Abstract
Males and females differ in the outcome of influenza A virus (IAV) infections, which depends significantly on age. During a typical seasonal influenza epidemic, young children (<5 years of age) and aged adults (65+ years of age) are at greatest risk for severe disease, and among these age groups, males tend to suffer a worse outcome from IAV infection than females. Following infection with either pandemic or outbreak strains of IAVs, females of reproductive ages (i.e., 15–49 years of age) experience a worse outcome than their male counterparts. Among females of reproductive ages, pregnancy is one factor linked to an increased risk of severe outcome of influenza, although it is not the sole factor explaining the femalepreponderance of severe disease. Small animal models of influenza virus infection illustrate that inflammatory immune responses and repair of damaged tissue following IAV infection also differ between the sexes and impact the outcome of infection. There also is growing evidence that sex steroid hormones, including estrogens, progesterone, and testosterone, directly impact immune responses during IAV infection to alter outcomes. Greater consideration of the combined effects of sex and age as biological variables in epidemiological, clinical, and animal studies of influenza pathogenesis is needed.
Keywords: estrogen, gender, immunopathology, pandemic, progesterone, testosterone
Among humans, the prevalence (i.e., number of infected individuals within a population) and intensity (i.e., viral load within an individual) of viral infections, including influenza, differs between males and females (1). Behavioral gender-associated factors, including occupation, personal hygiene (e.g., hand washing), and familial responsibilities (e.g., caring for children or the elderly), can influence exposure to viruses, including influenza A viruses (IAVs) (2). Several studies further illustrate that biological sex differences can lead to differential immune responses and outcomes following infection. Females often are less susceptible to viral infections because they mount stronger immune responses than males. The innate recognition and response to viruses as well as downstream adaptive immune responses differ between males and females during viral infections (3). Sex differences in immune responses to viral infections may further depend on age-related factors and differ prior to puberty, after puberty, or after reproductive senescence (4).
Influenza is a significant public health threat, with influenza A viruses (IAVs) causing seasonal epidemics, occasional outbreaks, and sporadic pandemics. Pulmonary disease following infection with IAV is caused by excessive and aberrant inflammatory responses to the virus, which leads to immunopathology and tissue damage (5). Effective therapies for limiting severe pulmonary disease following IAV infection often limit the ‘cytokine storm’ and pulmonary inflammation (6, 7), in addition to controlling viral replication. The severity of influenza disease is typically worse for young children, aged adults, individuals with compromised immune function, and pregnant women. Rarely are biological sex or gender-related factors considered when assessing the pathogenesis of influenza. In this review, we aim to illustrate that in humans, the age, sex, and reproductive status (i.e., sex steroid hormonal milieu) of an individual can predict the outcome of IAV infection, but rarely are these biological factors considered together in either epidemiological or clinical studies. Furthermore, age, biological sex, and reproductive status are rarely reported or systematically evaluated in studies utilizing animal models. The primary goal of this review is to summarize available data on the effects of biological sex and sex steroid hormones on influenza pathogenesis, with consideration of how these factors change over the life course.
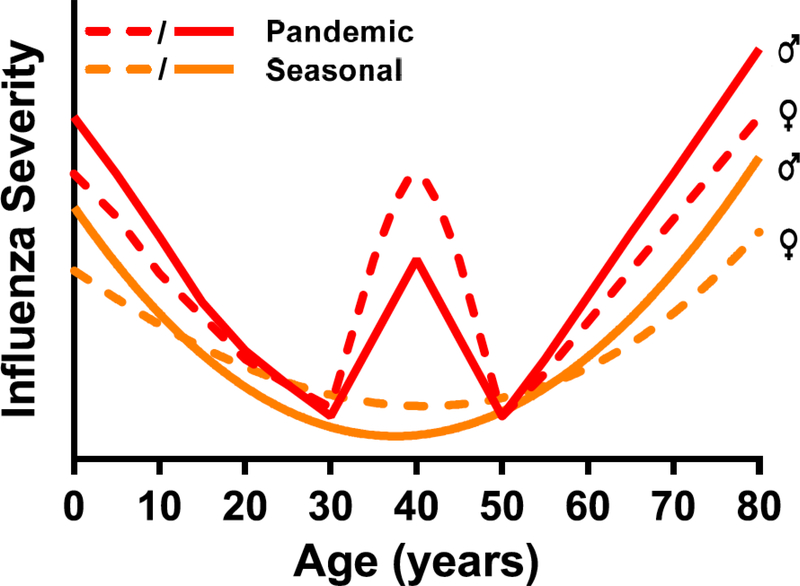
Age-associated sex differences in influenza pathogenesis (Figure 1)
Figure 1. Differential severity of seasonal and pandemic influenza A viruses (IAVs) based on age and sex.
During typical seasonal IAV epidemics, young children (<10 years of age) and aged adults (>65 years of age) are at greatest risk of severe disease. In contrast, during pandemics of IAVs (e.g., the 2009 H1N1 pandemic), in addition to children and aged adults, young adults of reproductive ages often experience disproportionately more severe outcomes. Layered on top of the age distribution is the observation that among children and aged adults, males tend to suffer more severe outcomes than females, whereas females experience more severe outcomes of both seasonal and pandemic IAVs during the reproductive years. Although pregnancy is one factor that impacts female-biased susceptibility to IAVs during the reproductive years, other factors, including greater risk of inflammation and tissue damage, may also contribute (2).
Birth to puberty.
The severity of influenza is generally greater prior to the onset of puberty than during adulthood. For example, hospitalization rates were higher in children under the age of 10 than in older age groups during the 2009 H1N1 pandemic (8). Studies utilizing mice further illustrate that pre-pubertal animals typically suffer greater disease than adult animals following IAV infections (9, 10). In contrast, pre-pubertal male ferrets have milder disease than adult males following IAV infection (11). In ferrets, influenza virus infection is primarily an upper respiratory tract infection, whereas in mice the lower airways are involved (12).
Most case reports of seasonal influenza do not analyze data for differences between males and females or if they do, then they often do not consider the interaction between sex and age. Examination of the limited available reported seasonal influenza cases that required hospitalization reveals that the severity of infection is typically greater in pre-pubertal males compared with age-matched females (13–15) (Figure 1). Data from Denmark suggest that differences between males and females in the risk of hospitalization from seasonal influenza virus shift at puberty. Thus, males are more likely to have severe seasonal influenza illness before puberty, whereas females are more likely to have severe seasonal influenza illness after puberty and before menopause (16). To date no animal studies have evaluated whether the pathogenesis of influenza differs between the sexes prior to puberty.
Reproductive ages.
The 1918 H1N1 influenza pandemic was the most deadly influenza pandemic to date (17). This influenza pandemic was disproportionately fatal in young adult males (i.e., 20–40 years of age) and was exacerbated by co-infection with tuberculosis, which is also considered to be a male-dominant disease (18, 19). Unlike the 1918 H1N1 pandemic, the 1957 H2N2 pandemic was the first pandemic that was lethal without a secondary bacterial infection. The 1957 H2N2 pandemic resulted in higher fatality rates among adult females than males (i.e., <50 years of age), despite the widespread use of vaccine therapy (20, 21). Many of the fatal cases during the H2N2 pandemic had underlying cardiac or pulmonary conditions; thus, sex-biased co-morbid conditions may have contributed to the increased rates of severe disease and mortality among young adult females during the 1957 H2N2 pandemic (21).
During the 2009 H1N1 pandemic in the United States, females were more likely to develop severe disease than males (53.2% female vs. 46.8% male hospitalizations) (2) (Figure 1). Age at the time of infection, however, was strong predictor of the male-female differences in the incidence, severity of 2009 H1N1 pandemic infection, and mortality rates. Among adults, females were at a higher risk of hospitalization and death from 2009 H1N1 infection than males (22). In Japan, a similar female bias was observed in clinical disease following infection with 2009 H1N1 influenza among adults of reproductive ages, which contrasted with the male bias observed prior to puberty (23). In Canada, during the first wave of the pandemic, a majority of critically ill patients with confirmed or probable 2009 H1N1 influenza were young adult females (24) (Figure 1). The reason for the greater proportion of hospitalized adult females than males in Canada is not known, but many cases involved comorbid conditions, including chronic lung disease (e.g., asthma), which is typically more severe in young adult females than males (25). The Canadian government has led the way by requiring disaggregation and analysis of data by sex and gender, which is the most parsimonious explanation for why male-female differences were so widely reported in Canada, and not in other countries, including the United States, during the 2009 H1N1 pandemic.
Avian H5N1 is a highly pathogenic influenza virus that affects the lower respiratory tract in humans and is primarily transmitted from diseased poultry to humans, with rare person-toperson transmission. Worldwide, the incidence and severity of H5N1 infection and mortality caused by H5N1 infection has been greater among young adult females (10–39 years of age) than males (26). There is annual, as well as country, variation in the male-female differences suggesting that gender-related factors, including occupational exposure, play a significant role. Furthermore, during the 2013–14 H7N9 avian influenza outbreak, while aged males were more likely than any other sex and age-matched cohort to be hospitalized with severe disease, it was females of reproductive ages (i.e., 18–50 years of age) who were most likely to die from H7N9 infection (27).
Sex differences in influenza pathogenesis have been systematically evaluated using small animal models (28–32). Using murine models, most, but not all (33), studies have shown that females develop higher pulmonary inflammatory responses and experience a more severe outcome from IAV infection (i.e., with infection with H1N1, H3N1, H3N2, or H7N9 IAVs) than males, despite the sexes having comparable virus titers (28, 29, 34–36). For example, pulmonary concentrations of proinflammatory cytokines (e.g., TNF-α, IFN-γ, IL-6, and IL-12) and chemokines (e.g. CCL2, CCL5, and CCL12) are greater during IAV infection in females than males (27, 28). Male mice also repair damaged pulmonary tissue faster than females (37). Repair of the damaged lung tissue following IAV infection is generally orchestrated by both immune cells (e.g., regulatory T cells and macrophages) and epithelial cells and involves the production of cytokines and growth factors (38, 39). In response to damage, epithelial cells release factors, including amphiregulin (AREG), that can promote repair and integrity of lung tissue damaged during IAV infection (40). Expression of AREG is greater in lung tissue as well as in respiratory epithelial cells derived from males as compared with females during IAV (37). Males also depend on AREG more than females for faster recovery from IAV, because when AREG is deleted from mice, the impact on pulmonary inflammation and function is significantly greater for male than for female mice (37). Importantly, infection of young adult female mice with IAVs reduces ovarian function and concentrations of sex hormones (28, 41) suggesting that inhibition of sex hormones, including estrogens and progesterone (P4) may contribute to severe outcomes from IAV in females (see below).
Reproductive senescence.
During a typical seasonal influenza epidemic, among older individuals (i.e., 65 years of age and older), males are at a higher risk of hospitalization from severe IAV disease than their female counterparts (42) (Figure 1). Of the cases of H7N9 have been reported throughout China, aged males (>50 years of age) were at the greatest risk for contracting H7N9 infection, comprising approximately two-thirds of the cases of H7N9 infection (43, 44). Surveillance studies indicated that approximately 70% of all H7N9 cases were male, with around 75% of confirmed H7N9 cases being males over 45 years of age (45–49).
Therefore, both male sex and older age are risk factors for infection with H7N9 influenza virus. Whether older males are more likely to come in contact with diseased poultry or migratory birds has not been shown.
In animal models of IAV pathogenesis, advanced age is generally associated with increased morbidity (i.e. hypothermia and clinical disease) and mortality, which appears to be caused by reduced control of IAV replication and delayed resolution of pulmonary inflammation (50, 51). Consistent with this, both the innate and adaptive immune responses are slower to initiate, reach lower peak magnitude, and are slower to resolve in aged compared with adult mice (sex either not specified or only female mice used) (50, 52–55). Despite a plethora of studies evaluating the mechanisms mediating the effects of aging on IAV pathogenesis, to date, no studies have evaluated the influence of biological sex on age-related changes in IAV pathogenesis.
Hormonal mechanisms mediating sex differences in influenza pathogenesis
Estrogens.
Estrogens, including 17β-estradiol (E2), occur in high concentrations in nonpregnant as well as pregnant females. E2 is responsible for the majority of the ‘classic’ estrogenic effects in reproductive and non-reproductive tissues. Murine models of IAV pathogenesis demonstrate that E2 treatment protects females against infection-induced morbidity and mortality (28, 56, 57). Treatment of mice with E2 protects against IAV disease by dampening the inflammatory responses associated with tissue damage, including excessive production of IFNγ, TNFα, and CCL2, and by promoting higher antibody responses to influenza vaccination (28, 56, 57). Some (57), but not all (28, 30), studies suggest that treatment of females with E2 affects type I IFN responses and virus replication in the lungs. Treatment with E2 also increases production of chemoattractants for neutrophils, including CCL3 and CXCL1, pulmonary infiltration of neutrophils, and cytokine production by virus-specific CD8+ T cells as compared with placebo-treated females (30). Neutrophils are critical regulators of inflammation, virus clearance, and tissue repair during influenza infection (58, 59). Depletion of neutrophils in E2-treated females reverses the protective effects of E2 on the outcome of IAV infection and increases inflammatory cytokine production (30).
Estriol (E3) is an endogenous estrogen in females with broad biological activity. Exogenous E3 treatment of either female or male mice improves the outcome of IAV infection (41). Further, E3 significantly reduces the transcriptional activity of genes associated with proinflammatory cytokines and chemokines during early IAV infection, which was associated with reduced recruitment of immune cells into the lungs (41). Reduced pulmonary inflammation in E3-treated mice correlates with improved pulmonary function and reduced morbidity and clinical disease. E3 treatment, however, does not alter the kinetics of virus replication and clearance, suggesting that E3-treated animals maintain sufficient antiviral immunity despite reduced overall inflammation (41). Together, these data suggest that exogenous E2 or E3 treatment confers protection during IAV infection through immunomodulatory mechanisms and, thus, suggest that elevated concentrations in females do not cause severe IAV outcomes.
Progestins.
Natural P4 is produced by the corpus luteum during the menstrual cycle in non-pregnant females and its production is sustained at high levels by the placenta during pregnancy. Synthetic analogs of P4, termed progestins, are used as hormonal contraceptives. In C57BL/6 mice infected with IAV, treatment with either P4 or LNG prevents severe outcome by decreasing pulmonary inflammation and promoting faster recovery during a primary infection (31, 60); in contrast, studies utilizing outbred CD-1 mice report minimal effects of P4 morbidity, mortality, and pulmonary inflammation following IAV infection (61). P4-based treatments also promote pulmonary repair following clearance of IAVs by elevating levels of TGF-β, IL-6, IL-22 and increasing numbers of regulatory CD39+ Th17 cells in the lungs. Production of AREG is increased following P4 treatment, which promotes proliferation and repair of respiratory epithelial cells during IAV infection (31). Treatment with either P4 of levonorgestrel also reduces IAV-specific antibody titers as well as IAV-specific memory CD8+ T cells numbers, which results in worse outcome following secondary IAV challenge in female mice (60). While the antiinflammatory effects of P4-based compounds protect against a primary virus infection, the reduction in memory T cell responses increase susceptibility to secondary influenza A virus challenge.
Androgens.
Androgens, specifically testosterone and dihydrotestosterone (DHT), modulate immune responses and susceptibility to a variety of infectious and non-infectious diseases (62, 63). For males, there is an initial surge of testosterone in early development, with concentrations increasing at puberty, reaching peak concentrations in early adulthood, and gradually declining with older age (64–66). In male mice, lower concentrations of testosterone, either associated with aging (i.e., 16–18 months of age) or caused by surgical castration of young males, are associated with increased IAV-associated pulmonary inflammation and morbidity, with no effect on the control of virus replication (37, 51). Importantly, administration of exogenous testosterone to either aged male or castrated young male mice significantly improves the outcome of IAV, independent of changes in pulmonary viral load (51). Although testosterone is protective in mouse models that are designed to allow for survival of infected mice, both testosterone and DHT minimally protect against lethal influenza virus infection (28). Because disease following infection with sub-lethal doses of IAV is largely immune-mediated, while at higher doses of IAV, disease is driven by an inability to control viral replication, these data further illustrate that androgens affect IAV pathogenesis by modulating inflammation and not control of virus replication (67).
Conclusions
Sex is a biological variable that should be considered in studies of IAV pathogenesis. There are gaps in our understanding of the precise mechanisms mediating sex-biased immune responses and how this affects the outcome of IAV infection. Future research must continue to define the pathways mediating how hormones, as reviewed here, but also genes and even the microbiota differently affect immunity to IAV in males compared with females. Future studies should continue to consider the age and reproductive status of individuals as well as whether females or even males are using exogenous hormones (either through contraceptives or replacement therapy) at the time of infection. We recommend that clinicians, epidemiologists, and basic biomedical scientists design experiments that include both males and females, develop a priori hypotheses that the sexes will differ in their responses to and the outcome of IAV infection across the life course, and statistically analyze outcome data by sex and age. The end goal should be that clinicians and researchers alike consider the sex of their patients or animals when interpreting data pertaining to IAV pathogenesis.
Acknowledgements
The writing of this review was supported by the NIH/NIAID Center of Excellence in Influenza Research and Surveillance contract HHS N272201400007C (SLK).
References
- 1.vom Steeg LG, Klein SL. 2016. SeXX Matters in Infectious Disease Pathogenesis. PLoS Pathog 12: e1005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein SL, Pekosz A, Passaretti C, Anker M, Olukoya P. 2010. Sex, gender and influenza, World Health Organization, Geneva: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klein SL, Flanagan KL. 2016. Sex differences in immune responses. Nat Rev Immunol 16: 626–38 [DOI] [PubMed] [Google Scholar]
- 4.Flanagan KL, Fink AL, Plebanski M, Klein SL. 2017. Sex and Gender Differences in the Outcomes of Vaccination over the Life Course. Annu Rev Cell Dev Biol 33: 577–99 [DOI] [PubMed] [Google Scholar]
- 5.Peiris JS, Hui KP, Yen HL. 2010. Host response to influenza virus: protection versus immunopathology. Curr Opin Immunol 22: 475–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cloutier A, Marois I, Cloutier D, Verreault C, Cantin AM, Richter MV. 2012. The prostanoid 15-deoxy-Delta12,14-prostaglandin-j2 reduces lung inflammation and protects mice against lethal influenza infection. J Infect Dis 205: 621–30 [DOI] [PubMed] [Google Scholar]
- 7.Walsh KB, Teijaro JR, Wilker PR, Jatzek A, Fremgen DM, Das SC, Watanabe T, Hatta M, Shinya K, Suresh M, Kawaoka Y, Rosen H, Oldstone MB. 2011. Suppression of cytokine storm with a sphingosine analog provides protection against pathogenic influenza virus. Proc Natl Acad Sci U S A 108: 12018–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chitnis AS, Truelove SA, Drunkenmiller JK, Hefferman RT, Davis JP. 2010. Epidemiologic and clinical features among patients hospitalized in Wisconsin with 2009 H1N1 Influenza A virus infections, April to August 2009. Wisconsin Medical Journal 109: 201–9 [PubMed] [Google Scholar]
- 9.Yasui H, Kiyoshima J, Hori T. 2004. Reduction of influenza virus titer and protection against influenza virus infection in infant mice fed Lactobacillus casei Shirota. Clin Diagn Lab Immunol 11: 675–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun S, Zhao G, Xia W, Hu J, Guo Y, Wu X, Tan Y, Zhou Y. 2011. Age-related sensitivity and pathological differences in infection by 2009 pandemic influenza A (H1N1) virus. Virology Journal 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang SS, Banner D, Degousee N, Leon AJ, Xu L, Paquette SG, Kanagasabai T, Fang Y, Rubino S, Rubin B, Kelvin DJ, Kelvin AA. 2012. Differential pathological and immune responses in newly weaned ferrets are associated with a mild clinical outcome of pandemic 2009 H1N1 infection. J Virol 86: 13187–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouvier NM, Lowen AC. 2010. Animal Models for Influenza Virus Pathogenesis and Transmission. Viruses 2: 1530–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quach C, Piche-Walker L, Platt R, Moore D. 2003. Risk factors associated with severe influenza infections in childhood: implication for vaccine strategy. Pediatrics 112: e197201. [DOI] [PubMed] [Google Scholar]
- 14.Crighton EJ, Moineddin R, Mamdani M, Upshur RE. 2004. Influenza and pneumonia hospitalizations in Ontario: a time-series analysis. Epidemiol Infect 132: 1167–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crighton EJ, Elliott SJ, Kanaroglou P, Moineddin R, Upshur RE. 2008. Spatio-temporal analysis of pneumonia and influenza hospitalizations in Ontario, Canada. Geospat Health 2: 191–202 [DOI] [PubMed] [Google Scholar]
- 16.Jensen-Fangel S, Mohey R, Johnsen SP, Andersen PL, Sorensen HT, Ostergaard L. 2004. Gender differences in hospitalization rates for respiratory tract infections in Danish youth. Scand J Infect Dis 36: 31–6 [DOI] [PubMed] [Google Scholar]
- 17.Noymer A, Garenne M. 2000. The 1918 influenza epidemic’s effects on sex differentials in mortality in the United States. Popul Dev Rev 26: 565–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nhamoyebonde S, Leslie A. 2014. Biological differences between the sexes and susceptibility to tuberculosis. J Infect Dis 209 Suppl 3: S100–6 [DOI] [PubMed] [Google Scholar]
- 19.Noymer A 2009. Testing the influenza-tuberculosis selective mortality hypothesis with Union Army data. Soc Sci Med 68: 1599–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serfling RE, Sherman IL, Houseworth WJ. 1967. Excess pneumonia-influenza mortality by age and sex in three major influenza A2 epidemics, United States, 1957–58, 1960 and 1963. Am J Epidemiol 86: 433–41 [DOI] [PubMed] [Google Scholar]
- 21.Kilbourne ED. 2006. Influenza pandemics of the 20th century. Emerg Infect Dis 12: 9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobs JH, Archer BN, Baker MG, Cowling BJ, Heffernan RT, Mercer G, Uez O, Hanshaoworakul W, Viboud C, Schwartz J, Tchetgen Tchetgen E, Lipsitch M. 2012. Searching for sharp drops in the incidence of pandemic A/H1N1 influenza by single year of age. PLoS One 7: e42328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eshima N, Tokumaru O, Hara S, Bacal K, Korematsu S, Tabata M, Karukaya S, Yasui Y, Okabe N, Matsuishi T. 2011. Sex- and age-related differences in morbidity rates of 2009 pandemic influenza A H1N1 virus of swine origin in Japan. PLoS ONE 6: e19409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar A, Zarychanski R, Pinto R, Cook DJ, Marshall J, Lacroix J, Stelfox T, Bagshaw S, Choong K, Lamontagne F, Turgeon AF, Lapinsky S, Ahern SP, Smith O, Siddiqui F, Jouvet P, Khwaja K, McIntyre L, Menon K, Hutchison J, Hornstein D, Joffe A, Lauzier F, Singh J, Karachi T, Wiebe K, Olafson K, Ramsey C, Sharma S, Dodek P, Meade M, Hall R, Fowler RA. 2009. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. Jama 302: 1872–9 [DOI] [PubMed] [Google Scholar]
- 25.Townsend EA, Miller VM, Prakash YS. 2012. Sex differences and sex steroids in lung health and disease. Endocr Rev 33: 1–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.WHO. 2013. Update on human cases of influenza at the human-animal interface, 2012. [PubMed] [Google Scholar]
- 27.Hoffmann J, Otte A, Thiele S, Lotter H, Shu Y, Gabriel G. 2015. Sex differences in H7N9 influenza A virus pathogenesis. Vaccine 33: 6949–54 [DOI] [PubMed] [Google Scholar]
- 28.Robinson DP, Lorenzo ME, Jian W, Klein SL. 2011. Elevated 17beta-estradiol protects females from influenza A virus pathogenesis by suppressing inflammatory responses. PLoS Pathog 7: e1002149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson DP, Huber SA, Moussawi M, Roberts B, Teuscher C, Watkins R, Arnold AP, Klein SL. 2011. Sex chromosome complement contributes to sex differences in Coxsackievirus B3 but not Influenza A virus pathogenesis. Biol Sex Differ 2: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robinson DP, Hall OJ, Nilles TL, Bream JH, Klein SL. 2014. 17beta-estradiol protects females against influenza by recruiting neutrophils and increasing virus-specific CD8 T cell responses in the lungs. J Virol 88: 4711–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hall OJ, Limjunyawong N, Vermillion MS, Robinson DP, Wohlgemuth N, Pekosz A, Mitzner W, Klein SL. 2016. Progesterone-Based Therapy Protects Against Influenza by Promoting Lung Repair and Recovery in Females. PLoS Pathog 12: e1005840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.vom Steeg LG, Vermillion MS, Hall OJ, Alam O, McFarland R, Chen H, Zirkin B, Klein SL. 2016. Age and testosterone mediate influenza pathogenesis in male mice. Am J Physiol Lung Cell Mol Physiol 311: L1234–L44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Celestino I, Checconi P, Amatore D, De Angelis M, Coluccio P, Dattilo R, Alunni Fegatelli D, Clemente AM, Matarrese P, Torcia MG, Mancinelli R, Mammola CL, Garaci E, Vestri AR, Malorni W, Palamara AT, Nencioni L. 2018. Differential Redox State Contributes to Sex Disparities in the Response to Influenza Virus Infection in Male and Female Mice. Front Immunol 9: 1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lorenzo ME, Hodgson A, Robinson DP, Kaplan JB, Pekosz A, Klein SL. 2011. Antibody responses and cross protection against lethal influenza A viruses differ between the sexes in C57BL/6 mice. Vaccine 29: 9246–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larcombe AN, Foong RE, Bozanich EM, Berry LJ, Garratt LW, Gualano RC, Jones JE, Dousha LF, Zosky GR, Sly PD. 2011. Sexual dimorphism in lung function responses to acute influenza A infection. Influenza Other Respir Viruses 5: 334–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoffmann J, Otte A, Thiele S, Lotter H, Shu Y, Gabriel G. 2015. Sex differences in H7N9 influenza A virus pathogenesis. Vaccine [DOI] [PubMed] [Google Scholar]
- 37.Vermillion MS, Ursin RL, Kuok DIT, Vom Steeg LG, Wohlgemuth N, Hall OJ, Fink AL, Sasse E, Nelson A, Ndeh R, McGrath-Morrow S, Mitzner W, Chan MCW, Pekosz A, Klein SL. 2018. Production of amphiregulin and recovery from influenza is greater in males than females. Biol Sex Differ 9: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun J, Madan R, Karp CL, Braciale TJ. 2009. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat Med 15: 277–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tate MD, Schilter HC, Brooks AG, Reading PC. 2011. Responses of mouse airway epithelial cells and alveolar macrophages to virulent and avirulent strains of influenza A virus. Viral Immunol 24: 77–88 [DOI] [PubMed] [Google Scholar]
- 40.Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, Kubota M, Turner D, Diamond JM, Goldrath AW, Farber DL, Collman RG, Wherry EJ, Artis D. 2011. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol 12: 1045–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vermillion MS, Ursin RL, Attreed SE, Klein SL. 2018. Estriol reduces pulmonary immune cell recruitment and inflammation to protect female mice from severe influenza. Endocrinology [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang CS, Wang ST, Chou P. 2002. Efficacy and cost-effectiveness of influenza vaccination of the elderly in a densely populated and unvaccinated community. Vaccine 20: 2494–9 [DOI] [PubMed] [Google Scholar]
- 43.WHO. 2014. WHO Risk Assessment of human infection with avian influenza A(H7N9) virus, World Health Organization [Google Scholar]
- 44.Skowronski DM, Janjua NZ, Kwindt TL, De Serres G. 2013. Virus-host interactions and the unusual age and sex distribution of human cases of influenza A(H7N9) in China, April 2013. Euro Surveill 18: 20465. [PubMed] [Google Scholar]
- 45.Wang C, Yu H, Horby PW, Cao B, Wu P, Yang S, Gao H, Li H, Tsang TK, Liao Q, Gao Z, Ip DK, Jia H, Jiang H, Liu B, Ni MY, Dai X, Liu F, Van Kinh N, Liem NT, Hien TT, Li Y, Yang J, Wu JT, Zheng Y, Leung GM, Farrar JJ, Cowling BJ, Uyeki TM, Li L. 2014. Comparison of Patients Hospitalized With Influenza A Subtypes H7N9, H5N1, and 2009 Pandemic H1N1. Clin Infect Dis [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Q, Zhou L, Zhou M, Chen Z, Li F, Wu H, Xiang N, Chen E, Tang F, Wang D, Meng L, Hong Z, Tu W, Cao Y, Li L, Ding F, Liu B, Wang M, Xie R, Gao R, Li X, Bai T, Zou S, He J, Hu J, Xu Y, Chai C, Wang S, Gao Y, Jin L, Zhang Y, Luo H, Yu H, He J, Li Q, Wang X, Gao L, Pang X, Liu G, Yan Y, Yuan H, Shu Y, Yang W, Wang Y, Wu F, Uyeki TM, Feng Z. 2014. Epidemiology of human infections with avian influenza A(H7N9) virus in China. N Engl J Med 370: 520–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dudley JP, Mackay IM. 2013. Age-specific and sex-specific morbidity and mortality from avian influenza A(H7N9). J Clin Virol 58: 568–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cowling BJ, Jin L, Lau EH, Liao Q, Wu P, Jiang H, Tsang TK, Zheng J, Fang VJ, Chang Z, Ni MY, Zhang Q, Ip DK, Yu J, Li Y, Wang L, Tu W, Meng L, Wu JT, Luo H, Li Q, Shu Y, Li Z, Feng Z, Yang W, Wang Y, Leung GM, Yu H. 2013. Comparative epidemiology of human infections with avian influenza A H7N9 and H5N1 viruses in China: a population-based study of laboratory-confirmed cases. Lancet 382: 129–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arima Y, Vong S. 2013. Human infections with avian influenza A(H7N9) virus in China: preliminary assessments of the age and sex distribution. Western Pac Surveill Response J 4: 1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yager EJ, Ahmed M, Lanzer K, Randall TD, Woodland DL, Blackman MA. 2008. Ageassociated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. J Exp Med 205: 711–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.vom Steeg LG, Vermillion MS, Hall OJ, Alam O, McFarland R, Chen H, Zirkin B, Klein SL. 2016. Age and testosterone mediate influenza pathogenesis in male mice. American Journal of Physiology-Lung Cellular and Molecular Physiology 311: L1234–L44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gil A, Yassai MB, Naumov YN, Selin LK. 2015. Narrowing of human influenza A virusspecific T cell receptor alpha and beta repertoires with increasing age. J Virol 89: 410216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang J, Bennett AJ, Fisher E, Williams-Bey Y, Shen H, Murasko DM. 2009. Limited expansion of virus-specific CD8 T cells in the aged environment. Mech Ageing Dev 130: 713–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiang J, Fisher EM, Murasko DM. 2011. CD8 T cell responses to influenza virus infection in aged mice. Ageing Res Rev 10: 422–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parzych EM, DiMenna LJ, Latimer BP, Small JC, Kannan S, Manson B, Lasaro MO, Wherry EJ, Ertl HC. 2013. Influenza virus specific CD8(+) T cells exacerbate infection following high dose influenza challenge of aged mice. Biomed Res Int 2013: 876314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nguyen DC, Masseoud F, Lu X, Scinicariello F, Sambhara S, Attanasio R. 2011. 17betaEstradiol restores antibody responses to an influenza vaccine in a postmenopausal mouse model. Vaccine 29: 2515–8 [DOI] [PubMed] [Google Scholar]
- 57.Pazos MA, Kraus TA, Munoz-Fontela C, Moran TM. 2012. Estrogen mediates innate and adaptive immune alterations to influenza infection in pregnant mice. PLoS ONE 7: e40502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tate MD, Brooks AG, Reading PC, Mintern JD. 2012. Neutrophils sustain effective CD8(+) T-cell responses in the respiratory tract following influenza infection. Immunol Cell Biol 90: 197–205 [DOI] [PubMed] [Google Scholar]
- 59.Tate MD, Deng YM, Jones JE, Anderson GP, Brooks AG, Reading PC. 2009. Neutrophils ameliorate lung injury and the development of severe disease during influenza infection. J Immunol 183: 7441–50 [DOI] [PubMed] [Google Scholar]
- 60.Hall OJ, Nachbagauer R, Vermillion MS, Fink AL, Phuong V, Hirsh A, Krammer F, Klein SL. in press. Progesterone-based contraceptives reduce adaptive immune responses and protection against heterosubtypic infection with influenza A viruses. Journal of Virology [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Davis SM, Sweet LM, Oppenheimer KH, Suratt BT, Phillippe M. 2017. Estradiol and progesterone influence on influenza infection and immune response in a mouse model. Am J Reprod Immunol 78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Trigunaite A, Dimo J, Jorgensen TN. 2015. Suppressive effects of androgens on the immune system. Cell Immunol 294: 87–94 [DOI] [PubMed] [Google Scholar]
- 63.Gubbels Bupp MR, Jorgensen TN. 2018. Androgen-Induced Immunosuppression. Front Immunol 9: 794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zirkin BR, Tenover JL. 2012. Aging and declining testosterone: past, present, and hopes for the future. J Androl 33: 1111–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Krause W 2006. Androgens in the demography of male life course ‐ A review. Biodemography and Social Biology 53: 4–12 [DOI] [PubMed] [Google Scholar]
- 66.Handelsman DJ, Sikaris K, Ly LP. 2016. Estimating age-specific trends in circulating testosterone and sex hormone-binding globulin in males and females across the lifespan. Ann Clin Biochem 53: 377–84 [DOI] [PubMed] [Google Scholar]
- 67.Kadel S, Kovats S. 2018. Sex Hormones Regulate Innate Immune Cells and Promote Sex Differences in Respiratory Virus Infection. Frontiers in Immunology 9 [DOI] [PMC free article] [PubMed] [Google Scholar]