: a) instructions for using the HPV self-test developed by the HOME Project; and b) a photo story information brochure about HPV, cervical cancer, and cervical cancer screening. Guided by input from community members, we developed these materials to target constructs from the Protection Motivation Theory (PMT), including: a) perceived vulnerability to cervical cancer; b) perceived severity of cervical cancer; c) response efficacy of HPV self-testing; d) self-efficacy to use and return an HPV self-test; e) response costs to using and returning the self-test; and f) intrinsic and extrinsic rewards of not using and returning the self-test.
The HOME Project self-test instructions were based on those from the device manufacturer (i.e., the control group instructions) but included larger text and pictures, simplified language, and a more segmented sequence of steps for using and returning the self-test. The photo story was a narrative type of entertainment-education with pictures and simple dialogue text that told a story about women discussing cervical cancer screening. This type of information format can be effective in communicating health-related information,24 and our photo story included targeted information about HPV, cervical cancer, and cervical cancer screening for women living in Appalachian Ohio.
HPV Detection
All returned HPV self-tests were sent to CDC to be tested for the detection of high-risk HPV types. The Evalyn® Brushes were extracted with the QIAamp DNA Mini Kit (Qiagen, Valencia, CA), and extracts were tested using the cobas HPV test (Roche Molecular Systems, California) as modified for a sample type not validated by the manufacturer.25 The assay detects 14 high-risk HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68) along with an endogenous target indicating sample adequacy. Assay results were reported as positive for one or more high-risk HPV types, negative for high-risk HPV types, or inadequate.
After receiving HPV detection results from CDC, we sent the results to the Valley View Health Centers. We then sent a notification letter to participants indicating their results were available at the Valley View Health Centers and encouraging them to schedule an appointment to receive their results and discuss further cervical cancer screening (i.e., Pap testing). This notification approach was used at the request of the Valley View Health Centers. For participants who did not return their HPV self-test, we mailed each a notification letter encouraging them to schedule an appointment at the Valley View Health Centers to discuss cervical cancer screening. Thus, the HOME Project encouraged all women, regardless of HPV self-test return status or HPV detection results, to schedule an appointment to discuss cervical cancer screening to better align with the screening guidelines of “co-testing” with HPV testing and Pap testing.2
Surveys
We mailed participants three self-administered surveys during the study. The T1 survey was the baseline survey prior to randomization, and it focused on demographic and health-related characteristics. We sent the T2 survey following return (or non-return) of a participant’s completed HPV self-test but prior to the availability of HPV detection results. For women who returned their HPV self-test, the T2 survey was sent immediately upon receipt of the completed self-test. For women who did not return their HPV self-test, the T2 survey was sent one month after self-test distribution. The T2 survey focused on women’s reasons for returning (or not returning) their self-test and satisfaction/experience with HPV self-testing (among self-test returners only). The T3 survey was the end-of-study survey and was sent about two months after notification letters were sent to participants. The T3 survey focused on women’s Pap testing behaviors. Of the 103 randomized women, 94 (91%) returned their T2 survey, and 90 (87%) returned their T3 survey.
Measures
Study outcomes included (yes or no for each): a) women’s return of their HPV-self test; and b) women’s receipt of a Pap test during the study. Pap testing status was based on information from medical records for all participants since all returned a MRR form. Among women who returned their HPV self-test, we examined self-test sample adequacy (i.e., whether women were able to collect a sample adequate for HPV detection; yes or no) and detection of one or more high-risk HPV types in the self-test sample (yes or no).
Using data from the T2 survey, we examined women’s reasons for returning (or not returning) their self-test and satisfaction/experience with HPV self-testing. All satisfaction/experience items used a 5-point Likert scale with responses of strongly agree, agree, not sure, disagree, and strongly disagree. We dichotomized responses by grouping strongly agree and agree (i.e., “agree”) versus all other responses (i.e., “not agree”).
Data Analysis
We first examined if baseline characteristics differed between the intervention and control groups due to chance imbalance using chi-square tests for categorical variables and independent samples t-tests for continuous variables. We then used logistic regression models to compare study arms on outcomes. Logistic regression models produced odds ratios (ORs) and 95% confidence intervals (CIs). Lastly, we descriptively examined women’s reasons for returning (or not returning) their self-test and satisfaction/experience with HPV self-testing. Analyses were conducted using Stata version 14.0 (Statacorp, College Station, TX) and two-tailed statistical tests with a critical alpha of 0.05.
RESULTS
Participant Characteristics
Women were from seven Appalachian Ohio counties, with most residing in Scioto (38%), Pike (26%), or Jackson (18%) county. Most participants were non-Hispanic white (98%); never married, divorced, separated, or widowed (56%); and reported a household income of less than $20,000 in the past year (56%) (Table 1). The mean age of participants was 46.4 years old (standard deviation=9.1 years). Most participants had public health insurance (66%), with fewer having private health insurance (16%) or no health insurance (18%). About half of participants (46%) reported their most recent Pap test prior to study entry was more than five years ago. Intervention and control groups were equivalent on all baseline characteristics, except for education level.
Table 1.
Participant characteristics by study arm (n=103)
| Intervention (n=51) |
Control (n=52) |
|||
|---|---|---|---|---|
| n (%) | n (%) | p | ||
| Demographics | ||||
| Age (years), mean (SD) | 46.1 (9.1) | 46.7 (9.1) | 0.75 | |
| Race/ethncity | 0.99 | |||
| White, non-Hispanic | 50 (98) | 51 (98) | ||
| Other | 1 (2) | 1 (2) | ||
| Marital status | 0.78 | |||
| Married or living with a partner | 23 (45) | 22 (42) | ||
| Never married, divorced, separated, or widowed | 28 (55) | 30 (58) | ||
| Education level | 0.04 | |||
| High school degree or less | 34 (67) | 24 (46) | ||
| Some college or more | 17 (33) | 28 (54) | ||
| Household income | 0.87 | |||
| Less than $20,000 | 30 (59) | 28 (54) | ||
| $20,000 or more | 18 (35) | 21 (40) | ||
| Not reported | 3 (6) | 3 (6) | ||
| Health and healthcare | ||||
| Health insurance | 0.20 | |||
| None | 7 (14) | 12 (23) | ||
| Public insurance | 38 (75) | 30 (58) | ||
| Private insurance | 6 (12) | 10 (19) | ||
| Smoking status | 0.58 | |||
| Never smoker | 17 (33) | 17 (33) | ||
| Former smoker | 7 (14) | 4 (8) | ||
| Current smoker | 27 (53) | 31 (60) | ||
| Age at sexual debut | 0.39 | |||
| Younger than 16 years | 15 (29) | 16 (31) | ||
| 16 years or older | 33 (65) | 29 (56) | ||
| Not reported | 3 (6) | 7 (13) | ||
| Lifetime number of sexual partners | 0.33 | |||
| 6 or fewer | 24 (47) | 19 (37) | ||
| 7 or more | 19 (37) | 19 (37) | ||
| Not reported | 8 (16) | 14 (27) | ||
| Most recent Pap test | 0.77 | |||
| 3–5 years ago | 27 (53) | 29 (56) | ||
| More than 5 years ago | 24 (47) | 23 (44) | ||
Note. SD = standard deviation. Percentages may not total 100 due to rounding. P-values represent findings of analyses assessing differences between intervention and control groups using chi-square analyses for categorical variables and independent samples t-tests for continuous variables.
HPV Self-Testing
Overall, 80 women (78%) used and returned their HPV self-test. Self-test return was similar among the intervention and control groups (78% vs. 77%; OR=1.09, 95% CI: 0.43–2.76). All women who returned their HPV self-test collected a sample that was adequate for HPV detection. Among self-test returners, 26% had one or more high-risk HPV types detected in their self-test sample.
The most common reasons why women returned their HPV self-test were to protect their health (68%), to see if they have an HPV infection (62%), and because the test was convenient (52%). Among women who did not return their self-test, the most common reasons for non-return were forgetting to return the test (27%), worry the test might hurt (13%), lack of time (13%), worry they might use the self-test incorrectly (13%), and worry the test might show they have an HPV infection (13%).
Satisfaction and Experiences with HPV Self-Testing
Women who returned their HPV self-test reported high levels of satisfaction and positive experiences with the self-testing process (Table 2). About 85% of these women liked the appearance of the HPV self-test, while over 90% agreed that their self-test was easy to use, convenient, and that they were comfortable returning their self-test through the mail. Fewer than 10% indicated they were embarrassed to use the HPV self-test, the self-test was painful to use, or that they were worried they used the self-test incorrectly. Over 90% reported they would be willing to use an HPV self-test in the future and to recommend a self-test to other women.
Table 2.
Women’s satisfaction and experiences with HPV self-testing (n=79a)
| n (%) | |
|---|---|
| The HPV self-test was convenient to use | 78 (99) |
| The HPV self-test was easy to use | 76 (96) |
| I would be willing to use the HPV self-test in the future | 76 (96) |
| The HPV self-test is a good way for women to protect their health | 74 (94) |
| I was comfortable returning the HPV self-test through the mail | 74 (94) |
| I would recommend the HPV self-test to other women I know | 73 (92) |
| I liked how the HPV self-test looked | 67 (85) |
| I am worried that I used the HPV self-test in an incorrect way | 7 (9) |
| I was embarrassed to use the HPV self-test | 5 (6) |
| It was painful to use the HPV self-test | 4 (5) |
| I would rather go to the doctor to get tested for HPV than use the HPV self-test | 0 (0) |
Note. HPV = human papillomavirus. Table reports the percentage of women who indicated “agree” or “strongly agree” for each statement. Each statement used a 5-point response scale with responses of “strongly agree,” “agree,” “not sure,” “disagree,” and “strongly disagree.”
A total of 80 women returned an HPV self-test. This table includes data for 79 women who returned an HPV self-test and completed a T2 survey after self-test return. One woman returned an HPV self-test but did not complete a T2 survey.
Pap Testing
Overall, 11 women (11%) received a Pap test during the study. Pap testing was similar among women in the intervention and control groups (14% vs. 8%; OR=1.91, 95% CI: 0.52–6.97). All women who received a Pap test had returned their HPV self-test. Pap testing was higher among women who returned their self-test and had a high-risk HPV type detected in their sample compared to those who returned their HPV self-test but did not have a high-risk HPV type detected (33% vs. 7%; OR=6.88, 95% CI: 1.76–26.82).
DISCUSSION
Our pilot study of the HOME Project suggests that HPV self-testing is a potentially promising strategy for reaching underscreened women in Appalachian Ohio. Indeed, nearly 80% of participants in our study returned their HPV self-test, though it should be reiterated that the overall response rate for our study was 15% and it is not known how many women who did not participate would have returned a self-test (if one had been mailed to them). Among women who returned their self-test, over a quarter had a high-risk HPV type detected. Our results are similar to the few recent mail-based HPV self-testing programs in the US, which had self-test return rates of at least 64% and HPV prevalence estimates up to 18%.17,18 It is worth noting that the return rates in US studies, including our study, are noticeably higher than those from large international mail-based programs (pooled return rate of 24%).7 A potential reason for this is that the US studies required women to provide consent as part of the study process, and this may have resulted in these studies enrolling women who were highly interested in using an HPV self-test. In contrast, many of the international studies mailed HPV self-tests to women without any prior contact or consent. In our study, it is also possible that some participants may have felt an obligation to return their self-test as part of being in the study. These factors should be considered when interpreting our self-test return rate. An important next step for US studies is implementation of mail-based HPV self-testing programs that are larger and conducted under more “real-world” scenarios. These studies should also examine correlates of self-test return and strategies for optimizing mail-based programs.
Women who returned their HPV self-test indicated high levels of satisfaction and positive experiences with self-testing, with very few reporting negative feedback. These findings add to the growing literature of women reporting extremely positive feedback after using an HPV self-test.17,26,27 The positive feedback in our study is likely due in part to the HPV self-test device that was used. Prior to our pilot study, we conducted formative research with women from Appalachian Ohio to examine their acceptability of different HPV self-test devices. Acceptability differed greatly across the various devices, with the Evalyn® Brush being the most preferred device.15 Given the wide range of HPV self-test devices available, we believe this type of formative research is necessary and can identify a culturally acceptable device.
The materials sent with the HPV self-test did not have a meaningful effect on HPV self-test return or Pap testing, as these outcomes were similar between the intervention and control groups. To our knowledge, this is the first study to compare different materials sent with an HPV self-test. The lack of an effect may be attributable to the high quality of the control group materials, which included HPV self-test instructions developed by the device manufacturer and an information brochure about cervical cancer created by CDC. Such materials may be sufficient for mail-based HPV self-testing programs, though future efforts should examine the impact of different information formats (e.g., video instructions versus text instructions).
Despite all women receiving a recommendation to schedule an appointment to discuss cervical cancer screening, few received a Pap test during the study, including those who had a high-risk HPV type detected in their self-test sample. Interestingly, no participants in our study received a Pap test without returning their HPV self-test, which occurred among about 10% of women in a recent Canadian study.28 If HPV self-testing becomes an approved and recommended screening approach in the US, it will be critical to identify strategies for ensuring women attend subsequent visits for Pap testing or other follow-up care. One potential strategy is patient navigation, which can address barriers and improve cancer screening behaviors and attendance at follow-up visits.29
Study strengths include our focus on underscreened women from a geographic area with cervical cancer disparities,21 input from community members to develop the intervention and select the HPV self-test device, and outcomes related to both HPV self-testing and Pap testing. Limitations include a modest response rate and a sample size that did not permit more sophisticated analyses (e.g., identifying correlates of HPV self-test return). All participants were from seven Appalachian Ohio counties, and we were not able to compare the characteristics of participants to characteristics of women who did not participate (i.e., the larger clinic population) due to lack of data on these women. However, the characteristics (race/ethnicity, education level, lack of health insurance, etc.) of our participants are similar to those of residents throughout Appalachian Ohio.30 We also lacked data on individual HPV types, as the cobas HPV test results were reported only as whether or not women had a high-risk HPV type detected. HPV self-testing is currently not an approved or recommended screening strategy in the US, and most laboratories do not currently perform HPV testing on self-collected samples. Further work is needed to validate HPV results for self-collected samples since current FDA-approved HPV tests have only been validated for provider-collected samples.
Mail-based HPV self-testing is a potentially promising strategy for reaching underscreened women from Appalachian Ohio, about a quarter of whom are infected with a high-risk HPV type. Most women returned an HPV self-test as part of our mail-based program and subsequently reported very positive feedback about the self-testing process. Future efforts are needed to better understand how to optimize the success of mail-based HPV self-testing programs, including how to increase women’s attendance at follow-up visits after returning an HPV self-test.
Acknowledgements:
We thank Mariela Scarbrough and Troy Querec for their contributions to this study.
Source of Funding: This study was supported by a research grant from Pelotonia with additional support provided by the National Cancer Institute (P30CA016058; Behavioral Measurement Shared Resource at The Ohio State University Comprehensive Cancer Center).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
Trials Registration: The trial is registered at ClinicalTrials.gov: identifier NCT02460237.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Gillison ML, Chaturvedi AK, Lowy DR. HPV prophylactic vaccines and the potential prevention of noncervical cancers in both men and women. Cancer 2008; 113(10 Suppl): 3036–3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin 2012; 62(3): 147–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.U.S. Food and Drug Administration. FDA approves first human papillomavirus test for primary cervical cancer screening 2014. Available at: http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm394773.htm.
- 4.United States Preventive Services Task Force. Draft recommendation statement. cervical cancer: Screening 2017. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement/cervical-cancer-screening2.
- 5.Zhao FH, Lewkowitz AK, Chen F, et al. Pooled analysis of a self-sampling HPV DNA test as a cervical cancer primary screening method. J Natl Cancer Inst 2012; 104(3): 178–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arbyn M, Verdoodt F, Snijders PJ, et al. Accuracy of human papillomavirus testing on self-collected versus clinician-collected samples: A meta-analysis. Lancet Oncol 2014; 15(2): 172–183. [DOI] [PubMed] [Google Scholar]
- 7.Verdoodt F, Jentschke M, Hillemanns P, Racey CS, Snijders PJ, Arbyn M. Reaching women who do not participate in the regular cervical cancer screening programme by offering self-sampling kits: A systematic review and meta-analysis of randomised trials. Eur J Cancer 2015; 51(16): 2375–2385. [DOI] [PubMed] [Google Scholar]
- 8.Racey CS, Withrow DR, Gesink D. Self-collected HPV testing improves participation in cervical cancer screening: A systematic review and meta-analysis. Can J Public Health 2013; 104(2): e159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arbyn M, Castle PE. Offering self-sampling kits for HPV testing to reach women who do not attend in the regular cervical cancer screening program. Cancer Epidemiol Biomarkers Prev 2015; 24(5): 769–772. [DOI] [PubMed] [Google Scholar]
- 10.Smith M, Lew JB, Simms K, Canfell K. Impact of HPV sample self-collection for underscreened women in the renewed cervical screening program. Med J Aust 2016; 204(5): 1941e–7. [DOI] [PubMed] [Google Scholar]
- 11.Madzima TR, Vahabi M, Lofters A. Emerging role of HPV self-sampling in cervical cancer screening for hard-to-reach women: Focused literature review. Can Fam Physician 2017; 63(8): 597–601. [PMC free article] [PubMed] [Google Scholar]
- 12.Scarinci IC, Litton AG, Garces-Palacio IC, Partridge EE, Castle PE. Acceptability and usability of self-collected sampling for HPV testing among African-American women living in the Mississippi Delta. Womens Health Issues 2013; 23(2): e123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vanderpool RC, Jones MG, Stradtman LR, Smith JS, Crosby RA. Self-collecting a cervico-vaginal specimen for cervical cancer screening: An exploratory study of acceptability among medically underserved women in rural Appalachia. Gynecol Oncol 2014; 132(Suppl 1): S21–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reiter PL, McRee AL. Cervical cancer screening (pap testing) behaviours and acceptability of human papillomavirus self-testing among lesbian and bisexual women aged 21–26 years in the USA. J Fam Plann Reprod Health Care 2015; 41(4): 259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reiter PL, Richardson M, Zimmermann BJ, et al. Acceptability of human papillomavirus self-test devices among women from high-risk populations. J Womens Health, Issues Care 2016; 5(1). [Google Scholar]
- 16.Richman AR, Brewer NT, Liebman AK, Rinas AC, Smith JS. Optimising human papillomavirus self-testing for high risk women. Sex Transm Infect 2011; 87(2): 118–122. [DOI] [PubMed] [Google Scholar]
- 17.Smith JS, Des Marais AC, Deal AM, et al. Mailed human papillomavirus self-collection with papanicolaou test referral for infrequently screened women in the United States. Sex Transm Dis 2018; 45(1): 42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nelson EJ, Hughes J, Oakes JM, Thyagarajan B, Pankow JS, Kulasingam SL. Human papillomavirus infection in women who submit self-collected vaginal swabs after internet recruitment. J Community Health 2015; 40(3): 379–386. [DOI] [PubMed] [Google Scholar]
- 19.Katz ML, Zimmermann BJ, Moore D, Paskett ED, Reiter PL. Perspectives from health-care providers and women about completing human papillomavirus (HPV) self-testing at home. Women Health 2017; 57(10): 1161–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howard M, Lytwyn A, Lohfeld L, Redwood-Campbell L, Fowler N, Karwalajtys T. Barriers to acceptance of self-sampling for human papillomavirus across ethnolinguistic groups of women. Can J Public Health 2009; 100(5): 365–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reiter PL, Fisher JL, Hudson AG, Tucker TC, Plascak JJ, Paskett ED. Assessing the burden of HPV-related cancers in Appalachia. Hum Vaccin Immunother 2013; 9(1): 90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Association for Public Opinion Research. Standard definitions: Final dispositions of case codes and outcome rates for surveys 9th ed. AAPOR; 2016. [Google Scholar]
- 23.Centers for Disease Control and Prevention (CDC). Inside knowledge: Cervical cancer 2012. Available at: https://www.cdc.gov/cancer/cervical/pdf/cervical_facts.pdf.
- 24.Unger JB, Cabassa LJ, Molina GB, Contreras S, Baron M. Evaluation of a fotonovela to increase depression knowledge and reduce stigma among Hispanic adults. J Immigr Minor Health 2013; 15(2): 398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. Laboratory procedure manual 2014. Available at: https://www.cdc.gov/Nchs/Data/Nhanes/Nhanes_13_14/HPVSWR_H_MET_COBAS.pdf.
- 26.Karjalainen L, Anttila A, Nieminen P, Luostarinen T, Virtanen A. Self-sampling in cervical cancer screening: Comparison of a brush-based and a lavage-based cervicovaginal self-sampling device. BMC Cancer 2016; 16: 221–016-2246–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sultana F, Mullins R, English DR, et al. Women’s experience with home-based self-sampling for human papillomavirus testing. BMC Cancer 2015; 15: 849–015-1804-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Racey CS, Gesink DC, Burchell AN, Trivers S, Wong T, Rebbapragada A. Randomized intervention of self-collected sampling for human papillomavirus testing in under-screened rural women: Uptake of screening and acceptability. J Womens Health (Larchmt) 2016; 25(5): 489–497. [DOI] [PubMed] [Google Scholar]
- 29.Krok-Schoen JL, Oliveri JM, Paskett ED. Cancer care delivery and women’s health: The role of patient navigation. Front Oncol 2016; 6: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pollard K, Jacobsen LA, Population Reference Bureau. The Appalachian region: A data overview from the 2012–2016 American Community Survey; 2018. Available at: https://www.arc.gov/assets/research_reports/DataOverviewfrom2012to2016ACS.pdf. [Google Scholar]


