Abstract
Introduction:
Gestational ethanol exposure is associated with multiple developmental abnormalities, collectively termed fetal alcohol spectrum disorder (FASD). While the majority of women abstain from ethanol following knowledge of pregnancy, one contributing factor to the high FASD prevalence is that pregnancy is not detected until 4–6 weeks. Thus, ethanol consumption continues during the initial stages of fetal development.
Methods:
An experimental protocol is described in which rhesus macaques self-administer 1.5 g/kg/day ethanol (or isocaloric maltose-dextrin) prior to pregnancy and through the first 60 days of a 168-day gestation term. Menstrual cycles were monitored, including measurements of circulating estradiol and progesterone levels. The latency to consume 1.5g/kg ethanol and blood ethanol concentration (BEC) were measured.
Results:
Twenty-eight fetuses (14 ethanol, 14 controls) were generated in this study. Ethanol did not affect menstrual cycles, or the probability of successful breeding. No ethanol-induced gross adverse effects on pregnancy were observed. Individual variability in latency to complete drinking translated into variability in BEC, measured 90 minutes following session start. Drinking latencies in controls and ethanol-drinkers were longer in the second gestational month than in the first. All pregnancies reached the planned experimental time point of G85, G110, or G135, when in utero MRIs were performed, fetuses were delivered by cesarean section, and brains were evaluated with ex vivo procedures, including slice electrophysiology. Fetal tissues have been deposited to the Monkey Alcohol Tissue Research Resource (MATRR).
Conclusion:
This FASD model takes advantage of the similarities between humans and rhesus macaques in gestational length relative to brain development, as well as similarities in ethanol self-administration and metabolism. The daily 1.5 g/kg dose of ethanol through the first trimester does not influence pregnancy success rates. However, pregnancy influences drinking behavior during the second month of pregnancy. Future publications using this model will describe the effect of early-gestational ethanol exposure on anatomical and functional brain development at subsequent gestational ages.
Keywords: Ethanol self-administration, rhesus macaque, fetal alcohol spectrum disorder, in vivo fetal imaging
Introduction:
Fetal alcohol spectrum disorder (FASD) is a prevalent neurodevelopmental disorder, affecting as many as 2–5% of school-aged children in the United States (May et al., 2009, Lange et al., 2017). A potential contribution to the high prevalence of FASD may be from women that consume alcohol prior to knowing that they are pregnant, when they would otherwise choose not to drink alcohol during pregnancy (CDC, 2013). Epidemiological data from the United States and Canada show that approximately half of reproductive-age women drink alcohol, and because the precise timing of the beginning of pregnancy is usually not known, a similar proportion drink during the first 5 to 6 weeks of pregnancy, prior to pregnancy awareness (Kesmodel, 2001, Tough et al., 2006, Strandberg-Larsen et al., 2008). This early exposure might interfere with the initiation of fetal neurogenesis (Stiles and Jernigan, 2010).
FASD is an umbrella term for the collection of fetal alcohol-induced effects that include physical abnormalities as well as neurocognitive and/or behavioral impairments (Hoyme et al., 2016) such as deficits in executive function, sustained attention tasks, and learning and memory (Hamilton et al., 2003, Kodituwakku, 2009, Manji et al., 2009). These symptoms are not detectable until several years after birth, and as a result, deficits arising from FASD are difficult to identify at early ages. Nevertheless, early detection is critical, because the risk for adverse long-term life outcomes associated with FASD is significantly diminished for individuals identified early in life (Streissguth et al., 2004). Therefore, it would be ideal to detect the effects of early-gestation alcohol exposure on the developing brain during pregnancy. Recent advances in fetal magnetic resonance imaging (MRI) have enabled non-invasive, quantitative study of fetal brain growth throughout the second half of gestation (Rajagopalan et al., 2011, Scott et al., 2011). One advanced MRI method, termed diffusion MRI, has demonstrated the potential for detecting abnormal morphological development of neurons in the developing cerebral cortex of rodents (Leigland et al., 2013a). In order to test the capability of fetal MRI to detect the effects of ethanol exposure on the developing brain in the second half of gestation, an animal model that is similar in gestational length to humans is required. Herein, we describe a non-human primate (NHP) model that has been designed to develop a non-invasive method for identifying neuroanatomical developmental abnormalities that arise from early-life alcohol exposure.
The use of NHPs, particularly rhesus macaques, has several advantages for generating results with high translatability to human clinical contexts. First, the length of gestation, relative to central nervous system development, exhibits higher similarity to humans than other animal model systems such as rodents (Workman et al., 2013). Throughout the second half of gestation, rhesus macaque brains undergo similar phases of brain growth to humans at the same gestational stage. This is a period in which the surface area of the cerebral cortex increases rapidly, the cortex folds, and myelination of many white matter fiber systems is initiated, exhibiting close similarity to human gestational timing relative to brain development. Therefore, macaque monkeys are ideal for assessing the potential of in utero MRI in the detection of brain developmental abnormalities associated with fetal exposure to ethanol. Second, rhesus macaques drink ethanol in a similar manner to humans. Specifically, when given open-access, a large proportion voluntarily drink ethanol in amounts similar to the chronic drinking levels exhibited by human alcoholics (Mello and Mendelson, 1972, Baker et al., 2014, Baker et al., 2017). The rate of ethanol absorption and clearance are also more similar between humans and macaques than rodents (Green et al., 1999, Vivian et al., 2001). To date, the majority of NHP fetal alcohol exposure studies have administered alcohol via intragastric gavage (Clarren and Bowden, 1982, Scott and Fradkin, 1984, Clarren and Astley, 1992, VandeVoort et al., 2015). The remaining studies report administration via a permanently implanted intragastric cannula (Altshuler and Shippenberg, 1981), intravenous delivery (Farber et al., 2010), or self-administration (Elton and Wilson, 1977, Schneider et al., 2001). The rhesus macaque model described here leverages the ability to induce oral intake of a controlled amount of ethanol (Vivian et al., 2001, Grant et al., 2008) with the highly relevant gestational and neurodevelopmental profiles in combination with multidisciplinary and longitudinal techniques that are already available in routine prenatal care settings. We demonstrate the ability to induce drinking of 1.5 g/kg/day of ethanol (an approximate human equivalent of 6 drinks/day) beginning prior to pregnancy and extending through the first 60 days of the 168-day gestational term of macaques. This model employs minimal use of anesthetics by training animals to comply with awake venipuncture and ultrasound, which can potentially confound studies of ethanol exposure in utero (Brambrink et al., 2010, Brambrink et al., 2012). This report examined the rate of drinking the 1.5 g/kg dose and the subsequent blood ethanol concentrations (BECs), the effect of ethanol on generating pregnancies, and the effect of pregnancy on drinking behavior. The data presented here will therefore serve as a reference for future publications and studies supported by tissue and data available through the Monkey Alcohol Tissue Research Resource (MATRR, http://www.matrr.com). Ultimately, this animal model is designed to assess whether fetal MRI possesses the requisite sensitivity to detect neurodevelopmental abnormalities in the second half of gestation, and if so, at which gestational age the highest degree of sensitivity to ethanol-induced damage is achieved. The feasibility of in utero fetal MRI, followed by ex vivo electrophysiological and MRI studies to validate the functional significance of potential anatomical findings, are also demonstrated herein. Future reports will focus on the effects of fetal alcohol exposure on anatomical and functional brain development in the context of this model.
Materials and Methods
Subjects
Forty-two multiparous female rhesus macaques were enrolled in this study. All procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and the NIH guidelines for the care and use of laboratory animal resources and approved by the Oregon National Primate Research Center (ONPRC) Institutional Animal Care and Use Committee.
Dams were individually housed in 76×60×60-cm stainless steel cages, with visual and auditory contact with conspecifics, except during breeding (see below). These subjects were maintained on a positive caloric diet of 1-g banana pellets (Test Diet, St. Louis, MO and BioServe, Flemington, NJ, USA) and housed in a temperature (21 ± 1°C) and humidity (30–50%) controlled environment with a 11:13-h light/dark cycle. A cross-sectional, rather than longitudinal design was employed for this study to avoid varying amounts of isoflurane and ketamine throughout pregnancy. Of the 42 recruited animals, 31 exhibited both consistent menstrual cycles conducive for scheduling time-mated breeding and reliable consumption of 1.5 g/kg/day ethanol or a caloric equivalent of maltose-dextrin solution, and hence were chosen to undergo time-mated breeding procedures. Animals that were not pregnant after 5 breeding attempts were removed from the study. This resulted in exclusion of 6 of the remaining 31 dams. Of these 6 animals, 5 were ethanol-drinking animals and one was a control. In addition, 4 of the dams initially assigned as control animals were re-enlisted in the ethanol group following their first cesarean section (remaining within the same gestational cohort). These 4 dams generated 3 additional ethanol-exposed fetuses (one did not become pregnant after 5 breeding attempts). Data regarding the age, weight, and number of prior pregnancies 25 assigned dams that produced the 28 fetuses are detailed in Table 1, according to each animal’s MATRR ID number. One control fetus was found to be a naturally occurring case of intrauterine growth restriction (IUGR) due to placental insufficiency (Lo et al., 2018), and in one of the ethanol cases, drinking was unintentionally allowed to continue until the 108th day of gestation (as described in “Pregnancy Confirmation”, below). The twenty-eight pregnancies were assigned as follows: G85 (n = 4 drinkers, n = 4 controls), G110 (n = 5 drinkers, n = 5 controls), and G135 (n = 5 drinkers, n = 5 controls). Specific information related to each fetus is summarized in Table 2, according to its MATRR ID number.
Table 1.
Information regarding dams used in this study.
| Age/Group | Dam IDa | # Prior Offspring | Age at Conception | Weight (kg) at Conception | Weight Gain (kg)b | Surgeries other than C-section prior to study |
|---|---|---|---|---|---|---|
| 84C | 10294 | 6 | 12.4 | 7.40 | 0.26 | |
| 87C | 10298c | 1 | 5.9 | 5.74 | 0.80 | |
| 84C | 10295c | 5 | 11.9 | 5.92 | 0.73 | |
| 87C | 10296 | 7 | 11.6 | 7.24 | 0.68 | |
| 83E | 10297 | 7 | 12.1 | 7.30 | 0.52 | |
| 84E | 10298c | 1 | 6.6 | 5.56 | 0.28 | |
| 84E | 10299 | 2 | 11.6 | 6.58 | 0.88 | Bone marrow and lymph node biopsy |
| 83E | 10295c | 6 | 13.0 | 6.06 | 0.76 | |
| 110C | 10300 | 7 | 18.2 | 5.60 | 0.68 | |
| 110C | 10301 | 6 | 16.1 | 6.22 | 0.80 | |
| 110C | 10302 | 7 | 17.1 | 8.06 | 1.40 | |
| 109C | 10303 | 6 | 11.8 | 8.74 | 1.16 | |
| 109C | 10304 | 4 | 10.8 | 6.72 | 0.66 | Bone marrow and lymph node biopsy |
| 111E | 10305 | 8 | 17.3 | 6.46 | 0.54 | |
| 110E | 10306 | 6 | 13.4 | 7.72 | 0.36 | |
| 110E | 10307 | 6 | 11.3 | 7.18 | 1.68 | |
| 110E | 10308 | 1 | 7.4 | 5.32 | 1.33 | |
| 110E | 10309 | 5 | 9.5 | 6.42 | 0.28 | |
| 135C | 10310 | 8 | 18.5 | 6.50 | 0.20 | |
| 136C | 10317c | 2 | 9.8 | 5.56 | 0.90 | Left oophorectomy |
| 134C | 10311 | 3 | 9.3 | 8.04 | 0.14 | |
| 134C | 10312 | 3 | 10.3 | 6.00 | 1.92 | |
| 134C | 10313 | 1 | 7.5 | 6.92 | 1.68 | |
| 135E | 10314 | 7 | 17.1 | 7.88 | 0.58 | |
| 133E | 10315 | 7 | 12.5 | 7.92 | 1.58 | |
| 133E | 10316 | 3 | 5.5 | 6.14 | 1.52 | |
| 136E | 10317c | 3 | 10.8 | 6.10 | 1.52 | |
| 134E | 10318 | 4 | 8.5 | 6.54 | 1.18 |
Monkey ID numbers refer to the MATRR naming convention (http://www.matrr.com).
Defined as the weight at time of C-section minus weight at time of conception.
These animals underwent two pregnancies within this study as described in the text
Table 2.
Fetal information.
| Fetal IDa | Sex | Age/Groupb | Dam IDa | Sire | Fetal Weight (g) | BPD Measures | Ephys | Notes |
|---|---|---|---|---|---|---|---|---|
| 10266 | F | 84C | 10294 | 1 | 71.4 | X | X | |
| 10267 | M | 87C | 10298 | 2 | 83.9 | X | ||
| 10268 | M | 84C | 10295 | 3 | 81.4 | X | ||
| 10269 | M | 87C | 10296 | 4 | 90.1 | X | ||
| 10270 | M | 83E | 10297 | 5 | 90.4 | X | X | |
| 10271 | F | 84E | 10298 | 4 | 66.9 | X | ||
| 10272 | F | 84E | 10299 | 1 | 72.6 | X | ||
| 10273 | M | 83E | 10295 | 6 | 68.0 | X | ||
| 10274 | F | 110C | 10300 | 7 | 193.7 | X | ||
| 10275 | M | 110C | 10301 | 5 | 225.3 | X | X | |
| 10276 | F | 110C | 10302 | 8 | 232.4 | X | X | |
| 10277 | F | 109C | 10303 | 4 | 183.4 | X | ||
| 10278 | F | 109C | 10304 | 6 | NA | X | ||
| 10279 | F | 111E | 10305 | 2 | 181.8 | X | X | |
| 10280 | F | 110E | 10306 | 5 | 165.7 | X | X | |
| 10281 | F | 110E | 10307 | 1 | 177.9 | X | X | |
| 10282 | M | 110E | 10308 | 5 | 205.7 | |||
| 10283 | M | 110E | 10309 | 9 | 205.9 | X | ||
| 10284 | F | 135C | 10310 | 1 | 193.1 | X | X | IUGR due to placental insufficiency. |
| 10285 | F | 136C | 10317 | 3 | 346.0 | |||
| 10286 | M | 134C | 10311 | 7 | 325.0 | X | X | |
| 10287 | F | 134C | 10312 | 8 | 328.0 | X | X | |
| 10288 | F | 134C | 10313 | 6 | 337.0 | X | ||
| 10289 | F | 135E | 10314 | 7 | 335.1 | X | X | Fetus was exposed to 108 days of ethanol drinking. |
| 10290 | M | 133E | 10315 | 2 | 302.6 | X | X | |
| 10291 | F | 133E | 10316 | 1 | 315.0 | X | ||
| 10292 | F | 136E | 10317 | 3 | 310.1 | X | X | |
| 10293 | F | 134E | 10318 | 1 | 338.4 |
Monkey ID numbers refer to the MATRR naming convention (http://www.matrr.com).
Actual gestation age at C-section and necropsy is given, followed by ethanol (E) or control (C) designation.
Blood collection
Each dam was trained to participate in awake blood collection as previously described (Jimenez et al., 2015). Briefly, animals were reinforced with food (fresh fruit, vegetables or nuts) for coming to the front of the cage and presenting their leg through a 10×10cm opening. Blood (3 ml) was collected from the femoral vein in an EDTA vacutainer tube through a 22-gauge needle. This procedure routinely demonstrates low levels of circulating stress hormones (Jimenez et al., 2015, Jimenez et al., 2017). For progesterone (P4) and estradiol (E2) measurements, blood was collected 1–3 times each week within the first 3 hours of the light cycle, and stored on ice for approximately 30 min until centrifuged for 15 min at 4°C (Beckman Colter, Model Allegra 21R). Plasma aliquots were stored at −80°C until assayed for P4 and E2 by the ONPRC Endocrine Technology Services Laboratory using a Roche Cobas e411 platform with 0.03–60 ng/ml and 5–4300 pg/ml sensitivity, respectively.
For blood ethanol concentration (BEC) measurements, blood was collected from the saphenous vein of ethanol-drinking animals only, every 3–7 days, 90 minutes after the daily drinking session began. This time point was chosen based on previous studies showing peak BEC following gavage of 1.5 g/kg ethanol is at 90 minutes (Green et al., 1999), and is consistent with the ethanol self-administration protocol used here (Vivian et al., 2001, Grant et al., 2008). Blood (20-μl) was diluted 25-fold with sterile water, which has been demonstrated to be a sufficient dilution to suppress effects of plasma constituents on ethanol concentration determinations (Tiscione et al., 2011, Tangerman, 1997), and frozen in a sealed glass vial until assayed. Duplicate standards of known ethanol concentration ranging from 25 to 400 mg/dl were used to generate a standard curve. Standards and samples were assayed on the same day using gas chromatography (Hewlett-Packard 5890 Series II, Avondale, PA), equipped with a headspace auto-sampler, flame ionization detector, and a Hewlett Packard 3392A integrator.
Ethanol availability
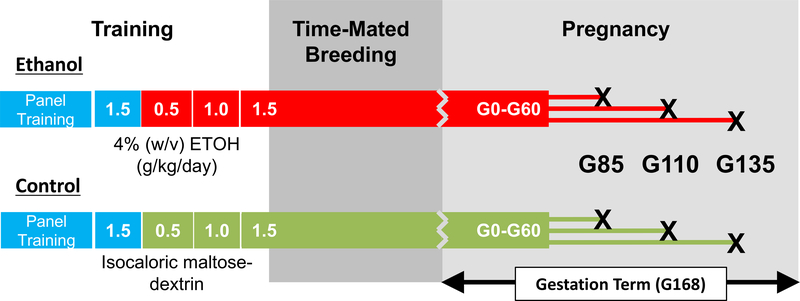
Details of the operant panel have been previously described (Vivian et al., 2001, Grant et al., 2008). Briefly, an operant panel replaced one wall of each cage. The operant panels had two sets of three lights (white, red and green) that signaled an active session, and food and fluid availability, respectively. Below each light set was a fluid spout (for a total of two fluid spouts per panel) that was activated by pulling a recessed dowel located near the center of the panel. Nalgene tubing connected each fluid spout (water and either ethanol or maltose-dextrin) to a 1-L fluid reservoir positioned on a digital scale (Ohaus Navigator Balances N1B110, Ohaus Corporation, Pine Brook, NJ). A 1-g banana food pellet was delivered on a fixed time 300-s (FT- 300) schedule until the predetermined volume of fluid was consumed, after which time water was freely available. Any remaining pellets were available on a FR-1 schedule after a 2-hr time-out by pulling the recessed dowel and activating an infrared finger-poke. Dowel pulls, finger pokes and fluid consumption were recorded every 500-ms via a computerized system using custom hardware and programming environment (National Instruments interface and Labview Software, Austin, TX, USA). The first phase of the induction procedure requires each animal to consume a volume of water that is equivalent to the volume of 1.5 g/kg of 4% ethanol (w/v). Next, animals were required to consume increasing volumes of 4% ethanol to achieve daily doses equal to 0.5 and then 1.0 g/kg in approximately 30-day increments. Animals were then maintained on 1.5 g/kg/day until gestational day 60 (G60) (Figure 1). Animals assigned to the control group experienced identical conditions, but an isocaloric maltose-dextrin solution was available in place of ethanol. To aid in palatability, a 1–2% (w/v) peach-flavored powder was added to both ethanol and maltose-dextrin solutions. Beginning on G61 ethanol was replaced with a 1.5 g/kg/day volume equivalent of the maltose-dextrin/peach juice vehicle and continued until the termination of the pregnancy. Daily sessions were 22-hr. Food and fluids were replenished daily.
Figure 1.
Experimental timeline. Animals were assigned as either ethanol-drinking or control during an approximately 2-month panel training period. Both groups began schedule-induced polydipsia with water (volume of water equivalent to 1.5 g/kg ethanol, indicated in blue) for a 1-month period. Subsequently, 4% w/v ethanol (red) was introduced for ethanol-drinking animals or an isocaloric maltose-dextrin solution (green) for control animals. In approximately 30-day increments, the volume required for each animal to drink was increased from 0.5, to 1.0 to 1.5 g/kg/day. Animals were maintained on 1.5 g/kg/day ethanol (or maltose dextrin) through breeding procedures and the first 60 days of gestation, corresponding to the first trimester. Fetuses were delivered by Cesarean section followed by necropsy at three endpoints indicated by X’s.
Time-mated breeding
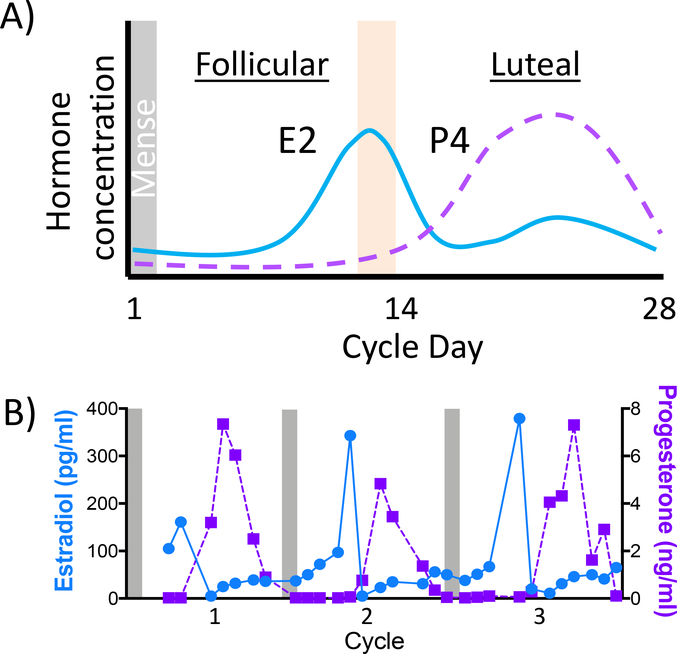
Each dam was trained to present their perineum for daily vaginal swabbing to monitor the length of each menstrual cycle. The first day of mense defined the start of a new cycle (day 1; Figure 2A). Ovulation was estimated as 14 days prior to the end of the average cycle and verified by tri-weekly P4 and E2 assays. Daily P4/E2 blood samples were obtained and assayed as the dam was suspected to be approaching ovulation. A mid-cycle rise in E2 (>100 pg/ml) was suggestive of upcoming ovulation (Figure 2A, orange bar). At this time, females were transferred to another housing room and pair-housed in a doublewide cage with one of nine breeding males as part of the ONPRC Time Mated Breeding program. For dams assigned to the ethanol group, each day the pair was separated for approximately two hours and a bottle containing 1.5 g/kg of alcohol was hung on the outside of the female’s cage (ethanol group only) and the male was provided a hanging bottle containing 150-g of the maltose-dextrin peach flavored solution. The pair was reunited after the two-hour window regardless of whether the 1.5 g/kg dose was consumed to allow the pair continuous opportunity to mate. Dams assigned to the control group were not separated from their partner daily. Breeding pairs were maintained until a rapid decline in estradiol (<100 pg/ml) was observed, and the dam was returned to her home cage. The first day of pairing was defined as gestational day zero (G0).
Figure 2.
Menstrual cycle monitoring for timed mating. A) Schematic of a 28-day menstrual cycle of a rhesus macaque. The cycle begins on the first day of mense, estradiol (E2) peaks at the end of the follicular phase and ovulation (orange bar). Progesterone (P4) rises and falls across the luteal phase. B) Three consecutive cycles from a rhesus macaque. Mense is indicated by grey bars, each cycle has a clear E2 peak followed by the rise and fall of progesterone during the luteal phase.
Pregnancy Confirmation
Circulating P4 and E2 levels were monitored to identify animals likely to be pregnant following pairing (example in Figure 2B). If P4 levels remained elevated (>1 ng/ml) following a mid-luteal phase peak and daily swabs were consistently negative for mense onset, then routine obstetrical ultrasound was performed at approximately G50 (range: G47 to G67) to confirm viability. For a subset of these fetuses, biparietal diameter measurements were performed (Table 2). Ultrasounds were performed in the home cage without sedation using a GE LOGIQ eVet portable ultrasound machine (SOUND, Carlsbad, CA) to determine pregnancy viability. If pregnancies were viable, then tri-weekly E2/P4 measurements were stopped. For animals that were not impregnated, the previously described steps were repeated over subsequent menstrual cycles until a pregnancy was achieved, for a maximum of five breeding attempts per dam. For one ethanol-drinking animal (Dam 10314), circulating hormone levels were not sufficiently sensitive to detect pregnancy until G108. At this point, this animal’s access to ethanol was immediately discontinued, however, this occurred at G108 rather than G60.
In utero MRI
At the time of pregnancy confirmation, the terminal MRI procedure, cesarean section, and necropsy were scheduled. For several cases, it was not feasible to schedule procedures on the exact gestation day that matched the G85, G110, or G135 age cohort to which the fetus was assigned. In these cases, the nearest available day was scheduled, which was always within two days of the planned gestational day (actual gestational ages are listed in Table 2). Pregnant females underwent anatomical and diffusion MRI examination of the fetal brain on a Siemens 3T Tim Trio scanner equipped with a 15-element human knee RF coil. Detailed anesthesia procedures and MRI protocols are described elsewhere (Wang and Kroenke, 2015).
Cesarean section and fetal brain collection:
Following MRI, the dam was maintained on 1–1.5% isoflurane and transported to a surgery suite. For the cesarean section, the dam was placed in dorsal recumbency. The fetus was removed via fundal transverse hysterotomy following umbilical venous and arterial cord blood collection. The umbilical cord was clamped with alligator forceps and transected. The hysterotomy and abdominal incisions were closed in standard fashion and the dam was recovered on the operating table until extubation. Heart rate, respiratory rate, pulse oximetry, expelled carbon dioxide, blood pressure, and body temperature were monitored throughout the procedure.
Prior to fetal necropsy and brain harvesting, standard measurements were taken including whole body weight, crown-rump length, right foot length, right hand length and transtemporal diameter. The chest was bisected and the left cardiac ventricle was cannulated. The brain and cephalic structures were perfused with ice-cold aerated perfusion solution as previously described (Cuzon Carlson et al., 2011). The skullcap was removed and the dura reflected using a scalpel and iris or mayo scissors, the brain was hemisected, and the left hemisphere was immersed in ice-cold aerated perfusion solution and transported to the laboratory for electrophysiological experiments. Perfusion of the right hemisphere continued with 4% paraformaldehyde until the brain tissue stiffened (approximately 5 minutes). The right hemisphere was then immersed in 4% paraformaldehyde overnight and then stored in phosphate buffered saline (PBS) for future post mortem MRI and immunohistochemical procedures (Wang and Kroenke, 2015).
Tissue preparation and slice electrophysiology
Fetal tissue preparation and electrophysiological studies were as previously described for experiments performed in adult rhesus macaques (Cuzon Carlson et al., 2011, Cuzon Carlson et al., 2018). In brief, the left hemisphere of the fetal brain was sectioned coronally (approximately 3–5 mm thick) to reveal the dorsal striatum. From the coronal section, a tissue block containing the striatum and overlying white matter was micro-dissected and immersed in ice-cold sucrose-based cutting solution. Coronal sections (250-μM thin) were obtained with a ceramic blade (Camden Instruments Limited, Lafayette, IN) attached to a Vibroslicer (Leica, Buffalo Grove, IL). Slices were equilibrated for 1-hr in artificial cerebral spinal fluid (aCSF; containing in mM: NaCl, 124; KCl, 4.5; MgCl2, 1; NaHCO3, 26; NaH2PO4, 1.2; glucose, 10; CaCl2, 2) at a temperature of 33°C, after which they were kept at room temperature until experimental use.
Slices were transferred to a recording chamber fixed to the stage of an upright microscope (Axioskop2, Zeiss, Thornwood, NY) and continuously perfused with solution maintained at a temperature of 28–32°C (Automatic Temperature Controller, Warner Instruments, Hamden, CT). Recording patch pipettes were pulled from borosilicate glass capillaries (1.5 mm outer diameter, 0.86 mm inner diameter, Sutter Instruments Co., Novato, CA) that when filled with a CsCl-based internal solution (contained in mM: CsCl, 150; HEPES, 10; MgCl2, 2; Na-GTP, 0.3; Mg-ATP, 3; BAPTA-4K, 0.2) and had a resistance of 4–6 MΩ. Slices were constantly perfused with aCSF supplemented with D-(−)2-amino-5-phosphonopentanoic acid (D-APV; 50μM; Tocris, Ellisville, MO) and 2,3-Dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide (NBQX; 5μM; Tocris) to isolate spontaneous inhibitory postsynaptic currents (sIPSCs). Recordings were made using a Multiclamp 700B amplifier (Molecular Devices, Foster City, CA) and whole-cell membrane currents were filtered at 2 kHz, digitized using Clampex v10.
Data Analysis
Comparisons between control and ethanol-drinking subjects were made using unpaired Student’s t-tests. A one-way ANOVA was used when comparisons were made between more than two groups. When repeated measures were necessary, a linear mixed effect model using the LME package (Pinheiro, 2018) in RStudio (R Core Team, 2015) was used with individual as the subject variable and cohort (three levels: G85, G110, G135), time (two levels: pre and post pregnancy or first and second 30-days) and their interaction as independent variables. Alpha < 0.05 was set as the threshold for statistical significance. All data are presented as mean ± standard error of the mean, unless otherwise noted.
Results
Alcohol did not alter menstrual cycle or rate of conception
On average, both control and ethanol drinking animals had similar menstrual cycle lengths (control: 32 ± 1 days; drinkers: 31 ± 2 days, t(21) = 0.68, p = 0.51). Of successful pregnancies, both control and ethanol drinking animals had a comparable number of time-mated breeding sessions (controls: 2.4 ± 1.6; drinkers: 2.3 ± 1.4; t(26) = 0.12, p = 0.90). Although there were more ethanol-drinking monkeys that were excluded from the study due to 5 unsuccessful breeding attempts (n=5, compared to n=1 control animal excluded in this manner), this was not a statistically significant difference (Fisher exact probability test, p = 0.20). No spontaneous miscairrages were observed in the viable pregnancies identified by ultrasound at G50. The only notable adverse event was the isolated case of IUGR secondary to placental insufficiency, observed in a control animal (animal 10284, Table 2). The age for the control dams (11.7 ± 4.1 years) did not significantly differ from that of ethanol-drinking dams (10.7 ± 3.6 years) at G1 (t(26) = 0.69, p = 0.67).
Pregnancy alters fluid intake that is not specific for ethanol
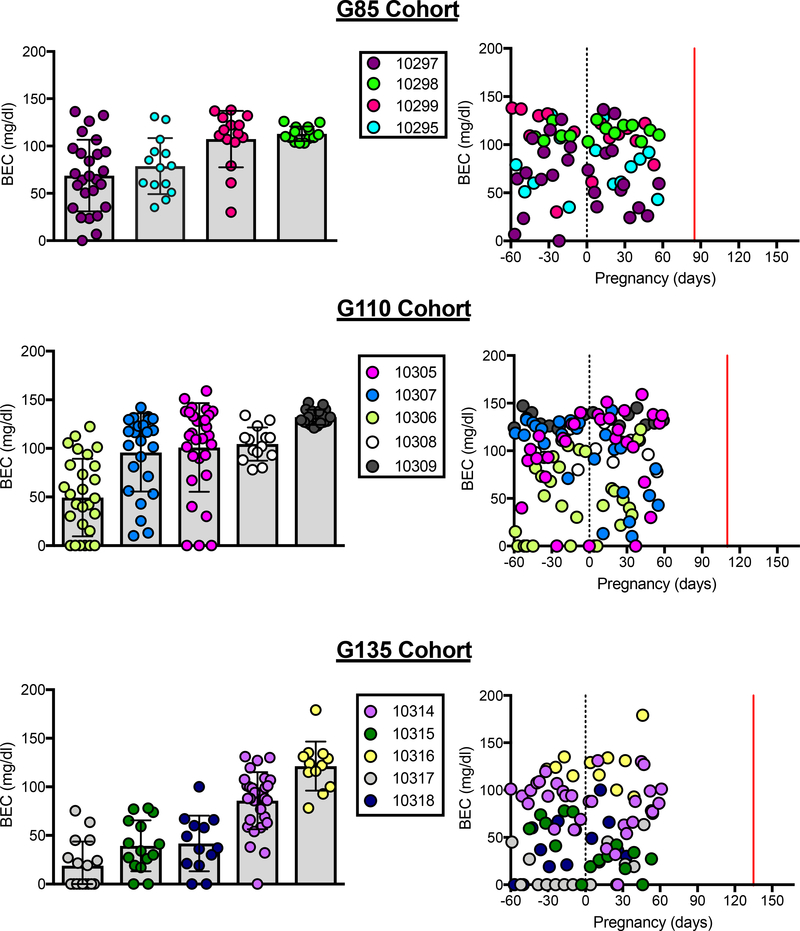
Each ethanol-drinking animal consumed 1.5 g/kg of ethanol per day, however, the time taken to consume the dose varied by individual and influenced BEC measured at 90-minutes following the initiation of drinking session (Figure 3). Despite this variability, a BEC exceeding 80 mg/dl was captured at least once in 13 of the 14 ethanol drinking dams. Overall, no differences were found in BEC across gestation groups (F(2,11) = 1.58, p = 0.25) or within the 60-days before and after pregnancy (F(1,11) = 1.00, p = 0.34). Additionally, while the number of days of ethanol consumption varied prior to pregnancy, there were no differences in lifetime ethanol consumed by dams across the gestational age cohorts (G85: 271.9 ± 33.4 g/kg, G110: 238.2 ± 15.0 g/kg, G135: 259.6 ± 36.3 g/kg; F(2,11) = 0.33, p = 0.73).
Figure 3.
Blood ethanol concentration 90-minutes after the daily drinking session began for all ethanol-drinking monkeys. Animals are individually colored with mean (SEM) presented on the left and individual BECs across gestational day shown on the right. The lines on the scatterplot indicate pregnancy (G0, black) and the cesarean section and fetal necropsy (red). Data presented include samples collected 60-days before and after pregnancy during 1.5 g/kg/day ethanol induction.
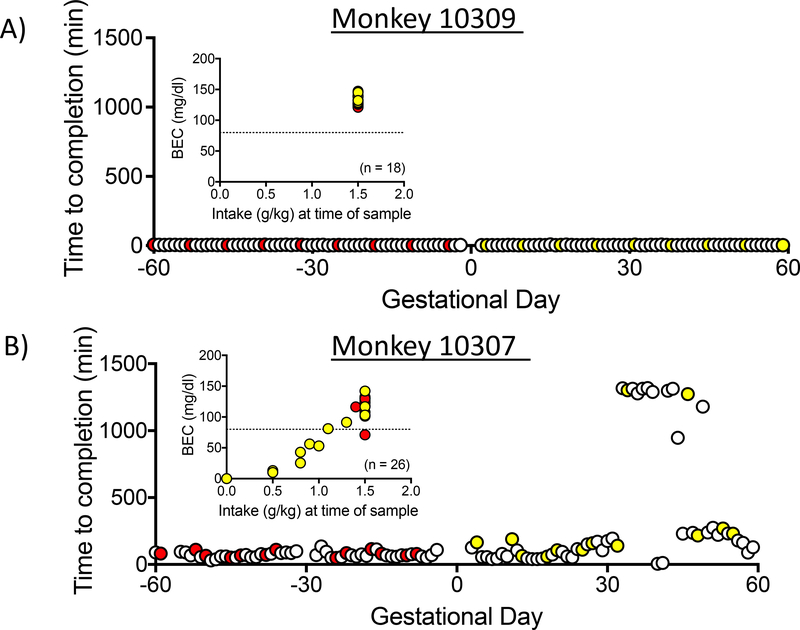
To illustrate the effect of drinking rate on BEC, Figure 4 shows the time to complete the ethanol dose for each drinking session for two monkeys during the 60-days before and after pregnancy (data are not available for the 3–6 day period surrounding day G1 because drinking took place outside of the dams home cage). Monkey 10309 (Figure 4A) reliably and rapidly completed the daily 1.5 g/kg dose with a median time to completion of 4.00 ± 0.55 minutes for the 60 days prior to pregnancy and 3.82 ± 0.54 minutes during the first 60 days of pregnancy. In contrast, monkey 10307 (Figure 4B) took a considerably longer period of time, 70.50 ± 10.43 minutes over the 60 days prior to pregnancy, and 159.10 ± 48.33 minutes during the first 60 days of pregnancy. As mentioned above, blood collection for BEC occurs 90 minutes after the start of the drinking session, and therefore the BEC values reflect the amount and pattern of ethanol consumed at 90 minutes. For example, animal 10309 (Figure 4A inset) completed 1.5 g/kg prior to 90 minutes for all samples taken, leading to consistent BECs over 80 mg/dl. However, animal 10307 (Figure 4B inset) consumed less than 1.5 g/kg at 90 minutes for 30% of the sessions, leading to lower BECs. Notably, animal 10307 exhibited low intakes at 90 minutes, particularly during the first 60 days of pregnancy when BECs fell below 80 mg/dl.
Figure 4.
Latency to consume 1.5g/kg of ethanol for two animals during the 60 days before and after pregnancy. BEC values, measured 90 minutes after the initiation of the drinking session are shown as insets. Pre-pregnancy BECs and latencies are shown in red, post-pregnancy shown in yellow. The subject represented in A drank the daily requirement faster when pregnant compared to the subject represented in B. Inset: The BEC values for the monkey 10309 consistently reflects consumption of the full 1.5 g/kg of ethanol, whereas for monkey 10307, drinking took place over an interval longer than 90 minutes, and as a consequence the 90-minute BEC was lower.
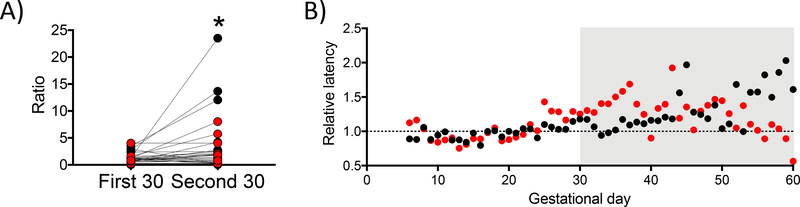
There was a relationship between gestational day and time to complete the 1.5 g/kg dose. As shown in Figure 4B, there are multiple occurrences when time to completion exceeded 16 hours during the second month of pregnancy. A similar pattern was observed for several other animals within both the ethanol and control groups. In order to characterize this pattern, the relative latency to consume 1.5 g/kg ethanol or isocaloric dose of maltose-dextrin was calculated across 30-day bins. The relative latency is the ratio defined as the median latency over the first (days 1–30) and second (days 31–60) 30-days of pregnancy divided by the median baseline latency during the 30 days prior to pregnancy for each animal. Figure 5A shows the median relative latencies for all animals, and Figure 5B shows median relative latencies averaged across control (black) and ethanol-drinking animals (red). A linear mixed-effects model with repeated measures revealed the median relative latency was significantly increased by time (first 30 days: 1.30 ± 0.18, second 30 days: 3.44 ± 0.98; F(1,28) = 1.74, p = 0.024) but that this was unrelated to ethanol or control gestational group (F(1,28) = 5.66, p = 0.20) or an interaction between the two factors (group*time: F(1,28) = 2.14, p = 0.15). These data indicate that during the second month after pregnancy, the latency to complete the daily volume of fluid (ethanol or maltose-dextrin) significantly increased.
Figure 5.
Pregnancy influenced the relative latency to consume the daily fluid volume. A) The median relative latency, the ratio of the median of the first (days 1–30) and second (days 31–60) 30-days of pregnancy divided by the median baseline latency during the 30 days prior to pregnancy for each animal to complete daily fluid requirement averaged by group (red: ethanol, black: maltose-dextrin). The second 30 days of pregnancy (days 31–60) had a significantly longer median relative latency than the first 30 (days 0–30). Student’s t-test, *p<0.05. B) Each data point is the median relative latency across all ethanol-drinking (red) or maltose-dextrin (black) animals.
Assessments of fetal growth
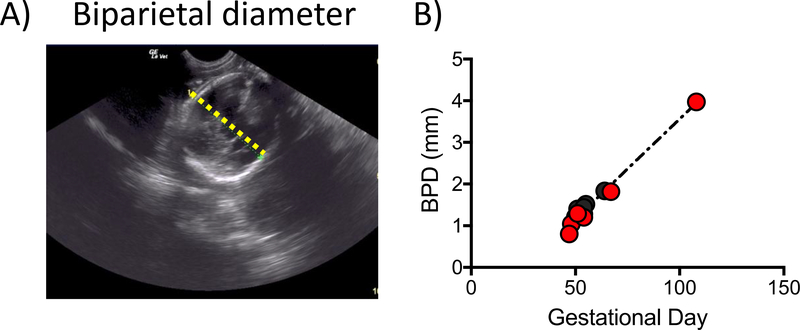
Ultrasounds were performed on awake animals for pregnancy confirmation at approximately G50 (range: G47–67). For a subset of the animals (Table 2), the biparietal diameter (BPD), a standard measurement used for fetal biometry, was measured (Figure 6A). BPD closely tracked the normative growth profile (Tarantal and Hendrickx, 1988) (black line, Figure 6B) for both ethanol-exposed and control fetuses. Although the ethanol-exposed animals were observed to have a smaller BPD than controls in a previous study for a subset of the G110 cohort (Lo et al., 2017), ethanol and control fetuses did not differ with regard to difference between observed BPD and the gestational age-corrected normative value in a combined analysis of all fetuses (Figure 6B).
Figure 6.
Representative ultrasound BPD measurement for a G48 fetus (A). Images were obtained from an awake dam trained to sit calmly for the procedure. (B) The BPD measurements for 13 fetuses fit the normative growth curve for rhesus macaques previously published by Tarantal et al., 1988 (dashed black line). Ethanol-naïve controls shown in black and ethanol exposed in red.
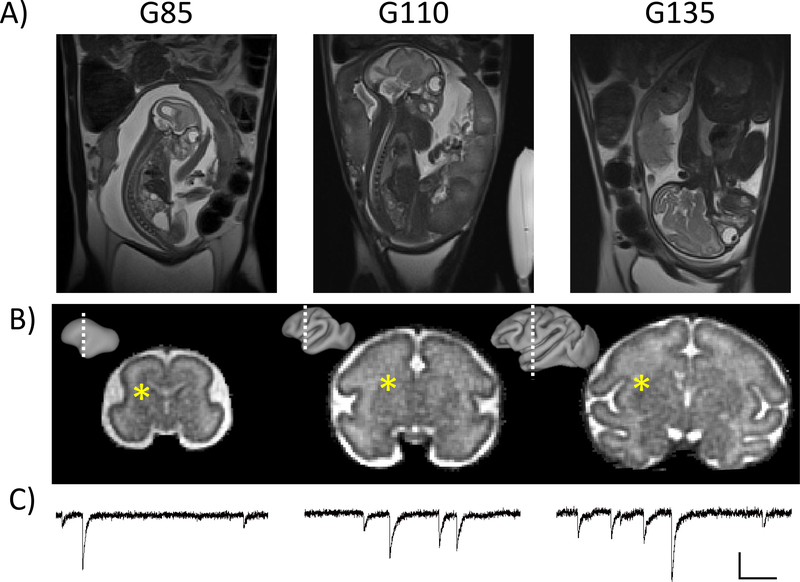
Fetal brain and placental MRIs were performed on all 28 pregnancies (Figure 7A,B). In each case, T2 and diffusion-weighted brain measurements were of suitable quality to perform retrospective motion correction procedures to reconstruct 3D images of each brain (Fogtmann et al., 2014), as exemplified for one animal at each gestational age in Figures 7A and 7B. Results from quantitative analyses will be presented in a future report. Placental MRI findings from a subset of the 28 have been reported previously (Lo et al., 2017).
Figure 7.
In-utero fetal brain MRI and ex vivo electrophysiology. A) T2-weighted images showing a G85 (left), G110 (middle) and G135 (right) fetus. B) High resolution T2-weighted images were acquired on the fetal brain and subjected to retrospective motion correction procedures for reconstructing 3D images of the brain. Lateral views of cerebral cortical hemispheres generated using the brain T2-weighted images are shown as insets, and white dashed lines indicate the position of the coronal views of the 3D images for each brain. C) Representative electrophysiological analysis of spontaneous inhibitory postsynaptic currents (sIPSC) from the caudate nucleus recorded from fetal tissues at G85, G110 and G135. The approximate area is indicated by the asterisks. Vertical scale 30pA, horizontal 150ms.
Immediately following fetal necropsy, ex vivo whole-cell patch clamp electrophysiological measures of intrinsic membrane properties, and excitatory and inhibitory postsynaptic currents were obtained from fetal brain tissue at the three gestational endpoints. Postsynaptic inhibitory current measurements are illustrated in Figure 7C. These are the first electrophysiological recordings from the fetal rhesus brain and demonstrate mature inhibitory synapses in the caudate nucleus (shown) as well as the putamen and somatosensory cortex (not shown) in fetuses as young as G85. Data from a subset of animals (indicated in Table 2) are currently being prepared for a future publication.
Discussion
A primary objective for generating this animal model of FASD was to facilitate the development of neuroimaging biomarkers of abnormal brain development that arise from exposure to ethanol early in gestation, and that can be measured in human subjects. Several animal models of FASD have previously utilized MRI to characterize the effect of fetal alcohol exposure on early brain development (Astley et al., 1995, Godin et al., 2010, Leigland et al., 2013b, O’Leary-Moore et al., 2010, Parnell et al., 2009, Parnell et al., 2013, Parnell et al., 2014, Tang et al., 2018) and see (O’Leary-Moore et al., 2010, Wang and Kroenke, 2015) for review. However, these studies primarily focused on rodents, which are born at earlier stages of CNS development than humans. Therefore, the MRI procedures performed during the early postnatal period, or on ex vivo brain tissue, are not easily translatable to human subjects. One notable exception acquired MRI measurements in macaques, however not until they were 2.4 to 4.1 years of age (Astley et al., 1995), which is the human equivalent to early to late adolescence.
A similarity between humans and NHPs is that NHPs will voluntarily drink alcohol over long periods of time in a manner that closely resembles human drinking (Vivian et al., 2001, Grant et al., 2008). Previously, NHP models of FASD have first established pregnancies and then used methods of ethanol administration, including controlled amounts of ethanol at weekly intervals via intragastric gavage (Clarren et al., 1987, Astley et al., 1995). A potential confound associated with this method is that it is non-voluntary and therefore induces stress, which can negatively affect fetal development (Schneider et al., 2002). Another NHP model of FASD relied on voluntary drinking for alcohol administration (Burke et al., 2009), but in this case, the alcohol dose along with other biological variables such as age, timing in pregnancy, and variables relevant to nutrition were not tightly controlled. The NHP model of FASD developed here provides self-paced drinking with precisely-controlled measures of alcohol intake and resultant BEC during a defined period of early gestation.
In previous studies of fetal alcohol exposure in rhesus macaques, the severity of outcomes on pregnancy and fetal development depended on the dose of ethanol administered. The occurrence of adverse pregnancy outcomes such as miscarriage were observed in animals that were gavaged weekly with ethanol doses of 1.8 g/kg or greater (Clarren et al., 1987, Clarren and Astley, 1992). The anatomical consequences of ethanol exposure worsened with ethanol dose (Bonthius et al., 1996). Corresponding cognitive and behavioral deficits were also observed in these animals (Clarren et al., 1988, Clarren and Astley, 1992). In the above studies, the timing with respect to gestation, as well as the ethanol dose, influenced the severity of ethanol-related insults, with early exposure having a larger impact on fetal development than later exposure. For example, doses of 2.5 g/kg or higher, introduced prior to 5 weeks gestation, consistently produced nonviable pregnancies, whereas viable pregnancies for doses up to 4.1 g/kg were possible beginning after 5 weeks gestation (Clarren and Astley, 1992). It has also been demonstrated that early gestation is associated with greater sensitivity to long-term consequences on cerebral cortical and white matter structure (Miller, 2007). In the weekly gavage paradigm, it was repeatedly observed that dams that experienced peak BEC values of 140 mg/dl and higher were at greater risk of a poor fetal developmental outcome (Clarren et al., 1988, Clarren and Astley, 1992). There are several differences between our study and the experimental conditions in which the 140 mg/dl BEC threshold was observed, such as the daily (as opposed to weekly) exposure in our study. However, we note that a BEC of 140 mg/dl on at least one occasion during pregnancy was documented in multiple dams (4 of 14, Figure 3).
In general, inducing monkeys to drink 1.5 g/kg ethanol in this study did not influence the establishment or maintenance of pregnancy, but rather, pregnancy influenced drinking patterns. Specifically, both ethanol and maltose-dextrin drinking animals exhibited increased relative latency to finish the 1.5 g/kg ethanol (or caloric equivalent) dose. This finding resembles previous observations that pregnant rhesus macaques have an increased likelihood for rejecting food in the second month of pregnancy, compared to earlier and later stages of gestation (Czaja, 1975). Lower food intake was attributed to changes in circulating ovarian hormone levels and specifically associated with increasing E2 and decreasing P4 during the second month of gestation. This pattern is consistent with relatively lower incidence of food rejection in the luteal phase, characterized by high P4 and low E2 levels, of the rhesus menstrual cycle (Czaja, 1975). A similar pattern in voluntary ethanol self-administration in female rhesus macaques is observed, in which for regularly menstruating animals, ethanol intake is higher in the luteal phase when P4 levels are high (Dozier et al., under review). Thus, for pregnant animals, high incidence of food rejection coincides with less rapid drinking, and in non-pregnant animals, low incidence of food rejection coincides with increased ethanol drinking. Although further investigation is needed to more firmly link drinking behavior to P4 levels in pregnant rhesus macaques, this subtle aspect of ethanol self-administration observed in our animal model may also influence the typical pattern of ethanol exposure in women early in pregnancy prior to pregnancy knowledge.
Three important variables in animal models of FASD are the timing of ethanol exposure during gestation, the ethanol dose, and the route of administration. The gestational timing selected for this model (the first 60 days of pregnancy) was motivated, as described above, by the desire to model human drinking prior to pregnancy awareness. The daily 1.5 g/kg ethanol dose was chosen based on previous reports of this dose having a significant effect on offspring development (Clarren and Astley, 1992). The route of administration, relying on self-motivated drinking, has positive attributes of inducing minimal stress and facilitating analysis of latencies to complete drinking throughout the first 60 days of pregnancy, but it is not compatible with ensuring equal peak BEC values for all animals. Animals that complete their drinking procedures faster achieve a higher peak BEC than animals that drink more slowly (Figure 4). In future studies to examine the effect of ethanol exposure on fetal brain development, analyses will be performed to determine whether outcome measures scale with BEC at 90 minutes following the initiation of daily drinking.
An additional limitation of studies involving NHP subjects is that they are costly to maintain compared to other animal model species, and it is therefore not possible to obtain data from large group sizes. This study was able to generate 14 ethanol-exposed and 14 control fetuses, but these were split across 3 time points for each group, and final gestation age and treatment groups consisted of 4–5 animals. Anomalous cases are present in the G135 control and ethanol-exposed groups. In the former, a case of IUGR secondary to placental insufficiency was observed (Lo et al., 2018). In the latter, an animal was exposed to ethanol over a lengthier gestational age range than intended (108 days of drinking, Table 2). Due to the value of each of these animals, the results obtained from them will be included in future reports, and the details of these deviations from the planned experiments documented here will provide important reference information.
It was possible to collect in utero MRI data on all fetuses generated in this study, at three timepoints throughout the second and third trimesters (G85, G110, and G135). Due to its non-invasive nature, MRI is a method that could, in principle, be used to study human fetuses that have been exposed to alcohol in utero to address the challenge of early detection. Studies of humans affected by FASD have identified many anatomical and functional brain abnormalities associated in childhood through adult-aged individuals (Norman et al., 2009). However, to date in utero, postnatal or childhood MRI studies of human subjects have not been referenced to details of alcohol drinking, such as dose, frequency, and timing of use. Further, the gestational stage in which MRI possesses the greatest sensitivity to impaired CNS development is not currently known. The model described in this study will be used to evaluate these questions and extend the in utero MRI imaging to functional consequences of anatomical abnormalities. Shown in Figure 7 are the first electrophysiological investigations of excitatory and inhibitory synapses in the fetal striatum and cortex of rhesus macaques. Thus, this macaque model also provides the unique opportunity to complement the in utero MRI measures with ex vivo functional measures of synaptic function. Importantly, the ex vivo electrophysiology can be adapted to incorporate immunohistochemistry and genomic approaches that further characterize the regions, populations and specific cellular adaptations that result from early gestation ethanol exposure.
While the current focus is on investigating fetal brain development, the opportunity to apply molecular and synaptic underpinnings of in vivo imaging and ultimately behavioral outcomes following birth represent a rich area for understanding the trajectory of FASD. We anticipate that through antenatal and postnatal investigations, it will be possible to implement and evaluate potential therapeutic interventions.
Acknowledgments
Support: NIH Grants AA021981, AA019431, and OD011092
REFERENCES
- Altshuler HL, Shippenberg TS (1981) A subhuman primate model for fetal alcohol syndrome research. Neurobehav Toxicol Teratol 3:121–126. [PubMed] [Google Scholar]
- Astley SJ, Weinberger E, Shaw DW, Richards TL, Clarren SK (1995) Magnetic resonance imaging and spectroscopy in fetal ethanol exposed Macaca nemestrina. Neurotoxicol Teratol 17:523–530. [DOI] [PubMed] [Google Scholar]
- Baker EJ, Farro J, Gonzales S, Helms C, Grant KA (2014) Chronic alcohol self-administration in monkeys shows long-term quantity/frequency categorical stability. Alcohol Clin Exp Res 38:2835–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker EJ, Walter NA, Salo A, Rivas Perea P, Moore S, Gonzales S, Grant KA (2017) Identifying Future Drinkers: Behavioral Analysis of Monkeys Initiating Drinking to Intoxication is Predictive of Future Drinking Classification. Alcohol Clin Exp Res 41:626–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonthius DJ, Bonthius NE, Napper RM, Astley SJ, Clarren SK, West JR (1996) Purkinje cell deficits in nonhuman primates following weekly exposure to ethanol during gestation. Teratology 53:230–236. [DOI] [PubMed] [Google Scholar]
- Brambrink AM, Evers AS, Avidan MS, Farber NB, Smith DJ, Martin LD, Dissen GA, Creeley CE, Olney JW (2012) Ketamine-induced neuroapoptosis in the fetal and neonatal rhesus macaque brain. Anesthesiology 116:372–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambrink AM, Evers AS, Avidan MS, Farber NB, Smith DJ, Zhang X, Dissen GA, Creeley CE, Olney JW (2010) Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology 112:834–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke MW, Palmour RM, Ervin FR, Ptito M (2009) Neuronal reduction in frontal cortex of primates after prenatal alcohol exposure. Neuroreport 20:13–17. [DOI] [PubMed] [Google Scholar]
- Clarren SK, Astley SJ (1992) Pregnancy outcomes after weekly oral administration of ethanol during gestation in the pig-tailed macaque: comparing early gestational exposure to full gestational exposure. Teratology 45:1–9. [DOI] [PubMed] [Google Scholar]
- Clarren SK, Astley SJ, Bowden DM (1988) Physical anomalies and developmental delays in nonhuman primate infants exposed to weekly doses of ethanol during gestation. Teratology 37:561–569. [DOI] [PubMed] [Google Scholar]
- Clarren SK, Bowden DM (1982) Fetal alcohol syndrome: a new primate model for binge drinking and its relevance to human ethanol teratogenesis. J Pediatr 101:819–824. [DOI] [PubMed] [Google Scholar]
- Clarren SK, Bowden DM, Astley SJ (1987) Pregnancy outcomes after weekly oral administration of ethanol during gestation in the pig-tailed macaque (Macaca nemestrina). Teratology 35:345–354. [DOI] [PubMed] [Google Scholar]
- Cuzon Carlson VC, Grant KA, Lovinger DM (2018) Synaptic adaptations to chronic ethanol intake in male rhesus monkey dorsal striatum depend on age of drinking onset. Neuropharmacology 131:128–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzon Carlson VC, Seabold GK, Helms CM, Garg N, Odagiri M, Rau AR, Daunais J, Alvarez VA, Lovinger DM, Grant KA (2011) Synaptic and morphological neuroadaptations in the putamen associated with long-term, relapsing alcohol drinking in primates. Neuropsychopharmacology 36:2513–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czaja JA (1975) Food rejection by female rhesus monkeys during the menstrual cycle and early pregnancy. Physiol Behav 14:579–587. [DOI] [PubMed] [Google Scholar]
- Elton RH, Wilson ME (1977) Changes in ethanol consumption by pregnant pigtailed macaques. J Stud Alcohol 38:2181–2183. [DOI] [PubMed] [Google Scholar]
- Farber NB, Creeley CE, Olney JW (2010) Alcohol-induced neuroapoptosis in the fetal macaque brain. Neurobiol Dis 40:200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogtmann M, Seshamani S, Kroenke C, Xi C, Chapman T, Wilm J, Rousseau F, Studholme C (2014) A unified approach to diffusion direction sensitive slice registration and 3-D DTI reconstruction from moving fetal brain anatomy. IEEE Trans Med Imaging 33:272–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin EA, O’Leary-Moore SK, Khan AA, Parnell SE, Ament JJ, Dehart DB, Johnson BW, Allan Johnson G, Styner MA, Sulik KK (2010) Magnetic resonance microscopy defines ethanol-induced brain abnormalities in prenatal mice: effects of acute insult on gestational day 7. Alcohol Clin Exp Res 34:98–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Leng X, Green HL, Szeliga KT, Rogers LS, Gonzales SW (2008) Drinking typography established by scheduled induction predicts chronic heavy drinking in a monkey model of ethanol self-administration. Alcohol Clin Exp Res 32:1824–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KL, Szeliga KT, Bowen CA, Kautz MA, Azarov AV, Grant KA (1999) Comparison of ethanol metabolism in male and female cynomolgus macaques (Macaca fascicularis). Alcohol Clin Exp Res 23:611–616. [PubMed] [Google Scholar]
- Hamilton DA, Kodituwakku P, Sutherland RJ, Savage DD (2003) Children with Fetal Alcohol Syndrome are impaired at place learning but not cued-navigation in a virtual Morris water task. Behav Brain Res 143:85–94. [DOI] [PubMed] [Google Scholar]
- Hoyme HE, Kalberg WO, Elliott AJ, Blankenship J, Buckley D, Marais AS, Manning MA, Robinson LK, Adam MP, Abdul-Rahman O, Jewett T, Coles CD, Chambers C, Jones KL, Adnams CM, Shah PE, Riley EP, Charness ME, Warren KR, May PA (2016) Updated Clinical Guidelines for Diagnosing Fetal Alcohol Spectrum Disorders. Pediatrics 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez VA, Helms CM, Cornea A, Meshul CK, Grant KA (2015) An ultrastructural analysis of the effects of ethanol self-administration on the hypothalamic paraventricular nucleus in rhesus macaques. Front Cell Neurosci 9:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez VA, Porcu P, Morrow AL, Grant KA (2017) Adaptations in Basal and Hypothalamic-Pituitary-Adrenal-Activated Deoxycorticosterone Responses Following Ethanol Self-administration in Cynomolgus Monkeys. Front Endocrinol (Lausanne) 8:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesmodel U (2001) Binge drinking in pregnancy--frequency and methodology. Am J Epidemiol 154:777–782. [DOI] [PubMed] [Google Scholar]
- Kodituwakku PW (2009) Neurocognitive profile in children with fetal alcohol spectrum disorders. Dev Disabil Res Rev 15:218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange S, Probst C, Gmel G, Rehm J, Burd L, Popova S (2017) Global Prevalence of Fetal Alcohol Spectrum Disorder Among Children and Youth: A Systematic Review and Meta-analysis. JAMA Pediatr 171:948–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigland LA, Budde MD, Cornea A, Kroenke CD (2013a) Diffusion MRI of the developing cerebral cortical gray matter can be used to detect abnormalities in tissue microstructure associated with fetal ethanol exposure. Neuroimage 83:1081–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigland LA, Ford MM, Lerch JP, Kroenke CD (2013b) The influence of fetal ethanol exposure on subsequent development of the cerebral cortex as revealed by magnetic resonance imaging. Alcohol Clin Exp Res 37:924–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo JO, Roberts VHJ, Schabel MC, Wang X, Morgan TK, Liu Z, Studholme C, Kroenke CD, Frias AE (2018) Novel Detection of Placental Insufficiency by Magnetic Resonance Imaging in the Nonhuman Primate. Reprod Sci 25:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo JO, Schabel MC, Roberts VH, Wang X, Lewandowski KS, Grant KA, Frias AE, Kroenke CD (2017) First trimester alcohol exposure alters placental perfusion and fetal oxygen availability affecting fetal growth and development in a non-human primate model. Am J Obstet Gynecol 216:302 e301–302 e308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manji S, Pei J, Loomes C, Rasmussen C (2009) A review of the verbal and visual memory impairments in children with foetal alcohol spectrum disorders. Dev Neurorehabil 12:239–247. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, Hoyme HE (2009) Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev Disabil Res Rev 15:176–192. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH (1972) Drinking patterns during work-contingent and noncontingent alcohol acquisition. Psychosom Med 34:139–164. [DOI] [PubMed] [Google Scholar]
- Miller MW (2007) Exposure to ethanol during gastrulation alters somatosensory-motor cortices and the underlying white matter in the macaque. Cereb Cortex 17:2961–2971. [DOI] [PubMed] [Google Scholar]
- Norman AL, Crocker N, Mattson SN, Riley EP (2009) Neuroimaging and fetal alcohol spectrum disorders. Dev Disabil Res Rev 15:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary-Moore SK, Parnell SE, Godin EA, Dehart DB, Ament JJ, Khan AA, Johnson GA, Styner MA, Sulik KK (2010) Magnetic resonance microscopy-based analyses of the brains of normal and ethanol-exposed fetal mice. Birth Defects Res A Clin Mol Teratol 88:953–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnell SE, Holloway HE, Baker LK, Styner MA, Sulik KK (2014) Dysmorphogenic effects of first trimester-equivalent ethanol exposure in mice: a magnetic resonance microscopy-based study. Alcohol Clin Exp Res 38:2008–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnell SE, Holloway HT, O’Leary-Moore SK, Dehart DB, Paniaqua B, Oguz I, Budin F, Styner MA, Johnson GA, Sulik KK (2013) Magnetic resonance microscopy-based analyses of the neuroanatomical effects of gestational day 9 ethanol exposure in mice. Neurotoxicol Teratol 39:77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnell SE, O’Leary-Moore SK, Godin EA, Dehart DB, Johnson BW, Allan Johnson G, Styner MA, Sulik KK (2009) Magnetic resonance microscopy defines ethanol-induced brain abnormalities in prenatal mice: effects of acute insult on gestational day 8. Alcohol Clin Exp Res 33:1001–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2018) nlme: Linear and Nonlinear Mixed Effects Models. R package version 3:1–137.
- Rajagopalan V, Scott J, Habas PA, Kim K, Corbett-Detig J, Rousseau F, Barkovich AJ, Glenn OA, Studholme C (2011) Local tissue growth patterns underlying normal fetal human brain gyrification quantified in utero. J Neurosci 31:2878–2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Kraemer GW (2001) Moderate alcohol during pregnancy: learning and behavior in adolescent rhesus monkeys. Alcohol Clin Exp Res 25:1383–1392. [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Kraemer GW, Roberts AD, DeJesus OT (2002) The impact of prenatal stress, fetal alcohol exposure, or both on development: perspectives from a primate model. Psychoneuroendocrinology 27:285–298. [DOI] [PubMed] [Google Scholar]
- Scott JA, Habas PA, Kim K, Rajagopalan V, Hamzelou KS, Corbett-Detig JM, Barkovich AJ, Glenn OA, Studholme C (2011) Growth trajectories of the human fetal brain tissues estimated from 3D reconstructed in utero MRI. Int J Dev Neurosci 29:529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott WJ Jr., Fradkin R (1984) The effects of prenatal ethanol in cynomolgus monkeys. Teratology 29:49–56. [DOI] [PubMed] [Google Scholar]
- Stiles J, Jernigan TL (2010) The basics of brain development. Neuropsychol Rev 20:327–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandberg-Larsen K, Rod Nielsen N, Nybo Andersen AM, Olsen J, Gronbaek M (2008) Characteristics of women who binge drink before and after they become aware of their pregnancy. Eur J Epidemiol 23:565–572. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Bookstein FL, Barr HM, Sampson PD, O’Malley K, Young JK (2004) Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. J Dev Behav Pediatr 25:228–238. [DOI] [PubMed] [Google Scholar]
- Tang S, Xu S, Gullapalli RP, Medina AE (2018) Effects of Early Alcohol Exposure on Functional Organization and Microstructure of a Visual-Tactile Integrative Circuit. Alcohol Clin Exp Res 42:727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangerman A (1997) Highly sensitive gas chromatographic analysis of ethanol in whole blood, serum, urine, and fecal supernatants by the direct injection method. Clin Chem 43:1003–1009. [PubMed] [Google Scholar]
- Tarantal AF, Hendrickx AG (1988) Use of ultrasound for early pregnancy detection in the rhesus and cynomolgus macaque (Macaca mulatta and Macaca fascicularis). J Med Primatol 17:105–112. [PubMed] [Google Scholar]
- Tiscione NB, Alford I, Yeatman DT, Shan X (2011) Ethanol analysis by headspace gas chromatography with simultaneous flame-ionization and mass spectrometry detection. J Anal Toxicol 35:501–511. [DOI] [PubMed] [Google Scholar]
- Tough S, Tofflemire K, Clarke M, Newburn-Cook C (2006) Do women change their drinking behaviors while trying to conceive? An opportunity for preconception counseling. Clin Med Res 4:97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VandeVoort CA, Grimsrud KN, Midic U, Mtango N, Latham KE (2015) Transgenerational effects of binge drinking in a primate model: implications for human health. Fertil Steril 103:560–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivian JA, Green HL, Young JE, Majerksy LS, Thomas BW, Shively CA, Tobin JR, Nader MA, Grant KA (2001) Induction and maintenance of ethanol self-administration in cynomolgus monkeys (Macaca fascicularis): long-term characterization of sex and individual differences. Alcohol Clin Exp Res 25:1087–1097. [PubMed] [Google Scholar]
- Wang X, Kroenke CD (2015) Utilization of Magnetic Resonance Imaging in Research Involving Animal Models of Fetal Alcohol Spectrum Disorders. Alcohol Res 37:39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman AD, Charvet CJ, Clancy B, Darlington RB, Finlay BL (2013) Modeling transformations of neurodevelopmental sequences across mammalian species. J Neurosci 33:7368–7383. [DOI] [PMC free article] [PubMed] [Google Scholar]