Introduction
Approximately 18 million Americans with type 2 diabetes (T2D) do not follow recommended guidelines for physical activity,1 and inactivity is independently associated with major diabetes-related complications and mortality.2–4 _ENREF_9 Intensive multicomponent programs for activity and other health behaviors in T2D have had positive results in research trials but may be less feasible in clinical settings.5–8
Motivational interviewing (MI) is a patient-centered approach that has been used in many settings for more than 30 years9–12 and has resulted in increased physical activity in patients with T2D.13 However, MI alone may not be enough to substantially modify activity, and MI’s effects on physical activity in T2D have been relatively small (effect size average of ~0.2 in 3 recent trials).14 These limited effects on activity may not translate to fewer adverse events. Furthermore, specific subgroups may be less likely to benefit from MI, such as those with low expectations of improvement or low overall optimism.15–17
Positive psychology (PP) interventions have the potential to promote physical activity in T2D. PP interventions use structured exercises (e.g., recalling positive events, using personal strengths) to boost the frequency and intensity of positive states. PP is well-accepted, requires little staff training, and can be delivered remotely,18, 19 and PP interventions consistently reduce distress and improve well-being.19, 20 Furthermore, PP programs have increased activity and related behaviors in trials of patients with medical illness.21, 22 However, there has been minimal use of PP in T2D.23
A novel intervention combining PP and MI in T2D may be very effective. A PP intervention could enhance the effects of MI by promoting factors (optimism, perceived support, and confidence) linked to superior outcomes in health behavior interventions,24, 25 and PP could have direct effects on physical activity mediated by increased well-being and confidence (Figure 1).20, 23, 26 These combined and complementary effects of PP and MI may be much more powerful than either approach alone. Prior attempts to combine behavioral and psychological interventions have been successful in T2D,27 but have been designed only for the minority of patients with clinical depression. A PP-MI intervention could be much more widely applicable.
Figure 1.
Conceptual model outlining potential mechanisms by which PP-MI may increase physical activity
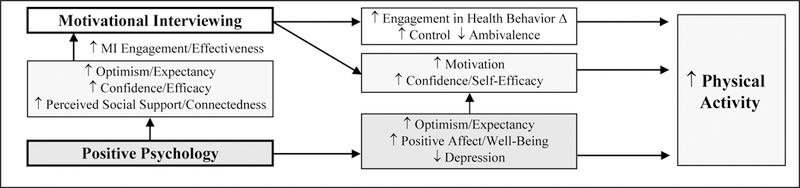
Legend. MI-motivational interviewing; PP=positive psychology
Accordingly, we developed a phone-based, 16-week PP-MI intervention customized for T2D using data from a PP-alone intervention trial in T2D,28 a PP-MI program in cardiac patients,29 and in-depth qualitative interviews with T2D patients. Specifically, we utilized the PP-alone intervention and added MI elements required for this T2D population. We selected a relatively long 16-week intervention, given that programs to promote health behaviors in those with, or at risk for, T2D have required interventions of at least this duration.29, 30 We chose to use a phone-based program based on interviews with medically ill patients, who preferred the combination of a personal connection via phone with the convenience of this modality.31, 32
In this article, we report the results of a proof-of-concept trial that examined the feasibility, acceptability, and initial impact of this novel PP-MI intervention.31, 32 We hypothesized that over 70% of all exercises would be completed, that participants would rate the activities as easy and beneficial (mean score >7.0 on ten-point scales), and that there would be pre-post improvement on psychological and behavioral outcomes.
Methods
Overview
This was a single-arm, proof-of-concept study for outpatients in an academic medical center recruited January-May 2017. Participants had a diagnosis of T2D and suboptimal baseline physical activity (≤150 minutes/week of moderate to vigorous physical activity [MVPA]) assessed via an adapted International Physical Activity Questionnaire (IPAQ).33, 34 All participants received a 16-week PP-MI intervention. Institutional Review Board approval was obtained prior to study initiation, and all participants completed written informed consent.
Participants
Study criteria.
We enrolled English-speaking adults who met the following inclusion criteria:
T2D (meeting American Diabetes Association [ADA] criteria35 for T2D [e.g., hemoglobin A1c [A1C]≥ 6.5% or fasting glucose ≥ 126 mg/dL]), assessed via record review. Participants were required to have T2D for ≥ 1 year with active illness (A1C of ≥ 6.5% within the past 6 months).
Low physical activity (≤150 minutes/week of MVPA [corresponding to ADA recommendations], over the prior week, assessed via the adapted IPAQ.33, 34
Prescribed a non-insulin glucose-lowering drug or lifestyle interventions (e.g., diet/exercise) to manage T2D. We excluded patients taking insulin to reduce heterogeneity of T2D severity in this initial trial.
Exclusion criteria were: (1) cognitive impairment precluding consent or participation, assessed using a six-item screen,36 (2) lack of phone availability, (3) additional medical conditions (e.g., severe arthritis, advanced heart failure) that precluded physical activity per patients or their medical providers, and (4) enrollment in mind-body programs, lifestyle intervention programs (e.g., cardiac rehabilitation), or other clinical trials. We did not exclude patients with psychiatric conditions to improve generalizability and because PP interventions have been effective in such populations.37, 38
Recruitment and enrollment.
Potential participants were adult outpatients in the primary care practices of an urban academic medical center. Upon receiving provider approval, the study team mailed potentially eligible patients opt-out letters that outlined the project; patients who did not opt out from study-related contact were then called by a study coordinator. During this call, the coordinator described the project and screened patients for eligibility (e.g., physical activity via IPAQ and cognitive deficits on six-item screen).
Those patients who met study criteria and were interested in the study then had an initial in-person study visit (In-person Visit #1) during which study details were reviewed, risks and benefits discussed, and written informed consent completed. Participants then completed baseline assessments for study outcome measures (including measurement of A1C and vital signs), and received an accelerometer (ActiGraph GT3X+) to assess baseline physical activity. Participants wore accelerometers on a waist-worn belt for one week before returning to the study clinic (In-person Visit #2), where they met with a study interventionist and began the program (see Study Procedures, below).
Study procedures
All participants received the 16-week PP-MI intervention in this one-arm proof-of-concept trial. During In-person Visit #2, study interventionists met with participants to provide a treatment manual, outline the structure and rationale of the intervention, and assign the first PP and MI exercises. The manual contained a PP and MI exercise for each session, with information about a PP or MI topic, instructions for completion of that week’s activities, space to write about the exercises and their effects, and space to track progress towards physical activity goals. Finally, participants were given a pedometer (Omron HJ-325) to allow them to track their own activity throughout the program.
The remainder of the intervention was completed by phone. Participants independently completed PP exercises, set physical activity goals each week, and reviewed the PP activities and physical activity accomplishments with their study trainer during weekly phone sessions. PP and MI components were delivered and reviewed sequentially within sessions (rather than intertwined) based on our experience, participant feedback, and pilot work. The calls were approximately 30 minutes in duration.
Study interventionists were psychologists with experience delivering PP interventions and MI. Study intervention supervisors (CC, EP) provided training on intervention delivery using a training manual, role-play, and didactic instruction. Weekly interventionist meetings reviewed cases and answered questions about intervention content. Phone sessions were recorded, and portions were reviewed by supervisors (CC, EP) for fidelity to ensure that the PP and MI intervention components were delivered as described in the protocol and trainer manual.
PP component details.
The PP portion of the sessions (15 mins) was structured to include: (i) review of the prior PP exercise, (ii) discussion of how to translate the PP exercise skills to daily life, and (iii) assignment of the next exercise via guided review of the treatment manual. Table 1 outlines the specific PP exercises, and Supplementary Figure 1 outlines pages from the treatment manual.
Table 1.
Positive Psychology Exercises (in order of completion)`
| Week | Session Title | Description |
|---|---|---|
| 1 | Gratitude for positive events34 |
After completing an assessment of their values and long-term goals, participants recall three events, small or large, in the preceding week that were associated with satisfaction, happiness, pride, or other positive states. |
| 2 | Expressing gratitude19 | Participants write a letter of gratitude thanking a person for their support or kindness. |
| 3 | Capitalizing on positive events35 |
Participants identify a positive event as it occurs in their daily life and then capitalize on it by telling another person, extending the good event, or celebrating the event in some other way. |
| 4 | Using gratitude in daily life |
This session focuses on implementing gratitude interventions and skills into daily life. |
| 5 | Remembering your past successes36 |
Participants recall an event in which they experienced success. They next write about the event, their contribution to the success, and the positive feelings elicited by recalling it. |
| 6 | Identifying your personal strengths19 |
Participants complete an assessment of personal strengths and observe their use of one strength during the week. |
| 7 | Using perseverance19 | Participants plan and then use perseverance to complete a specific goal that week. |
| 8 | Humor in everyday life |
Participants recall three funny things that happened to them over the past week, then write about the events and how they made them feel. |
| 9 | Strengths in daily life | This session focuses on implementing strengths-based interventions and skills into daily life. |
| 10 | Enjoyable and meaningful activities37 |
Participants complete three activities: an enjoyable activity alone, an enjoyable activity with another person, and a meaningful activity completed alone or with others. |
| 11 | Performing acts of kindness38 |
Participants complete three acts of kindness, planned or spontaneous, in a single day. |
| 12 | The good life39 | Participants write about a good life over the next year in one or more life domains. |
| 13 | Focusing on meaning in life |
This session focuses on implementing enjoyment- and values-based interventions and skills into daily life. |
| 14 | Planning for the future |
Participants review their favorite exercises and make a plan for continuing to use their PP-based skills in the future. |
MI component details.
The MI section of the intervention was developed from our PP-MI program in ACS patients,29 team members’ MI interventions,9–11 and qualitative data from T2D patients. The MI portion of the calls (15 mins) assessed participants’ motivation to increase physical activity, introduced a topic to enhance motivation and reduce barriers to successfully engage in activity, and developed a physical activity goal (see Table 2). Interventionists typically focused on walking, per patient preference, because brisk walking and other forms of moderate physical activity can improve both physical and mental health39–42—including depression—and because walking is a feasible first step in T2D patients becoming more active.43 The ultimate physical activity goal was typically at least 150 min/week moderate to vigorous physical activity (MVPA), per ADA and national-level guidelines.44 Goals were tailored to each participant based on their clinical status and preferences. Gradual, safe physical activity progression was ensured via a stepwise approach, symptom monitoring, and as-needed coordination with participants’ physicians.
Table 2.
Motivational Interviewing Sessions (in order of completion)
| All Sessions | ||
|---|---|---|
| 5 A’s Model | Interventionists: (a) Ask about progress on the prior cognitive (e.g., pros/cons of becoming active) or physical activity goal, (b) Advise about the benefits of physical activity on health and function, (c) Assess barriers/facilitators to change, (d) Assist with setting a goal, and (e) Arrange the next phone session. |
|
| Session-specific Topics | ||
| Session | Title | MI focus |
| 1 | Moving for better health and tracking your activity |
Participants learn about the benefits of activity and objective ways to track activity. |
| 2 | Setting a SMART physical activity goal |
Participants learn to set SMART (Specific, Measurable, Attainable, Relevant, and Time-based) goals |
| 3 | Barriers and problem solving |
Participants learn to identify barriers to physical activity and problem-solve around them. |
| 4 | Reviewing and reflecting on physical activity |
Participants review their progress thus far and revisit their overall activity goals. |
| 5 | Finding new routes | Participants use the Microscale Audit of Pedestrian Streetscapes-Mini (MAPS-Mini)43 to identify features in their neighborhood that promote walking, then use that information to plan a new walking route. |
| 6 | Using neighborhood resources |
Participants identify resources in their neighborhood (e.g., parks, indoor spaces) that promote activity. |
| 7 | Using equipment resources and making small changes |
Participants identify equipment resources that promote activity and learn ways to make small changes in their lives to increase activity. |
| 8 | Using social resources | Participants identify social resources (e.g., friends, walking groups) that promote activity. |
| 9 | Reviewing and reflecting on physical activity |
Participants review their progress thus far and revisit their overall activity goals. |
| 10 | Managing slips | Participants identify factors that increase the likelihood of “slipping” and problem-solve around them. |
| 11 | Reducing sitting time | Participants learn about the importance of sitting time and track their sitting time over the week. |
| 12 | Standing breaks | Participants schedule standing breaks to reduce sitting time in their routines. |
| 13 | Increasing strength through exercises |
Participants learn about strength training as an adjunct to aerobic exercise. |
| 14 | Planning for the future | Participants review their progress and make a plan for continuing to be active in the future. |
Study outcome measures
Baseline characteristics. We obtained participants’ baseline sociodemographic and medical characteristics from participant report and medical record review.
Feasibility/acceptability (Study Aim 1).
Feasibility was assessed via rates of dropout, proportion of sessions completed, and proportion of participants completing a majority of sessions. Study staff recorded rates of dropout (i.e., no self-report, medical [vital signs/A1C], or physical activity [accelerometer] data at the 16-week follow-up). Interventionists recorded completion of PP-MI sessions. A completed PP-MI session required: (i) PP exercise completion and phone discussion and (ii) creation of a specific physical activity-based goal for the next week. For feasibility, we set a priori thresholds of 80% retention, completion of 70% of total exercises, and completion of 9+ sessions by a majority of participants.
Acceptability was assessed via participants’ reports of ease and utility (provided via 0–10 Likert scales, with 10 being very easy/helpful) of the prior week’s PP exercise and MI-based topic during phone sessions, for four total ratings per session. We set a threshold for acceptability of mean participant ratings of 7.0/10 across all ratings/sessions. Though participants may have provided high ratings due to social desirability, we have previously had substantial variability in these ratings across different exercises.45
Pre-post changes in study outcome measures at 16 weeks (Study Aim 2).
We assessed changes in psychological and behavioral self-report outcome measures at baseline and via an in-person study assessment at 16 weeks.
For physical activity, we utilized both accelerometer-based and self-report data. Accelerometers objectively measure activity, though they only measure some activities (e.g., walking, but not bicycling or swimming), and intermittent accelerometer use may lead to Hawthorne-type effects (i.e., novelty of wearing an accelerometer may transiently increase activity). Self-report activity measures may also have accuracy challenges due to limited participant recall or investigator demand but can capture both more typical levels of activity and a broader scope of activity. We therefore used both accelerometers and self-report to measure activity.
We followed established protocols for the G3TX+ accelerometers.46 Participants were asked to wear the devices for 7 days, and we considered 5 valid days of wear (8+ hours/day) to be sufficient; participants re-wore devices if needed. We used standard cutoffs for MVPA (1952 counts/minute) and non-wear time.47, 48 For broader self-reported activity, we used the IPAQ, which has been well-validated in diverse settings.34
Psychological measures included the Positive and Negative Affect Schedule (PANAS)49 for positive affect, the Life Orientation Test-Revised (LOT-R)50 for optimism, and the Hospital Anxiety and Depression Scale (HADS) depression and anxiety subscales.51
For behavioral outcomes, overall diabetes self-care (e.g., diet, medication, foot care), was measured using the 11-item Summary of Diabetes Self-Care Activities (SDSCA),52 a well-validated measure associated with clinical outcomes.53, 54 T2D medication adherence was measured using a %-based self-report measure55 that correlates highly with pillcap measurement in T2D patients, allowing us to include patients with varied medications/dosing schedules. For dietary adherence, we analyzed the SDSCA dietary items.53
Medical outcomes (Specific Aim 3; exploratory).
At baseline and follow-up, we measured body mass index (BMI), as calculated via height/weight measured by trained study nurses and A1C, as measured by venous blood sampling at study visits.
Statistical analysis
Descriptive statistics were used to summarize baseline characteristics and rates of dropout and session completion. For mean participant ratings of intervention ease and utility, we utilized a mixed effects regression model; this allowed us to account for intra-participant variability, given that each participant could complete up to 13 sessions. For pre-post changes in physical activity, psychological outcomes, other self-report measures, and medical outcomes, we utilized paired t tests. Given the small sample, we did not expect statistically significant changes in study outcomes, and we also measured the effect size (ES; Cohen’s d) of changes in study outcomes. Alpha was set at p=.05, all statistical tests were two-tailed, and all statistical analysis was completed using Stata 15.0.
Results
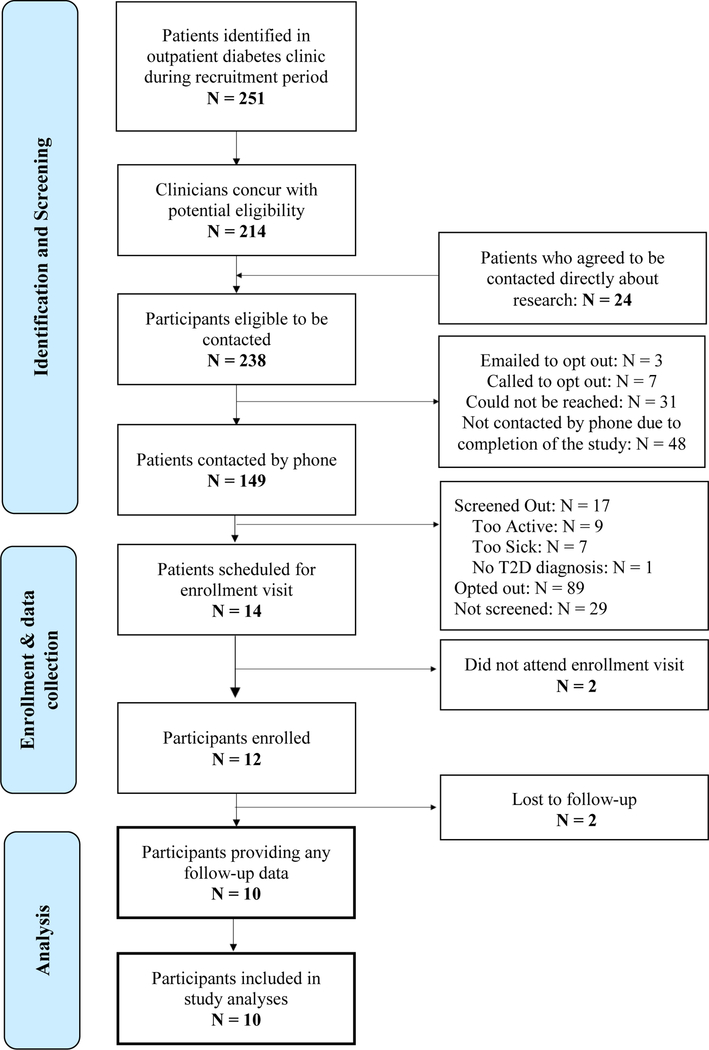
Twelve patients were eligible and enrolled; 10 participants (83%) provided baseline and follow-up questionnaire data (see Figure 2 for study flow diagram) and were analyzed for study outcomes. Of the two participants who dropped out, one expressed being too busy; the second provided no explicit reason for dropout. See Table 3 for participants’ baseline characteristics.
Figure 2.
Study Flow Diagram
Table 3.
Baseline characteristics of study cohort
| Characteristic (N=12) | N (%) |
|---|---|
| Sociodemographic | |
| Age in years (M [SD]) | 58.5 (11.4) |
| Male | 7 (58) |
| White race | 10 (83) |
| Married | 6 (50) |
| Employed | 7 (58) |
| Medical variables | |
| A1C (M [SD]) | 8.9 (1.7) |
| Duration of T2D in years (M [SD]) | 8.9 (4.4) |
| Body mass index (M [SD]) | 37.1 (6.9) |
| Current smoker | 0 (0) |
| Medical comorbidities | |
| Hypertension | 11 (92) |
| Hyperlipidemia | 11 (92) |
| Retinopathy | 0 (0) |
| Nephropathy | 3 (25) |
| Neuropathy | 3 (25) |
| Coronary artery disease | 2 (17) |
| Congestive heart failure | 0 (0) |
| Medications at enrollment | |
| Oral hypoglycemic | 12 (100) |
| Antidepressant | 3 (25) |
| Benzodiazepine | 1 (8) |
| Baseline study outcome measures (M [SD]) | |
| Positive affect (PANAS) | 36.5 (4.9) |
| Optimism (LOT-R) | 18.1 (5.1) |
| Anxiety (HADS-A) | 6.2 (3.5) |
| Depression (HADS-D) | 3.5 (1.7) |
| Dietary adherence (SDSCA diet item) | 2.4 (1.4) |
| Diabetes-related adherence (SDSCA) | 1.9 (0.8) |
| Medication adherence (SRMA) | 8.5 (2.1) |
| Self-reported activity (IPAQ; MET- min/week) |
2,063 (3,779) |
| Accelerometer-measured MVPA (minutes/day) |
18.5 (18.8) |
| Steps (per day; N=10)) | 5,952 (3,427) |
N (%) unless otherwise noted.
Legend. A1C=hemoglobin A1C; HADS-A: Hospital Anxiety and Depression Scale, anxiety subscale; HADS-D: Hospital Anxiety and Depression Scale, depression subscale; IPAQ: International Physical Activity Questionnaire; LOT-R: Life Orientation Test-Revised; MVPA: Moderate to vigorous physical activity; PANAS: Positive and Negative Affect Schedule, positive affect items; SD: Standard Deviation; SDSCA: Summary of Diabetes Self-Care Activities; SRMA: Self-Reported Medication Adherence
Primary aim (feasibility and acceptability)
In total, including all 12 enrollees, 78% (122/156; M=10.2/13) of all possible exercises were completed by participants. A total of 83% of participants (n=10) completed at least 9 of 13 exercises. Excluding the two participants who immediately dropped out after zero or one session, 93% (121/130; M=12.1/13) of all sessions were completed, and all participants completed a majority of the exercises. For acceptability, mean ratings of exercise ease and utility were all greater than 7/10 (PP: mean ease = 8.3 [standard deviation <SD> 1.7], mean utility = 9.1 [SD 1.4]; MI: mean ease = 8.1 [SD 2.1], mean utility = 9.1 [SD 1.8]).
Secondary aim (pre-post changes in study outcomes; see Table 4)
Table 4.
Study outcomes for secondary aim (psychological, behavioral, and health-related outcomes)*
| Outcomes | Baseline mean score (SD) |
Follow-up mean score (SD) |
Pre-post change |
Test- Statistic (t)** |
p-value | Effect size (12 week) |
|
|---|---|---|---|---|---|---|---|
| Psychological outcomes |
Positive affect (PANAS; range: 10–50) |
37.2 (5.1) | 37.4 (4.6) | 0.2 | 0.12 | .91 | .04 |
| Optimism (LOT-R; range: 0–24) |
19.0 (5.0) | 19.4 (3.7) | 0.4 | 0.34 | .74 | .11 | |
| Anxiety***
(HADS-A; range 0–21) |
5.8 (3.6) | 6.6 (4.4) | 0.8 | 0.73 | .48 | .23 | |
| Depression***
(HADS-D; range 0–21) |
3.1 (1.4) | 2.6 (1.8) | −0.5 | −0.64 | .54 | −.20 | |
| Health behavior outcomes |
Dietary adherence (SDSCA diet item; mean days/week) |
2.5 (1.4) | 4.1 (2.0) | 1.6 | 3.08 | .013 | .97 |
| Diabetes self-care composite (SDSCA; mean days/week) |
2.0 (0.7) | 3.8 (1.2) | 1.8 | 5.10 | < .001 | 1.61 | |
| Medication adherence in past 2 weeks (SRMA; range 0–10) |
8.2 (2.2) | 9.3 (1.1) | 1.1 | 2.01 | .075 | .64 | |
| Self-reported physical activity (IPAQ; MET-min/week) |
2,257 (4,135) | 3,099 (4,866) | 842 | 2.61 | .028 | .83 | |
| Accelerometer-measured physical activity (MVPA; minutes per day) |
18.5 (18.8) | 18.0 (17.0) | −0.6 | −0.12 | .91 | −.04 | |
| Accelerometer-measured physical activity, excluding outlier (N=9) (MVPA; minutes per day) |
15.5 (17.2) | 19.1 (17.6) | 3.6 | 1.44 | .19 | .48 | |
| Health outcomes |
Hemoglobin A1C (%) | 8.87 (1.77) | 8.48 (1.54) | −0.39 | −1.00 | .34 | −.32 |
| Body mass index (kg/m2) | 37.3 (6.1) | 37.0 (6.8) | −0.3 | −0.48 | .64 | −.15 | |
N=10 unless otherwise specified
Paired t-test.
Lower scores on these measures represents better psychological health; for all other measures, higher scores represent better status.
Legend. HADS-A: Hospital Anxiety and Depression Scale, anxiety subscale; HADS-D: Hospital Anxiety and Depression Scale, depression subscale; IPAQ: International Physical Activity Questionnaire; LOT-R: Life Orientation Test-Revised; MVPA: Moderate to vigorous physical activity; PANAS: Positive and Negative Affect Schedule, positive affect items; SD: Standard Deviation; SDSCA: Summary of Diabetes Self-Care Activities; SRMA: Self-Reported Medication Adherence.
Regarding physical activity, there were significant improvements in IPAQ self-reported activity (MET minutes/week) (2,257 [SD 4,135] vs. 3,099 [SD 4,866]; t=2.61; p=.03; ES=.83) but not on MVPA measured via accelerometer. One participant with an active job reported having an atypical week (sedentary vacation) for his post-intervention MVPA; removing this participant from analyses resulted in a greater improvement in MVPA (minutes per week) for the cohort, though this remained non-significant (15.5 [SD 17.2] [pre] vs. 19.1 [SD 17.6] [post], t=1.44, p=.19). Correlation between baseline accelerometer data and self-report was not significant (ρ= 0.22; p=.54). There were variable effects on psychological outcomes, with small improvements in positive affect and optimism but a small increase in anxiety. Regarding diabetes-specific self-care, there were large improvements in the overall SDSCA score (2.0 [SD 0.7] vs. 3.8 [SD 1.2]; t=5.10; p<.001; ES=1.61) and dietary adherence score (2.5 [SD 1.4] vs. 4.1 [SD 2.0]; t=3.08; p=.013; ES=0.97). Participants also reported moderate improvements in medication adherence in the past two weeks (8.2 [SD 2.2] vs. 9.3 [SD 1.1]; t=2.01; p=.08; ES=0.64). Finally, there were small pre-post improvements in BMI (37.3 kg/m2 [SD 6.1] to 37.0 kg/m2 [SD 6.8]), and mean A1C dropped from 8.87% (SD 1.77) to 8.48% (SD 1.54).
Discussion
Among patients with T2D and suboptimal physical activity, a 16-week, phone-delivered intervention combining PP content with an MI-based physical activity goal-setting program was feasible and well-accepted in an initial, small, proof-of-concept trial. Overall, 78% of sessions were completed, over 80% of participants completed at least 9/13 exercises, and participants rated intervention exercises as easy and useful.
For the secondary aim related to intervention impact, the intervention was associated with substantial improvements in self-reported activity, dietary adherence, and overall diabetes self-care; there were lesser and more mixed pre-post effects on accelerometer-measured MVPA and psychological outcomes. However, when one outlier (due to an unusual circumstance) was removed, there were medium size effects of the intervention on MVPA. Finally, there were modest but promising effects on BMI and A1C.
These findings are also consistent with prior studies of PP interventions in other medical populations that have found such interventions to be well-accepted and in some cases to improve health behavior outcomes.18, 21, 22 A pair of prior studies of a PP-alone intervention have had mixed effects on health behavior adherence in patients with T2D.23, 28 This was the first study in T2D to combine PP with a behavioral intervention to concurrently leverage the benefits of both intervention types. The magnitude of the effects on self-reported physical activity and overall disease self-care was similar to that seen in similar PP-MI studies, though effects on psychological outcomes were lesser in this small sample than in prior work.28, 29 That the intervention was associated with improvements in key health behaviors, overall diabetes self-care, and some medical outcomes suggests that this intervention may have promise.
If the findings of this pilot study persist in larger, controlled studies, they may have substantial public health importance. Nonadherence to critical T2D health behaviors is associated with more complications, greater functional loss, and diabetes-related mortality.56 Comprehensive health behavior interventions have been effective in research studies, but their complexity and intensity (e.g., requiring multiple in-person visits over a long period) have made them difficult to implement in real-world settings.57–59 In contrast, the PP exercises and MI-based content of this intervention are low-burden and well-accepted. In addition, they can be delivered remotely and require more limited provider training.18, 20, 60 The focus of PP on wellness and strengths, rather than illness, is also highly patient-centered.61
There were several important limitations to this initial study, including the lack of a control condition, the small sample size, a predominantly White cohort, and recruitment from an academic medical center. A substantial proportion of approached patients opted out from participation given the need for multiple in-person study visits and the relatively long intervention.
In summary, a PP-MI intervention in T2D patients was feasible, well-accepted, and had promising effects on clinical outcomes. Next-step studies will aim to include a larger, more diverse sample and to compare the intervention to a control condition to assess whether this simple intervention can have substantial benefits in the large, vulnerable, and growing population of people with T2D. This program may then be well-positioned for broad implementation, given that it can be remotely delivered, requires minimal provider training, and is simple for patients.
Supplementary Material
References.
- 1.Morrato EH, Hill JO, Wyatt HR and Sullivan PW. Physical activity in U.S. adults with diabetes and at risk for developing diabetes, 2003. Diabetes Care 2007; 30: 203–9. [DOI] [PubMed] [Google Scholar]
- 2.Blomster JI, Chow CK, Zoungas S, et al. The influence of physical activity on vascular complications and mortality in patients with type 2 diabetes. Diabetes, Obesity and Metabolism 2013; 15: 1008–12. [DOI] [PubMed] [Google Scholar]
- 3.Brown RE, Riddell MC, Macpherson AK, Canning KL and Kuk JL. All-cause and cardiovascular mortality risk in U.S. adults with and without type 2 diabetes: influence of physical activity, pharmacological treatment and glycemic control. Journal of Diabetes and Its Complications 2014; 28: 311–5. [DOI] [PubMed] [Google Scholar]
- 4.Reddigan JI, Riddell MC and Kuk JL. The joint association of physical activity and glycaemic control in predicting cardiovascular death and all-cause mortality in the US population. Diabetologia 2012; 55: 632–5. [DOI] [PubMed] [Google Scholar]
- 5.Carroll J, Winters P, Fiscella K, et al. Process evaluation of practice-based diabetes prevention programs: what are the implementation challenges? Diabetes Educator 2015; 41: 271–9. [DOI] [PubMed] [Google Scholar]
- 6.Matthews L, Kirk A, Macmillan F and Mutrie N. Can physical activity interventions for adults with type 2 diabetes be translated into practice settings? A systematic review. Translational Behavioral Medicine 2014; 4: 60–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bastiaens H, Sunaert P, Wens J, et al. Supporting diabetes self-management in primary care: pilot-study of a group-based programme focusing on diet and exercise. Primary Care Diabetes 2009; 3: 103–9. [DOI] [PubMed] [Google Scholar]
- 8.Osborn CY, Amico KR, Cruz N, et al. Development and implementation of a culturally tailored diabetes intervention in primary care. Translational Behavioral Medicine 2011; 1: 568–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park ER, Japuntich S, Temel J, et al. A smoking cessation intervention for thoracic surgery and oncology clinics: a pilot trial. Journal of Thoracic Oncology 2011; 6: 1059–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park ER, Quinn VP, Chang Y, et al. Recruiting pregnant smokers into a clinical trial: using a network-model managed care organization versus community-based practices. Preventive Medicine 2007; 44: 223–9. [DOI] [PubMed] [Google Scholar]
- 11.Park ER, Puleo E, Zorn M, et al. A process evaluation of a telephone-based peer-delivered smoking cessation intervention for adult survivors of childhood cancer. Preventive Medicine 2006; 42: 435–42. [DOI] [PubMed] [Google Scholar]
- 12.Miller WR and Rollnick S. Motivational interviewing: Preparing people for change, 3rd edition. New York, NY: Guilford Press, 2012. [Google Scholar]
- 13.Christie D and Channon S. The potential for motivational interviewing to improve outcomes in the management of diabetes and obesity: a clinical review. Diabetes, Obesity and Metabolism 2014; 16: 381–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avery L, Flynn D, van Wersch A, Sniehotta FF and Trenell MI. Changing activity behavior in type 2 diabetes: a systematic review and meta-analysis of behavioral interventions. Diabetes Care 2012; 35: 2681–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goossens ME, Vlaeyen JW, Hidding A, Kole-Snijders A and Evers SM. Treatment expectancy affects the outcome of cognitive-behavioral interventions in chronic pain. Clinical Journal of Pain 2005; 21: 18–26. [DOI] [PubMed] [Google Scholar]
- 16.Joseph CL, Havstad SL, Johnson D, et al. Factors associated with nonresponse to a computer-tailored asthma management program for urban adolescents with asthma. Journal of Asthma 2010; 47: 667–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scheier MF, Helgeson VS, Schulz R, et al. Moderators of interventions designed to enhance physical and psychological functioning among younger women with early-stage breast cancer. Journal of Clinical Oncology 2007; 25: 5710–4. [DOI] [PubMed] [Google Scholar]
- 18.Huffman JC, Mastromauro CA, Boehm JK, et al. Development of a positive psychology intervention for patients with acute cardiovascular disease. Heart International 2011; 6: e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seligman ME, Steen TA, Park N and Peterson C. Positive psychology progress: empirical validation of interventions. Am Psychol 2005; 60: 410–21. [DOI] [PubMed] [Google Scholar]
- 20.Bolier L, Haverman M, Westerhof GJ, Riper H, Smit F and Bohlmeijer E. Positive psychology interventions: a meta-analysis of randomized controlled studies. BMC Public Health 2013; 13: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogedegbe GO, Boutin-Foster C, Wells MT, et al. A randomized controlled trial of positive-affect intervention and medication adherence in hypertensive African Americans. Archives of Internal Medicine 2012; 172: 322–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peterson JC, Charlson ME, Hoffman Z, et al. A randomized controlled trial of positive-affect induction to promote physical activity after percutaneous coronary intervention. Archives of Internal Medicine 2012; 172: 329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohn MA, Pietrucha ME, Saslow LR, Hult JR and Moskowitz JT. An online positive affect skills intervention reduces depression in adults with type 2 diabetes. Journal of Positive Psychology 2014; 9: 523–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McClure JB, Ludman E, Grothaus L, Pabiniak C, Richards J and Mohelnitzky A. Immediate and short-term impact of a motivational smoking intervention using a biomedical risk assessment. Nicotine & Tobacco Research 2009; 11: 394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith DE, Kratt PP and Mason DA. Motivational interviewing to improve adherence to a behavioral weight-control program for older obese women with NIDDM. A pilot study. Diabetes Care 1997; 20: 52–4. [DOI] [PubMed] [Google Scholar]
- 26.Meevissen YM, Peters ML and Alberts HJ. Become more optimistic by imagining a best possible self: effects of a two week intervention. Journal of Behavior Therapy and Experimental Psychiatry 2011; 42: 371–8. [DOI] [PubMed] [Google Scholar]
- 27.Safren SA, Gonzalez JS, Wexler DJ, et al. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in patients with uncontrolled type 2 diabetes. Diabetes Care 2014; 37: 625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DuBois CM, Millstein RA, Celano CM, Wexler DJ and Huffman JC. Feasibility and Acceptability of a Positive Psychological Intervention for Patients With Type 2 Diabetes. Prim Care Companion CNS Disord 2016; 18:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huffman JC, Albanese AM, Campbell KA, et al. The Positive Emotions after Acute Coronary Events behavioral health intervention: Design, rationale, and preliminary feasibility of a factorial design study. Clinical trials 2017; 14: 128–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knowler WC, Fowler SE, Hamman RF, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009; 374: 1677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huffman JC, DuBois CM, Mastromauro CA, Moore SV, Suarez L and Park ER. Positive psychological states and health behaviors in acute coronary syndrome patients: a qualitative study. Journal of health psychology 2016; 21: 1026–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madva EN, Gomez-Bernal F, Millstein RA, et al. Magnitude and sources of distress in mid-life adults with chronic medical illness: an exploratory mixed-methods analysis. Psychol Health Med 2018; 23: 555–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee PH, Macfarlane DJ, Lam T and Stewart SM. Validity of the international physical activity questionnaire short form (IPAQ-SF): A systematic review. International Journal of Behavioral Nutrition and Physical Activity 2011; 8: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Booth ML, Ainsworth BE, Pratt M, et al. International physical activity questionnaire: 12-country reliability and validity. Med sci sports Exerc 2003; 195: 3508–1381. [DOI] [PubMed] [Google Scholar]
- 35.American Diabetes Association. Standards of medical care in diabetes: 2013. Diabetes Care 2013; 36 Suppl 1: S11–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Callahan CM, Unverzagt FW, Hui SL, Perkins AJ and Hendrie HC. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Medical Care 2002; 40: 771–81. [DOI] [PubMed] [Google Scholar]
- 37.Layous K, Chancellor J, Lyubomirsky S, Wang L and Doraiswamy PM. Delivering happiness: translating positive psychology intervention research for treating major and minor depressive disorders. J Altern Complement Med 2011; 17: 675–83. [DOI] [PubMed] [Google Scholar]
- 38.Celano CM, Gomez-Bernal F, Mastromauro CA, et al. A positive psychology intervention for patients with bipolar depression: a randomized pilot trial. J Ment Health 2018; in press. [DOI] [PMC free article] [PubMed]
- 39.Sung K and Bae S. Effects of a regular walking exercise program on behavioral and biochemical aspects in elderly people with type II diabetes. Nursing and Health Sciences 2012; 14: 438–45. [DOI] [PubMed] [Google Scholar]
- 40.Blumenthal JA, Babyak MA, Doraiswamy PM, et al. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med 2007; 69: 587–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ekkekakis P and Murri MB. Exercise as antidepressant treatment: Time for the transition from trials to clinic? Gen Hosp Psychiatry 2017; 49: A1–A5. [DOI] [PubMed] [Google Scholar]
- 42.Gujral S, Aizenstein H, Reynolds CF 3rd, Butters MA and Erickson KI. Exercise effects on depression: Possible neural mechanisms. Gen Hosp Psychiatry 2017; 49: 2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Feo P and Schwarz P. Is physical exercise a core therapeutical element for most patients with type 2 diabetes? Diabetes Care 2013; 36 Suppl 2: S149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Colberg SR, Sigal RJ, Fernhall B, et al. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care 2010; 33: e147–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huffman JC, DuBois CM, Healy BC, et al. Feasibility and utility of positive psychology exercises for suicidal inpatients. Gen Hosp Psychiatry 2014; 36: 88–94. [DOI] [PubMed] [Google Scholar]
- 46.Cain KL, Conway TL, Adams MA, Husak LE and Sallis JF. Comparison of actiGraph accelerometers with the normal filter and the low frequency extension. International Journal of Behavioral Nutrition and Physical Activity 2013; 10: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi L, Ward SC, Schnelle JF and Buchowski MS. Assessment of wear/nonwear time classification algorithms for triaxial accelerometer. Medicine and Science in Sports and Exercise 2012; 44: 2009–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Copeland JL and Esliger DW. Accelerometer assessment of physical activity in active, healthy older adults. Journal of Aging and Physical Activity 2009; 17: 17–30. [DOI] [PubMed] [Google Scholar]
- 49.Watson D, Clark LA and Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology 1988; 54: 1063–70. [DOI] [PubMed] [Google Scholar]
- 50.Scheier MF, Carver CS and Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the Life Orientation Test. Journal of Personality and Social Psychology 1994; 67: 1063–78. [DOI] [PubMed] [Google Scholar]
- 51.Bjelland I, Dahl AA, Haug TT and Neckelmann D. The validity of the hospital anxiety and depression scale. An updated literature review. Journal of psychosomatic research 2002; 52: 69–77. [DOI] [PubMed] [Google Scholar]
- 52.Toobert DJ, Hampson SE and Glasgow RE. The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care 2000; 23: 943–50. [DOI] [PubMed] [Google Scholar]
- 53.Tan SL, Juliana S and Sakinah H. Dietary compliance and its association with glycemic control among poorly controlled type 2 diabetic outpatients in Hospital Universiti Sains Malaysia. Malaysian Journal of Nutrition 2011; 17: 287–99. [PubMed] [Google Scholar]
- 54.Tol A, Shojaeezadeh D, Eslami A, et al. Evaluation of self-care practices and relative components among type 2 diabetic patients. Journal of Education and Health Promotion 2012; 1: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gonzalez JS, Schneider HE, Wexler DJ, et al. Validity of medication adherence self-reports in adults with type 2 diabetes. Diabetes Care 2013; 36: 831–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sadarangani KP, Hamer M, Mindell JS, Coombs NA and Stamatakis E. Physical activity and risk of all-cause and cardiovascular disease mortality in diabetic adults from Great Britain: pooled analysis of 10 population-based cohorts. Diabetes Care 2014; 37: 1016–23. [DOI] [PubMed] [Google Scholar]
- 57.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346: 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stevens VJ, Obarzanek E, Cook NR, et al. Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med 2001; 134: 1–11. [DOI] [PubMed] [Google Scholar]
- 59.Svetkey LP, Stevens VJ, Brantley PJ, et al. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA 2008; 299: 1139–48. [DOI] [PubMed] [Google Scholar]
- 60.Schueller SM and Parks AC. Disseminating self-help: positive psychology exercises in an online trial. Journal of Medical Internet Research 2012; 14: e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lauver DR, Ward SE, Heidrich SM, et al. Patient-centered interventions. Res Nurs Health 2002; 25: 246–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.